Introduction
Doxorubicin is one of the most widely used
anthracycline antibiotic/anticancer agents (1). It is an effective antineoplastic drug,
but its usage is limited by various complications, including its
cardiotoxicity (2). To solve this
problem, doxorubicin-loaded nanoparticles including liposomes were
developed and they could result in improved tumor-growth inhibition
with less general toxicity compared to the free drug molecules
(3). Liposomal formulations of
doxorubicin, can achieve locally high drug concentrations within a
tumor and tumor vasculature while maintaining low systemic toxicity
(4). In previous reports, various
liposomes showed sustained release of doxorubicin and produced a
reliable cure of local cancer in mouse models (4).
Three-dimensional culture system is more similar to
tissue conditions in vivo and can mimic native environments,
unlike the two-dimensional culture system (5). Three-dimensional culture models have
been proposed due to their various advantages over two-dimensional
culture systems (6).
Three-dimensional environment has been reported to enhance
reprogramming process (7).
Three-dimensional spherical spatial boundary conditions have been
reported to regulate the differentiation of mesenchymal stem cells
(8). Moreover, three-dimensional
culture system can be used for the evaluation of intercellular
interactions in the regulation of cell proliferation and
differentiation (9,10). Doxorubicin is reported to suppress
bone marrow stem cells while expanding cancer stem cells (11). However, the effects of
doxorubicin-loaded anionic, cationic, and neutral liposomes on the
stem cell spheroids derived from bone marrow and gingiva has not
been tested yet. The aim of this report is to evaluate the effects
of anionic, cationic, and neutral liposomes containing doxorubicin
on the cellular viability and stem cell surface maker expression of
three-dimensional stem cell spheroids. To the authors' knowledge,
this is the first report evaluating the effects of anionic,
cationic, and neutral liposomes containing doxorubicin on stem cell
spheroids consisting of human gingiva-derived stem cells and bone
marrow-derived stem cells.
Materials and methods
Materials
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC)
and 1,2-Dipalmitoyl-sn-glycero-3-phosphoserine, sodium salt (DPPS)
were purchased from Echelon Biosciences (Salt Lake City, UT, USA).
1,2-dipalmitoyl-3-trimethylammonium-propane [chloride salt (16:0
TAP)] was purchased from Avanti Polar Lipids (Birmingham, AL, USA).
Cholesterol was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Dichloromethane was purchased from Daejung
Chemical (Cheongwon, Korea). A dialysis membrane [pre-wetted RC
Tubing (MWCO: 25 kD)] was purchased from Spectrum (Spectra/Por;
Laguna Hills, CA, USA). Dimethyl sulfoxide, 99.0% (methyl
sulfoxide, DMSO) and Triton X-100 were purchased from Samchun Pure
Chemical (Pyeongtaek, Korea). Phosphate-buffered saline (PBS, pH
7.4) was purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Doxorubicin hydrochloride was purchased from LC
laboratories (Woburn, MA, USA).
Methods
Preparation of doxorubicin-loaded
liposomes
Liposomes were prepared by the
thin-lipid-film-hydration method from the mixture. For the neutral
liposome, cationic liposome, and anionic liposome, we used
DPPC:Cholesterol=10:1; DPPC: 16:0TAP:Cholesterol=5:5:1;
DPPC:DPPS:Cholesterol=5:5:1 (weight ratio), respectively. Briefly,
the lipids were dissolved in dichloromethane and the solvent was
removed via evaporation under a reduced pressure at 55°C. Then, a
thin film of lipids was dispersed in distilled water (lipid
concentration was 2.2 mg/ml) with doxorubicin hydrochloride by
sonication. Finally, unloaded doxorubicin was removed through
dialysis, using distilled water for 1 h. The amount of doxorubicin
in liposomes was evaluated based on the fluorescence of doxorubicin
(490/570 nm) after the complete disruption of liposomes by Triton
X-100.
Size and morphology of liposomes
The average size diameter, polydispersity, and zeta
potentials of the liposomes were determined using a Zetasizer Nano
ZS90 (Malvern Instruments, Malvern, UK) and Data Transfer
Assistance (DTA) software at 25°C. Zeta potential was measured in
PBS. The morphology of liposomes was observed by transmission
electron microscopy using negative staining with 2% (w/v) uranyl
acetate solution for 10 min.
