Introduction
Fragile X syndrome (FXS) is one of the most common
monogenic diseases that can lead to autism spectrum disorder and
intellectual disability (1). Genetic
diagnosis shows that FXS is an X-linked genetic disorder that is
characterized by behavioral problems and specific physical
dysmorphisms (2). Child patients
with FXS have ritualistic behaviors and social deficits and study
has provided for early recognition and diagnosis for the children
patients (3). Previous study
indicated that FXS included the clinical manifestations, such as
language delay, intellectual dysfunction, behavioral and social
problems, and other physical malformations (4). In recent years, although various
medical treatments have been applied for patients with FXS, a
spectrum of medical problems are commonly experienced by people
with FXS, such as otitis media, seizures, and gastrointestinal
problems (5). Therefore, the
efficacy of prenatal genetic diagnosis for FXS plays essential role
for prenatal diseases testing.
Currently, FXS is regarded as a monogenic disease
caused by expansion of a trinucleotide repeat in the 5′
untranslated region of the fragile X mental retardation 1
(FMR1) gene (6). The
FMR1 gene is a microsatellite locus that contains <55 CGG
repeats in its 5′-untranslated region on Xq27.3 (7). Bioinformatics has indicated that CGG
repeat (>200 CGG repeats) in its 5′-untranslated region is
associated with fragile X tremor and ataxia syndrome (FXTAS) by
silencing FMR1 gene and resulting in FXS (8,9). The
abnormal CGG expansion results in methylation and transcriptional
silencing of the FMR1 gene, which subsequently leads to a
reduction or loss of fragile X mental retardation 1 protein (FMRP)
(10). Additionally, previous study
has indicated that methylation of the FMR1 gene exon
1/intron 1 boundary positioned fragile X related epigenetic element
2 (FREE2) affects X-chromosome inactivation (XCI) in FXS full
mutation (11).
In recent years, antenatal diagnosis of FXS plays
essential role in prevention of FXS (12–14).
Study has found that single-tube polymerase chain reaction (PCR)
panel of highly polymorphic markers is an efficient method for
preimplantation genetic diagnosis of FXS (15). Interestingly, nested PCR presents
higher specificity than traditional PCR using two sets of primers
(16). However, the diagnostic
sensitivity of traditional PCR is not enough for FXS patients for
the reason that it can be only detect the smaller pre-mutation gene
(17). Notably, nested PCR can be
used to diagnose Preimplantation genetic diagnosis for FXS, which
has indicated that amplification efficiency was > or =96% for
the FMR1 CGG-repeat region and 5–10% for the polymorphic markers
(18). Although Southern blot
analysis is the gold-standard method for the molecular diagnosis of
FXS, low resolution and complex technology is not suitable for mass
screening for FXS patients (19).
Currently, gene detection using PCR is essential for
patients with suspicious FXS, which could give a good birth and
good care (20). In this study, we
evaluated the diagnostic efficacy of nested PCR for patient with
suspicious FXS. Our results provide a reliable method for diagnosis
of the FMR1 mutation and CGG repeat lengths using nested
PCR.
Materials and methods
Ethical statement
This study was performed in Jiaxing Maternity and
Child Health Care Hospital (Jiaxing, China from May 2015 to
December 2016. This study was carried out with approval by the
Ethics Committee of Jiaxing Maternity and Child Health Care
Hospital. All patients and healthy individuals provided written
informed consent before their participation. The use of their blood
samples were also approved with written informed consent of
participators.
DNA samples
A total number of 32 female patients with FXS and 32
healthy female donors were recruited for gene expression analysis.
The mean age was 28.4 (range, 23.2–36.4) and 28.8 (range,
22.6–35.8) years old in patients with FXS and healthy female
donors, respectively. Genomic DNA samples from female FXS carriers
and healthy female donors were analyzed in this study. Assays were
performed with 32 genomic DNA samples of known FMR1 mutation
(12, 18, 52, and 110 repeats) and 32 genomic DNA samples of healthy
individuals. All cell line DNA templates were purchased from the
Coriell Cell Repositories (Coriell Institute for Medical Research,
Camden, NJ, USA) with critical diagnostic cutoffs. DNA was
extracted using the EasyXpress Viral Nucleic Acid Release kit from
Express Biotech International, Inc. (Thurmont, MD, USA). DNA was
prepared for PCR products from with a master mix from Asuragen
containing GC-rich AMP buffer, FMR1 primers, and GC-rich
polymerase mix.
Nested PCR
The CGG repeat-containing ssDNAs were analyzed using
Cy3-labeled [5′-Cy3-CCGCCGCCGCCGCCG-3′; (CCG)5] repeat
probes and a Cy5-labeled probe
(5′-Cy5-CATCTTCTCTTCAGCCCTGCTAGCGCCGGGAGC-3′; Integrated DNA
Technologies, Coralville, IA, USA). The samples were heated to 95°C
and then cycled 100 times above and below the Tm of the repeat
probe to optimize binding in 100 mM NaCl, 25 mM Tris-Cl, pH 8.0.