Doxorubicin release from
liposomes
The release of doxorubicin from liposomes was
evaluated over time at room temperature in PBS. Doxorubicin-loaded
liposomes were put in a dialysis bag and the amount of remaining
doxorubicin in the bag was measured using points based on
fluorescence as time went on.
Formation of cell spheroids with human
gingiva-derived stem cells and bone marrow-derived stem cells
Stem cell spheroids were formed in the silicon
elastomer-based concave microwells (H389600, StemFIT 3D; MicroFIT,
Seongnam, Korea) with 600 µm diameters. Gingiva-derived stem cells
and bone marrow-derived stem cells in the amount of
9×105 were seeded and subsequently cultured to
investigate cellular behavior. Human bone marrow-derived
mesenchymal stem cells (Catholic MASTER Cells) were obtained from
Catholic Institute of Cell Therapy (CIC; Seoul, Korea). The
Institutional Review Board of Seoul St Mary's Hospital, College of
Medicine, Catholic University of Korea (Seoul, Korea), approved
this study (KC17SESI0290 and KC11SISI0348), and informed consents
from the study participants were obtained. All the methods used in
this study were performed in accordance with the relevant
guidelines and regulations. Gingival tissues were collected from
the patient (64-year-old female) visiting the Department of
Periodontics, Seoul St Mary's Hospital, on July 2013. In short,
gingivae were de-epithelialized, minced, and digested in an
α-modified minimal essential medium (α-MEM; Gibco; Thermo Fisher
Scientific, Inc.) containing collagenase IV (2 mg/ml;
Sigma-Aldrich; Merck KGaA) and dispase (1 mg/ml; Sigma-Aldrich;
Merck KGaA). The ratios between human gingiva-derived stem cells
and human bone marrow-derived mesenchymal stem cells were 2:1. Cell
aggregation and cell-spheroid formation were observed under an
inverted microscope (Leica DM IRM; Leica Microsystems, Wetzlar,
Germany), and the images were saved as JPGs. The groups consisted
of i) unloaded control group; ii) doxorubicin, 1 µg/ml (D1); iii)
doxorubicin, 10 µg/ml (D10); iv) unloaded anionic group (A0); v)
anionic group loaded with doxorubicin at 1 µg/ml (A1); vi) anionic
group loaded with doxorubicin at 10 µg/ml (A10); vii) unloaded
cationic group (C0); viii) cationic group loaded with doxorubicin
at 1 µg/ml (C1); ix) cationic loaded with doxorubicin at 10 µg/ml
(C10); x) unloaded neutral (N0); xi) neutral group loaded with
doxorubicin at 1 µg/ml (N1); and xii) neutral group loaded with
doxorubicin at 10 µg/ml (N10).
Determination of cell viability
The viability of spheroids was qualitatively
analyzed with the Live/Dead kit assay (Molecular Probes, Eugene,
OR, USA). The assay is based on the principle that the activity of
intracellular esterase causes non-fluorescent, cell-permeant
calcein acetoxymethyl ester to become intensely fluorescent, giving
the viable spheroids an intense, uniform, green fluorescence. The
ethidium homodimer enters into the damaged cell membrane and then
binds to nucleic acids, thereby producing a red fluorescence in the
dead cells.
Stem cell spheroids were cultured in an α-MEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 15% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml of
penicillin, and 100 µg/ml of streptomycin (Sigma-Aldrich; Merck
KGaA), 200 mM of L-Glutamine (Sigma-Aldrich; Merck KGaA), and 10 mM
of ascorbic acid 2-phosphate (Sigma-Aldrich; Merck KGaA). These
spheroids were washed twice with the growth media, followed by
suspension in 1 ml of α-MEM containing 2 µl of 50 mM calcein
acetoxymethyl ester working solution and 4 µl of the 2 mM ethidium
homodimer-1 for 30 min at room temperature. The spheroids stained
with calcein acetoxymethyl ester and ethidium homodimer-1 were
observed under a fluorescence microscope (Axiovert 200; Carl Zeiss
AG, Oberkochen, Germany) at days 1, 3 and 5.
A cell-viability analysis was performed on days 1,
3, 5, and 7. WST-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H
tetrazolium, monosodium salt] [Cell-Counting Kit-8 (CCK-8);
Dojindo, Tokyo, Japan] was added to the cultures, and the spheres
were incubated for 1 h at 37°C. Viable cells were identified by the
assay, which relies on the ability of mitochondrial dehydrogenases
to oxidize WST-8 into a formazan product. The spectrophotometric
absorbance of the samples was measured at 450 nm using a microplate
reader (BioTek Instruments, Inc., Winooski, VT, USA).
Paracrine effect evaluation of the
secretion of human vascular endothelial growth factor
The determination of human vascular endothelial
growth factor from three-dimensional systems was performed using a
commercially available kit (Quantikine® ELISA, cat. no.
DVE00; R&D Systems, Inc., Minneapolis, MN, USA). All reagents
and samples were prepared according to the manufacturer's
recommendations. The absorbance levels at 540 and 570 nm were
measured and the differences were used as the value.
Maintenance of stemness
After cultivation for 7 days, the spheroids were
retrieved. Antibodies were purchased from R&D Systems and then
diluted to a 50X concentration. Human SSEA-4 (Clone MC-813-70;
SC023) conjugated to NHL493 (green) and human TRA-1-60(R) (Clone
TRA-1-60; SC023) conjugated to NL557 (red) were used as positive
markers of human stem cells. After spheres were incubated for 1 h
at 37°C, the antibody-containing media were removed, and the cells
were rinsed with fresh media and re-fed with fresh media. The
spheroids were visualized under a fluorescence microscope (Axiovert
200; Carl Zeiss AG).
Western blot analysis
Stem cells were washed two times with ice-cold PBS
and solubilized in lysis buffer on day 7 for 30 min. The lysates
were centrifuged at 15,000 × g for 10 min at 4°C. These samples
were then separated by sodium dodecyl sulfate polyacrylamide gel
electrophoresis (Mini-PROTEAN® TGX™ Precast