PCR procedures were performed as follows: Denaturation step at 94°C
for 5 min; 40 cycles of denaturation at 94°C for 30 sec; annealing
at 56°C for 30 sec; extension at 72°C for 45 sec, with a final
extension at 72°C for 5 min; 4°C soak. For nested PCR, following
the same cycling procedures defined in traditional PCR. Nested PCR
was used to amplify different DNA copy number to increase the
sensitivity of PCR fragment detection.
Analysis of dried blood spots
Peripheral blood (5 ml) was collected from female
FXS carriers and healthy female donors using blood collection tube
(Qiagen, Hilden, Germany). To examine FMR1 gene, we spiked
1.5 µg genomic DNA and spiked blood samples were settled onto 3MM
Whatman filter paper and were dried for 12 h at 25°C. DNA was
extracted described in DNA samples and then eluted in 200 µl of the
supplied buffer. Both the normal and nested PCR assays were
performed to analyze FMR1 mutation.
Capillary electrophoresis
All samples were analyzed by capillary
electrophoresis described previously (21). Conditions for traditional PCR and
nested PCR for subsequent capillary electrophoresis were identical
to those for CCG repeat. Assays were performed by 5′ dTP-PCR
reaction used 0.15 mmol/l 7-deaza-dGTP, 0.05 mmol/l of dGTP,
SYBR-Green I, 5′ and 3′ flanking primers, end-labeled with
6-carboxyfluorescein and 3′ dTP-PCR primers, and thermocycling was
performed on the GeneAmp PCR System 9700 (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Electropherograms were analyzed with GeneMapper software (version