Gels; Bio-Rad Laboratories, Inc., Hercules, CA, USA), transferred
to polyvinylidene difluoride membranes (Immun-Blot®;
Bio-Rad Laboratories, Inc.) and immunoblotted with the
corresponding antibodies and enhanced chemiluminescent detection
kits. Primary antibodies against collagen I (ab6308) and
glyceraldehyde 3-phosphate (GAPDH; ab9485) and secondary antibodies
were purchased from Enzo Life Sciences, Inc. (Farmingdale, NY,
USA), Cell Signaling Technology, Inc. (Danvers, MA, USA), Thermo
Fisher Scientific, Inc., and Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). A quantitative analysis of the protein expressions
of Collagen I and GAPDH was conducted with image processing and
analyzing software (ImageJ; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
The data are represented as means ± standard
deviations of the experiments. A test of normality was performed
with Shapiro-Wilk test, and a two-way analysis of variance (ANOVA)
with a post hoc Tukey test was performed to determine the
differences between the groups using a commercially available
program (SPSS 12 for Windows; SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of doxorubicin-loaded
liposomes
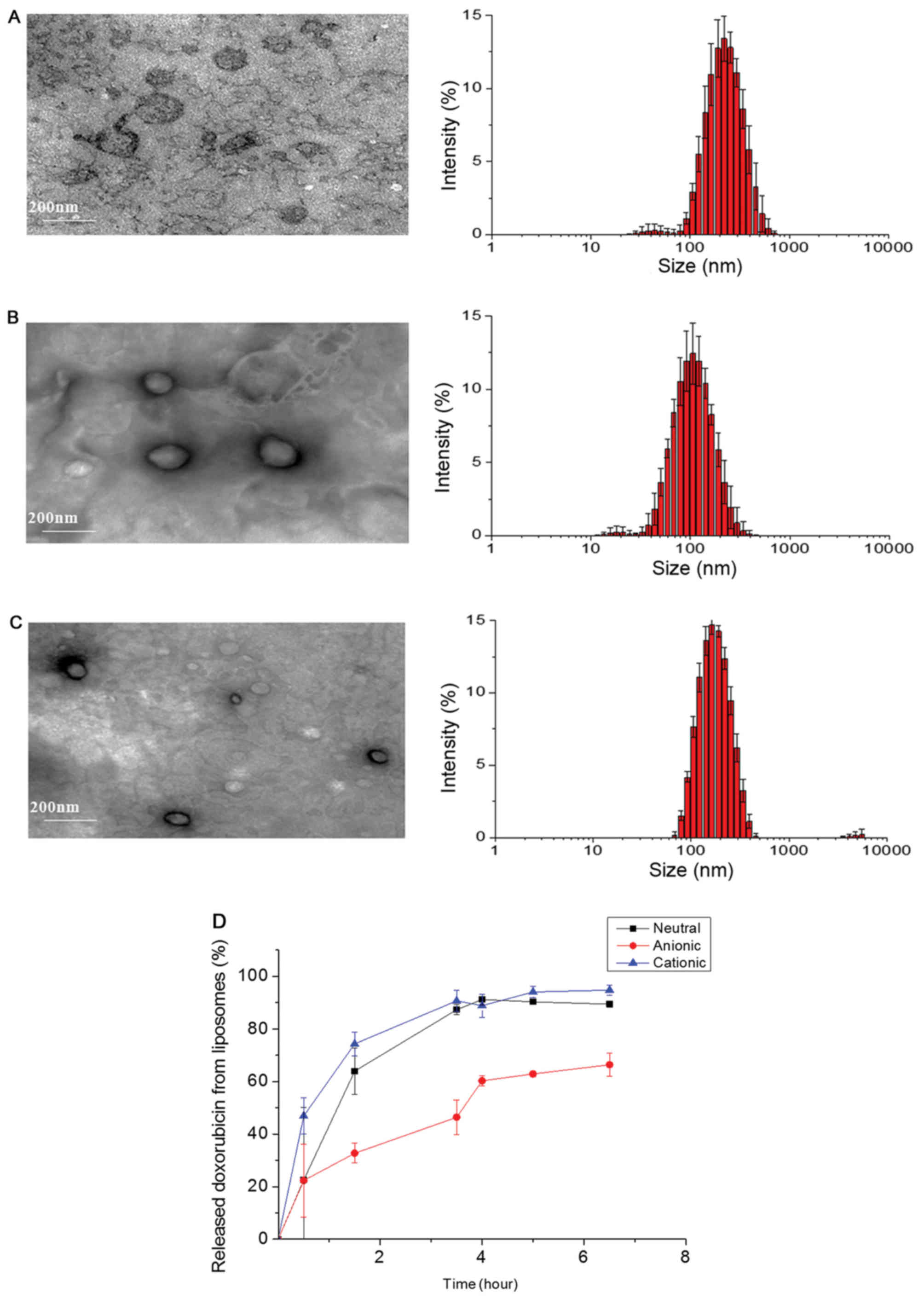
Fig. 1 shows the
transmission electron microscopy of anionic, cationic, and neutral
liposomes by film-hydration methods and doxorubicin loaded inside
of the liposomes. The sizes of fabricated liposomes were
approximately 100 to 200 nm, and they have spherical shapes in
aqueous condition (Fig. 1). Size
distribution of liposomes is shown in Table I. The zeta potential values were
−41.9, +24.4, and +0.176, for anionic, cationic, and neutral
liposomes showing the surface charges were properly controlled
based on lipid composition. During the in vitro release
test, doxorubicin was gradually released from liposomes (Fig. 1D). The release rate was slowest in
the case of anionic liposomes compared to the other two
samples.
 | Table I.Size distribution of liposomes. |
Table I.
Size distribution of liposomes.