4.0; Applied Biosystems; Thermo Fisher Scientific, Inc.).
DNA sequencing
PCR-amplified FMR1 productions were purified
by a QIAquick PCR Purification kit (no. 28104; Qiagen).
Concentration of PCR productions was calculated using NanoDrop
spectrophotometer. DNA sequencing reactions were completed with 2
to 5 ng of purified PCR products using BigDye Terminator v3.1 Cycle
Sequencing kits (Applied).
Statistical analysis
All data were analyzed by Statistical Package for
the Social Sciences (SPSS 19.0; IBM Corp., Armonk, NY, USA). ROC
curve analysis was determined by the accuracy of the temperature
cutoffs, classifies samples and expansion negative in the blinded
validation study. The distributions of the measured CGG repeat
values were fit with two Gaussian functions to determine the
average values and the relative fractions of the repeat
populations.
Results
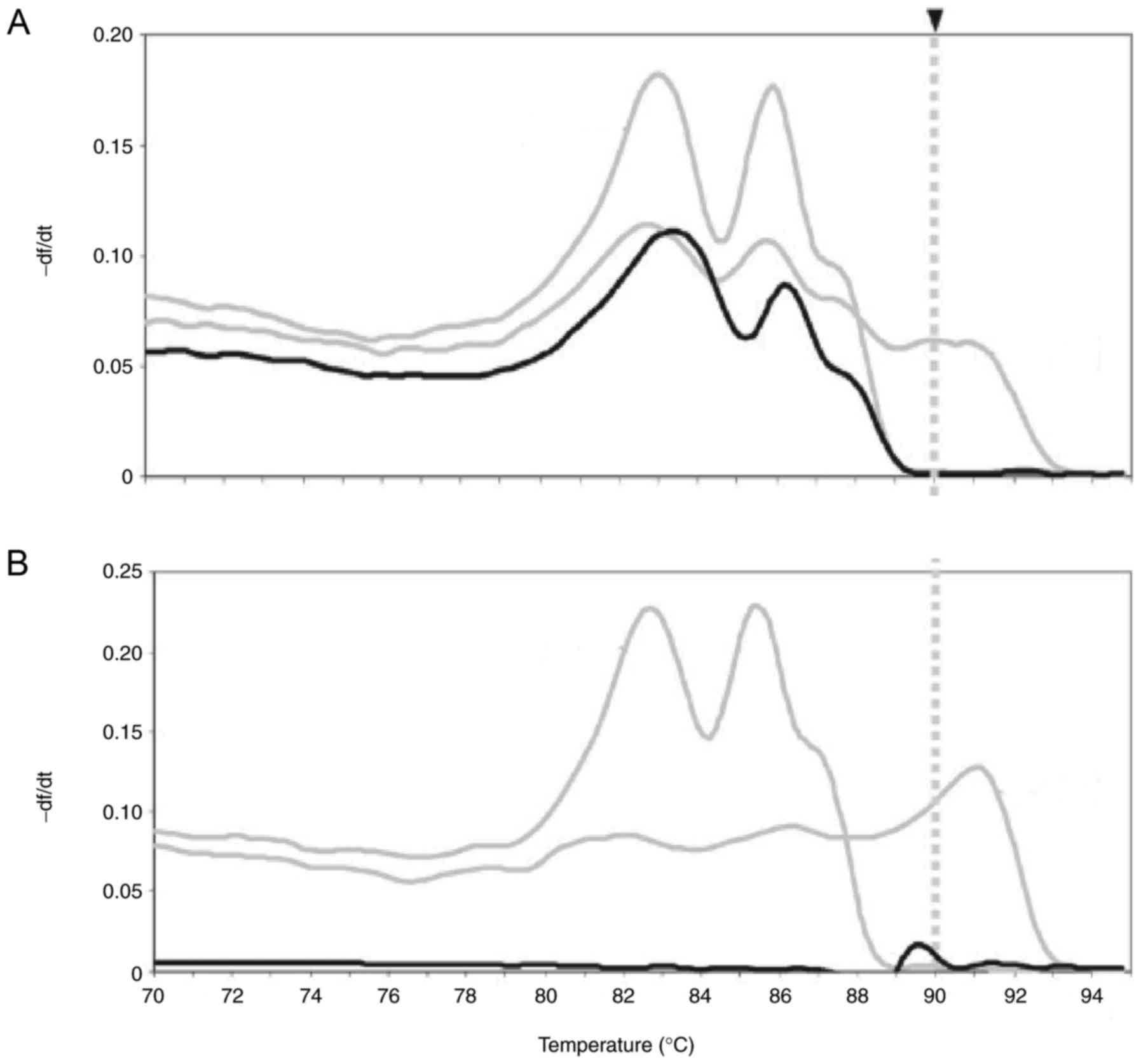
Comparison of nested PCR and PCR in
diagnosis of FXS
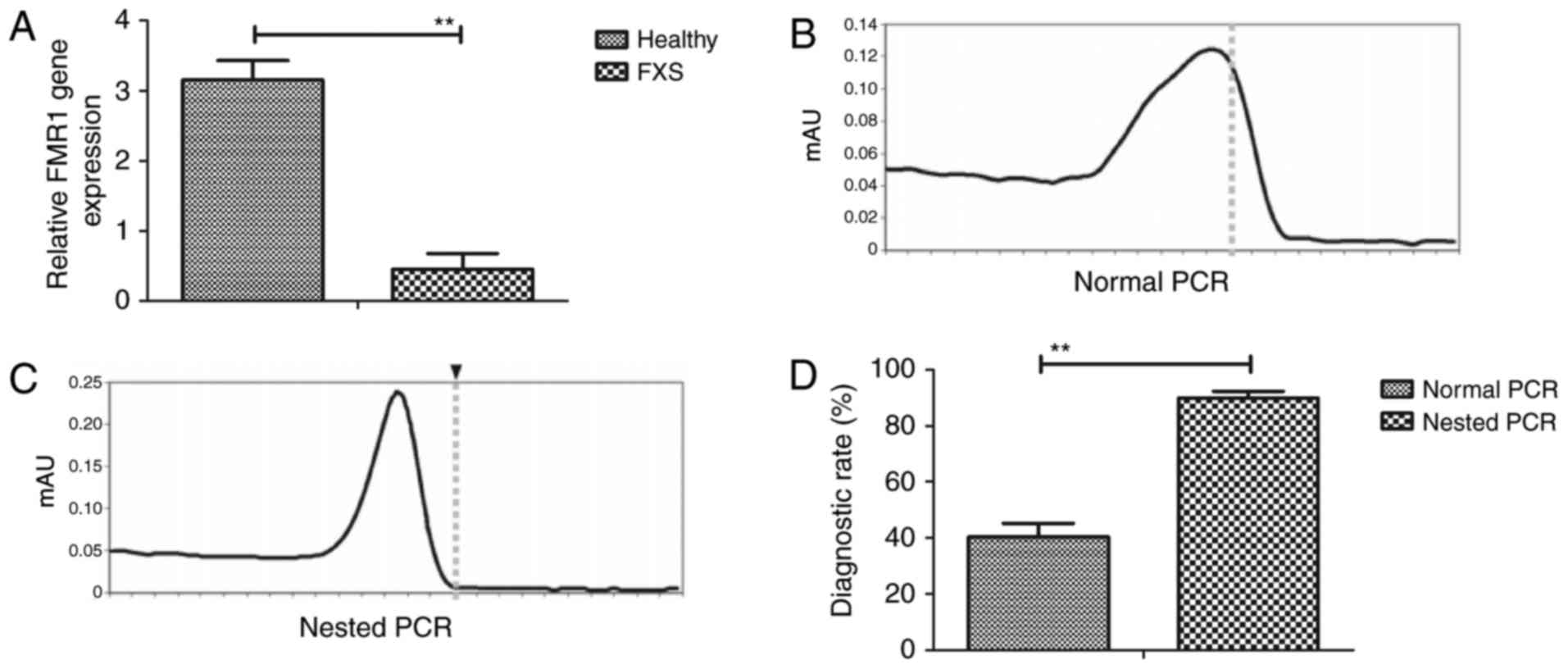
We first compared the efficacy of nested PCR and
traditional PCR in diagnosis of FXS. We showed patients with FXS
showed lowed FMR1 gene expression compared to healthy
individuals (Fig. 1A). As shown in
Fig. 1B and C, nested PCR was
different with PCR for samples, which could diagnose patients with
FXS. Results demonstrated that nested PCR presented more
sensitivity than PCR in diagnosing of FXS (Fig. 1D). These results show that nested PCR
is a potential method for diagnosis of FXS patients.
Analysis of CGG repeat length based on
nested PCR
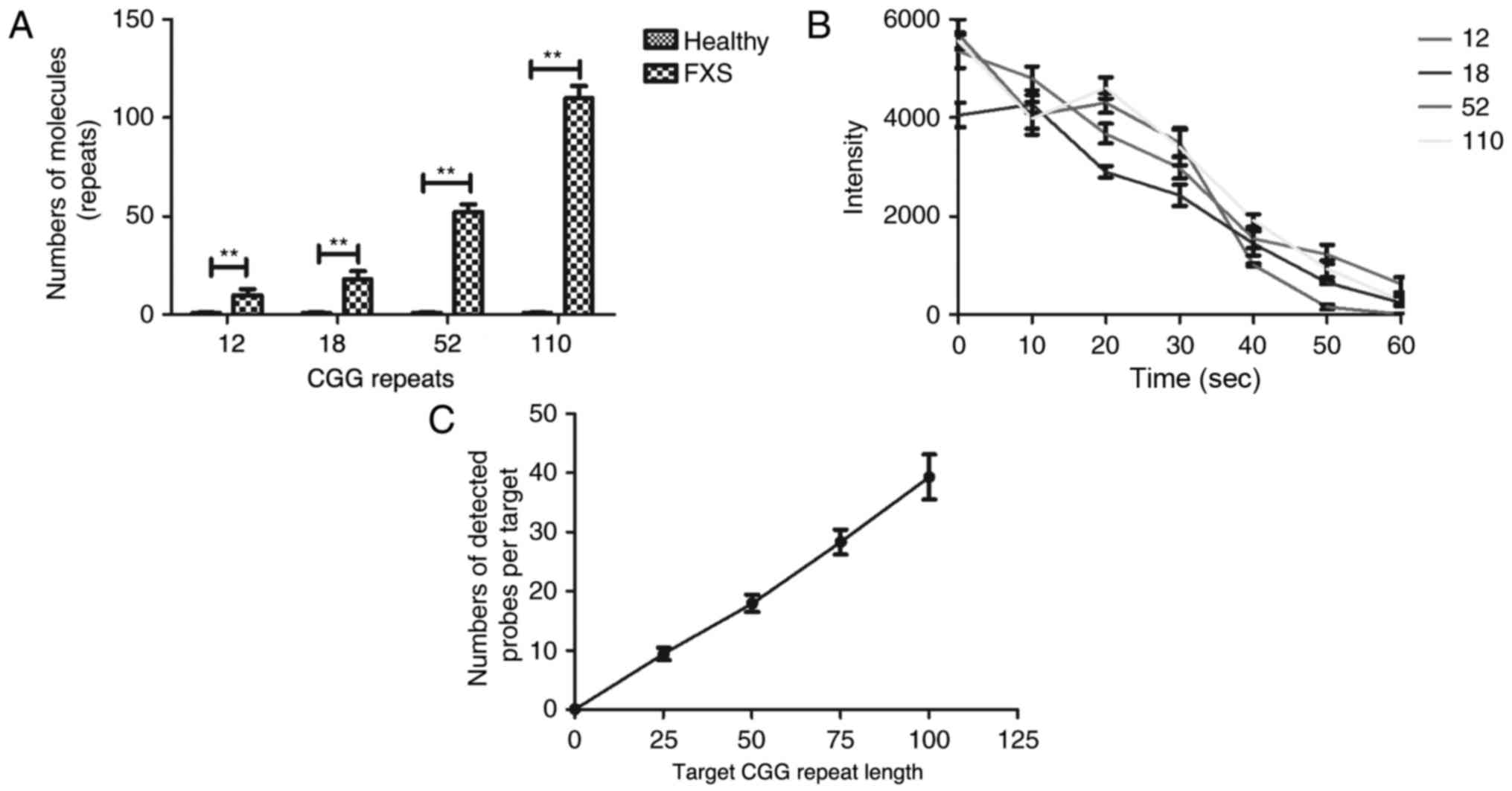
To validate the efficacy of nested PCR, we tested
DNA target strands spanning the normal and gray-zone and analyzed
CGG repeat sequences. We depicted that representative nested PCR
combined immobilized probe-bound targets with CGG repeat lengths of
12, 18, 52, and 110 (Fig. 2A). As
shown in Fig. 2B, nested PCR
combined immobilized probe-bound targets with CGG predominantly
exhibited a complete loss of fluorescence in FXS patients compared
to healthy individuals. As shown in Fig.
2C, target CGG repeat length is positively associated with the
average number of probes bound using nested PCR. These results
indicate that CGG repeat length can be detected based on nested
PCR.
Direct physical length measurement of
CGG repeats
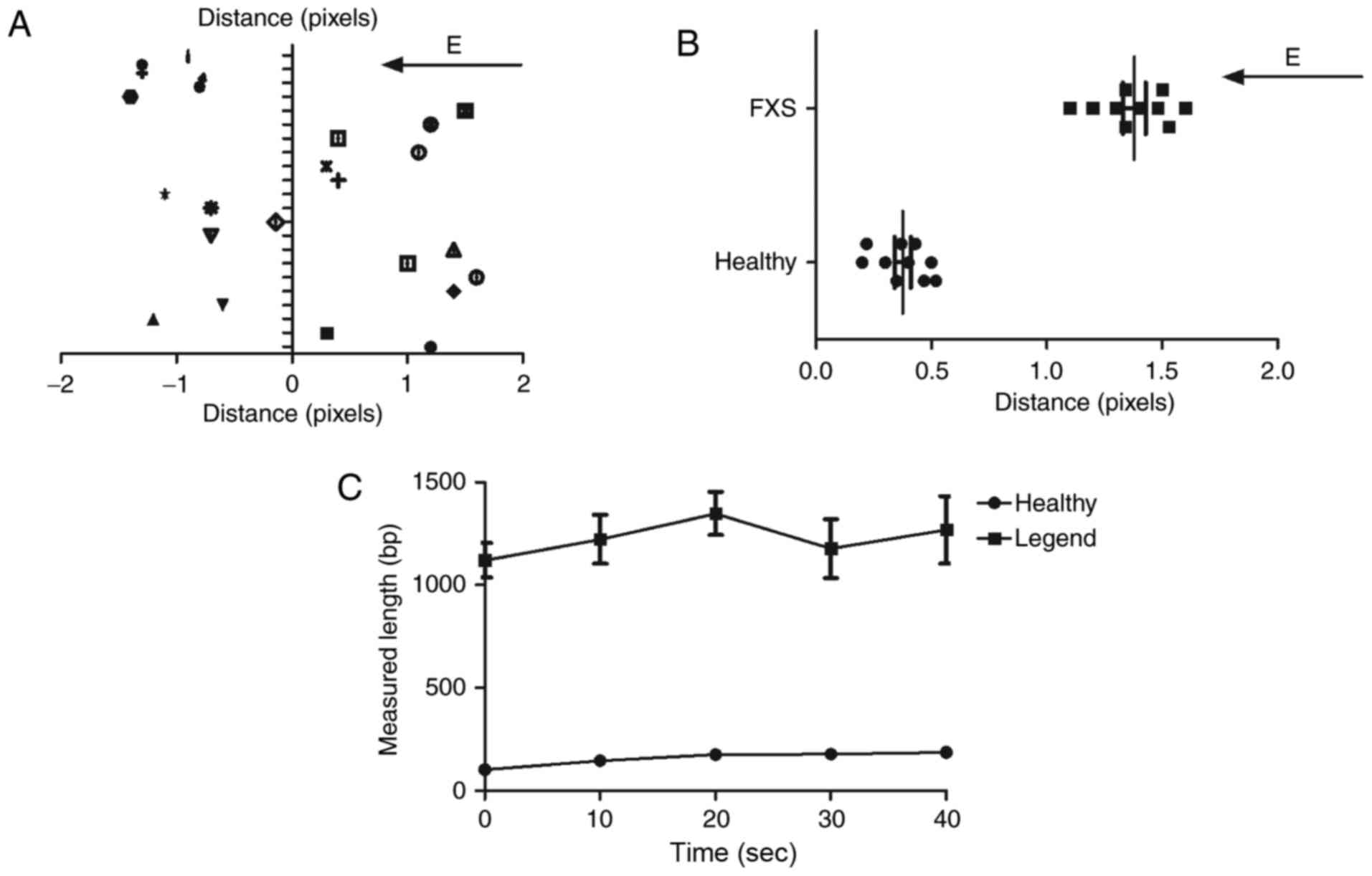
We next analyzed direct physical length measurement
of CGG repeats after nested PCR analysis. As shown in Fig. 3A, the immobilized DNA of FXS patients
was elongated and oriented in the electric field. We found that DNA
from FXS patients presented longer distance compared to DNA from
healthy individuals (Fig. 3B). We
observed that the averaged measured lengths (bp) of the molecules
in FXS patients were increased with the number of base pairs
compared to DNA from healthy individuals (Fig. 3C). These results indicate that the
shortening of the average length relative to the expected length
between FXS patients and healthy individuals may result from
incomplete elongation by the electric field and length
heterogeneities due to nested PCR of the repeat region.
Blinded validation study
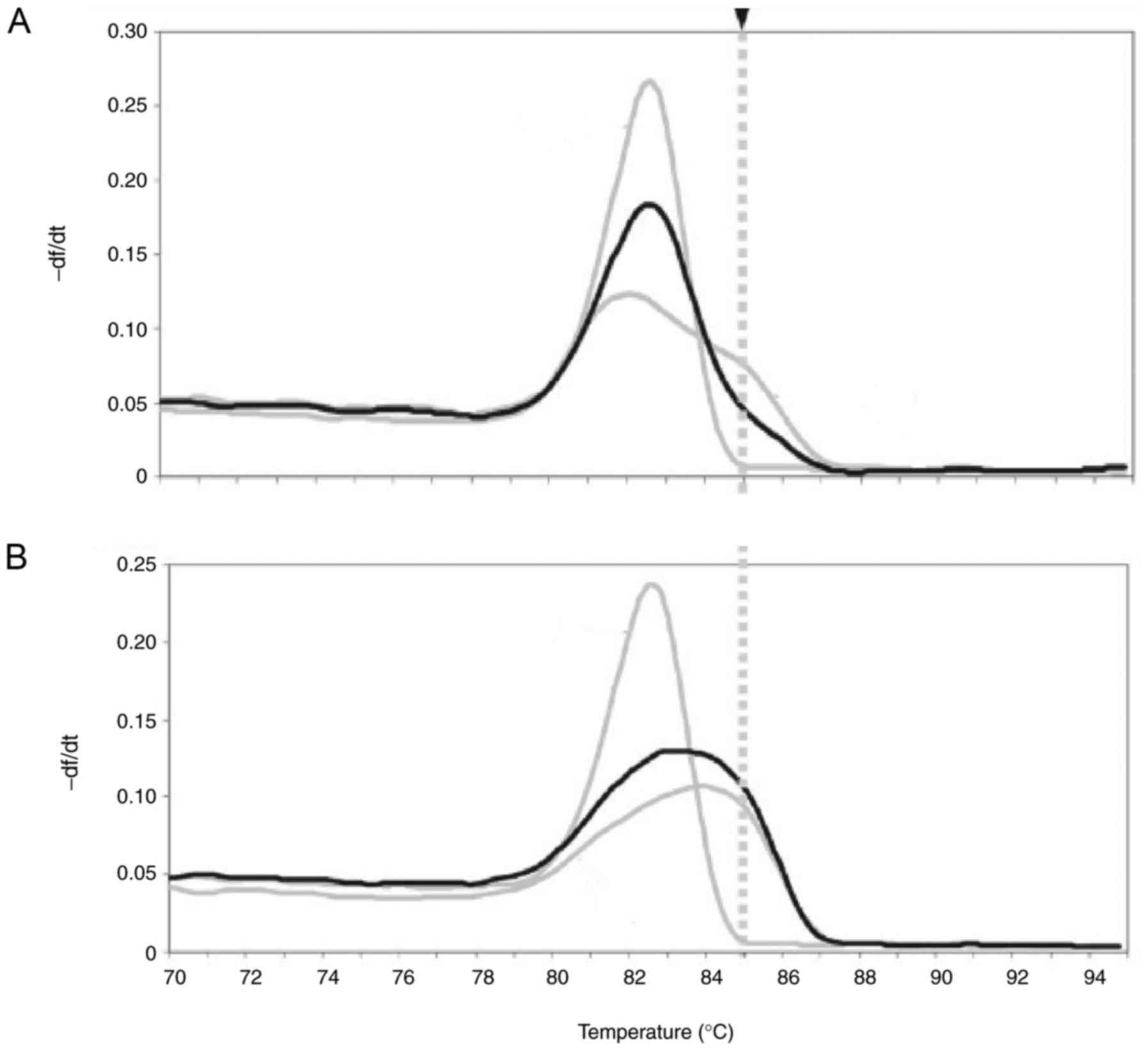
To evaluate the efficacy of nested PCR assay for
discriminating between FXS patients and healthy individuals, we
conducted a blinded validation study in 32 previously genotyped
clinical FXS samples and 32 healthy individuals' clinical samples.
As shown in Fig. 4A, nested PCR
clearly identified FXS. We also showed nested PCR also specified
healthy individuals' samples (Fig.
4B). These results suggest that nested PCR assay is an
efficient method for diagnosis of FXS patients.
Detection from spiked blood spots
We finally confirmed the efficacy of nested PCR
assay using spiked blood spots. We showed that all DNA from spiked
and control unspiked blood spots were correctly screened as
positive or negative for an expansion mutation when we used the
baseline temperature cutoffs of 85°C for the nested PCR (Fig. 5A). We showed that there was no
positive call from the blood spot sample spiked in healthy
individuals (Fig. 5B). These results
confirm the accuracy of nested PCR for diagnosis of FXS
patients.
Discussion
The full FMR1 mutation is associated with the
progression of FXS (22,23). Previous studies has indicated that
quantification of the number of (CGG)n repeats in the 5′
untranslated region of the FMR1 gene plays decisive role in
fragile X molecular diagnostic testing (24,25). The
current study has suggested that nested PCR assay for fragile X
diagnosis is a potential approach to detect and determine the
methylation states of FMR1 gene. In this study, we validated
the molecular genetic diagnosis of nested PCR for patients
suspected of FXS. We reported that nested PCR assay could
efficiently diagnose the patients with FXS. Our results suggest
that nested PCR is a potential method for diagnosis of FXS
patients, which could detect CGG repeat length.
Currently, PCR has been wildly used to detect
various human diseases, such as Alzheimer's disease and human
kidney diseases (26,27). Previous study has indicated that a
single-tube tetradecaplex PCR panel of highly polymorphic markers
can act as a genetic diagnosis of FXS (15). In this study, we indicated that
nested PCR is an efficient method in diagnosis of FXS patients,
which is more efficient than single-tube tetradecaplex PCR panel.
Our data suggested that nested PCR could analyze the CGG repeat
length, which further confirm the diagnosis of FXS. Rajan-Babu
et al have found that closed-tube triplet-primed PCR and
amplicon melt peak analysis is a simplified strategy for rapid
screening of FXS (17).
Additionally, FMR1 PCR technology can accurately categorize the
spectrum of low abundance expanded alleles and full mutations based
on infer homozygosis in clinical samples (28). In this study, we found that direct
physical length measurement of CGG repeats can be measured using
our nested PCR method, which can be used to different abundance
expanded alleles and full mutations in diagnosis of FXS. Malcov
et al have indicated that nested PCR could detect the
CGG-repeat region arrange from 55 to 200 (18). We reported that our nested PCR can
detect CGG repeat more than 200 CGG repeats. However, there are
some disadvantages of nested PCR, including tedious processes and a
higher risk of cross-contamination in the PCR reaction. Notably,
nested PCR presents higher sensitivity and specificity than
traditional PCR in diagnosing of FXS.
DNA from blood cells is used in molecular tests to
detect the fragile X mutation, which is characterized by an
unstable expansion of a CGG repeat in the FMR1 gene
(29). Chow et al have
identified the efficacy of feasibility of blood spot PCR in
large-scale screening of FXS in southern Taiwan and results
demonstrated that a simple PCR combined with blood spot sampling is
effective and feasible for large-scale screening of newborn boys
for fragile X carrier status (30).
In this study, spiked blood spots were conducted to identify the
diagnostic results of nested PCR. We reported that results detected
by nested PCR for diagnosis of FXS patients was consistent with
detection from spiked blood spots.
In conclusion, our results provided detailed
diagnostic data that may have particular application for FXS
clinical diagnosis. Findings indicate that the nested PCR
technology is more efficient for FXS diagnosis compared to
traditional PCR. As a result, the capabilities of the nested PCR
may provide an easy and fast method, which could support PCR-based
higher CGG repeat numbers of FMR1 in a blinded validation
study. However, more study should be performed in large number
populations.
Acknowledgements
This study was supported by Department of the Public
Technology Research and Social Development Project of Zhejiang
Province (2013C33108).
References
|
1
|
Hill MK, Archibald AD, Cohen J and
Metcalfe SA: A systematic review of population screening for
fragile X syndrome. Genet Med. 12:396–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Winarni Indah T, Chonchaiya W, Adams E, Au
J, Mu Y, Rivera SM, Nguyen DV and Hagerman RJ: Sertraline may
improve language developmental trajectory in young children with
fragile × syndrome: A retrospective chart review. Autism Res Treat.
2012:1043172012.PubMed/NCBI
|
|
3
|
Visootsak J, Warren ST, Anido A and Graham
JM Jr: Fragile X syndrome: An update and review for the primary
pediatrician. Clin Pediatr (Phila). 44:371–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferreira GC and Lamonica DA: Language
characterization in the X-fragile syndrome: A review study. Pro
Fono. 17:111–120. 2005.(In Portuguese). PubMed/NCBI
|
|
5
|
Kidd SA, Lachiewicz A, Barbouth D, Blitz
RK, Delahunty C, McBrien D, Visootsak J and Berry-Kravis E: Fragile
X syndrome: A review of associated medical problems. Pediatrics.
134:995–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cowley B, Kirjanen S, Partanen J and
Castrén ML: Epileptic electroencephalography profile associates
with attention problems in children with fragile X syndrome: Review
and case series. Front Hum Neurosci. 10:3532016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McCary LM and Roberts JE: Early
identification of autism in fragile X syndrome: A review. J
Intellect Disabil Res. 57:803–814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ciaccio C, Fontana L, Milani D, Tabano S,
Miozzo M and Esposito S: Fragile X syndrome: A review of clinical
and molecular diagnoses. Ital J Pediatr. 43:392017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lozano R, Azarang A, Wilaisakditipakorn T
and Hagerman RJ: Fragile X syndrome: A review of clinical
management. Intractable Rare Dis Res. 5:145–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Essop FB and Krause A: Diagnostic, carrier
and prenatal genetic testing for fragile X syndrome and other
FMR-1-related disorders in Johannesburg, South Africa: A 20-year
review. S Afr Med J. 103 12 Suppl 1:S994–S998. 2013. View Article : Google Scholar
|
|
11
|
Rueda JR, Ballesteros J and Tejada MI:
Systematic review of pharmacological treatments in fragile X
syndrome. BMC Neurol. 9:532009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xunclà M, Badenas C, Domínguez M,
Rodríguez-Revenga L, Madrigal I, Jiménez L, Soler A, Borrell A,
Sánchez A and Milà M: Fragile X syndrome prenatal diagnosis:
Parental attitudes and reproductive responses. Reprod Biomed
Online. 21:560–565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Romero-Espinoza P, Rosales-Reynoso MA,
Willemsen R and Barros-Nunez P: FMR1 protein expression in blood
smears for fragile X syndrome diagnosis in a Mexican population
sample. Genet Test Mol Biomarkers. 14:511–514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leehey MA: Fragile X-associated
tremor/ataxia syndrome: Clinical phenotype, diagnosis, and
treatment. J Investig Med. 57:830–836. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen M, Zhao M, Lee CG and Chong SS:
Identification of microsatellite markers <1 Mb from the FMR1 CGG
repeat and development of a single-tube tetradecaplex PCR panel of
highly polymorphic markers for preimplantation genetic diagnosis of
fragile X syndrome. Genet Med. 18:869–875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang WC, Chou YP, Kao PM, Hsu TK, Su HC,
Ho YN, Yang YC and Hsu BM: Nested-PCR and TaqMan real-time
quantitative PCR assays for human adenoviruses in environmental
waters. Water Sci Technol. 73:1832–1841. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rajan-Babu IS, Law HY, Yoon CS, Lee CG and
Chong SS: Simplified strategy for rapid first-line screening of
fragile X syndrome: Closed-tube triplet-primed PCR and amplicon
melt peak analysis. Expert Rev Mol Med. 17:e72015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malcov M, Naiman T, Yosef DB, Carmon A,
Mey-Raz N, Amit A, Vagman I and Yaron Y: Preimplantation genetic
diagnosis for fragile X syndrome using multiplex nested PCR. Reprod
Biomed Online. 14:515–521. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Curtis-Cioffi KM, Rodrigueiro DA,
Rodrigues VC, Cicarelli RM and Scarel-Caminaga RM: Comparison
between the polymerase chain reaction-based screening and the
Southern blot methods for identification of fragile X syndrome.
Genet Test Mol Biomarkers. 16:1303–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosales-Reynoso MA, Vilatela EA, Ojeda RM,
Arce-Rivas A, Sandoval L, Troyo-Sanromán R and Barros-Núñez P: PCR
approach for detection of fragile X syndrome and Huntington disease
based on modified DNA: Limits and utility. Genet Test. 11:153–159.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaughan MJ, Chanon A and Blakeslee JJ:
Using capillary electrophoresis to quantify organic acids from
plant tissue: A test case examining coffea arabica seeds. J Vis
Exp. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hagerman R and Hagerman P: Advances in
clinical and molecular understanding of the FMR1 premutation and
fragile X-associated tremor/ataxia syndrome. Lancet Neurol.
12:786–798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang JY, Hessl D, Schneider A, Tassone F,
Hagerman RJ and Rivera SM: Fragile X-associated tremor/ataxia
syndrome: Influence of the FMR1 gene on motor fiber tracts in males
with normal and premutation alleles. JAMA Neurol. 70:1022–1029.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Godler DE, Inaba Y, Schwartz CE, Bui QM,
Shi EZ, Li X, Herlihy AS, Skinner C, Hagerman RJ, Francis D, et al:
Detection of skewed X-chromosome inactivation in Fragile X syndrome
and X chromosome aneuploidy using quantitative melt analysis.
Expert Rev Mol Med. 17:e132015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo S, Huang W, Xia Q, Xia Y, Du Q, Wu L
and Duan R: Cryptic FMR1 mosaic deletion in a phenotypically normal
mother of a boy with fragile X syndrome: Case report. BMC Med
Genet. 15:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wilkinson R, Wang X, Kassianos AJ, Zuryn
S, Roper KE, Osborne A, Sampangi S, Francis L, Raghunath V and
Healy H: Laser capture microdissection and multiplex-tandem PCR
analysis of proximal tubular epithelial cell signaling in human
kidney disease. PLoS One. 9:e873452014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vignini A, Morganti S, Salvolini E,
Sartini D, Luzzi S, Fiorini R, Provinciali L, Di Primio R, Mazzanti
L and Emanuelli M: Amyloid precursor protein expression is enhanced
in human platelets from subjects with Alzheimer's disease and
frontotemporal lobar degeneration: A real-time PCR study. Exp
Gerontol. 48:1505–1508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Filipovic-Sadic S, Sah S, Chen L, Krosting
J, Sekinger E, Zhang W, Hagerman PJ, Stenzel TT, Hadd AG, Latham GJ
and Tassone F: A novel FMR1 PCR method for the routine detection of
low abundance expanded alleles and full mutations in fragile X
syndrome. Clin Chem. 56:399–408. 2010.PubMed/NCBI
|
|
29
|
Christofolini DM, Lipay MV, Ramos MA,
Brunoni D and Melaragno MI: Screening for fragile X syndrome among
Brazilian mentally retarded male patients using PCR from buccal
cell DNA. Genet Mol Res. 5:448–453. 2006.PubMed/NCBI
|
|
30
|
Chow JC, Chen DJ, Lin CN, Chiu CY, Huang
CB, Chiu PC, Lin CH, Lin SJ and Tzeng CC: Feasibility of blood spot
PCR in large-scale screening of fragile X syndrome in southern
Taiwan. J Formos Med Assoc. 102:12–16. 2003.PubMed/NCBI
|