| Liposome | Z-average (nm) | Polydispersity
index | Zeta potential
(mV) |
|---|
| Neutral | 162.1 | 0.159 | + 0.176 |
| Cationic | 91.40 | 0.229 | + 24.4 |
| Anionic | 186.2 | 0.210 | − 41.9 |
Evaluation of cell morphology and
cellular viability
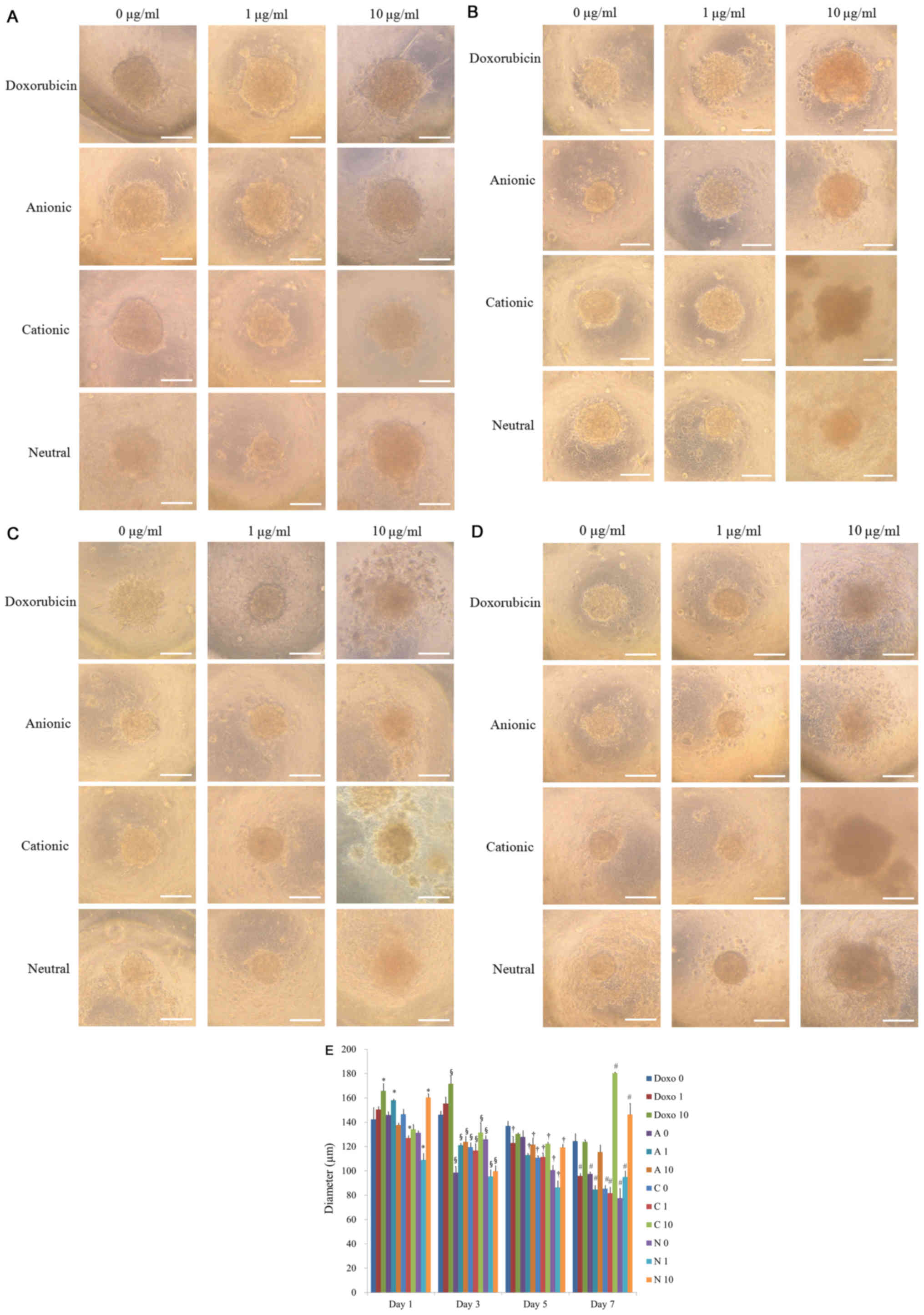
Spheroids were well formed in silicon
elastomer-based concave microwells on day 1 (Fig. 2A). There were no significant changes
in the morphology with the application of doxorubicin on day 1. The
results of morphology of days 3, 5, and 7 are shown in Fig. 2B-D, respectively. In general, the
shapes of the cells in the tested groups were similar to the
control group, except for the 10 µg/ml group. Noticeable changes in
the morphology were noted for D10 and C10 at day 3 (Fig. 2B). The shapes of the cells in these
groups were larger, and cells were adrift. There were no
significant changes in the morphology with the longer incubation
time (Fig. 2C and D). The diameters
of the spheroids for D0, D1, D10, A0, A1, A10, C0, C1, C10, N0, and
N1 on day 1 were shown in Fig. 2E.
The diameters of the spheroids were maintained throughout the
incubation time but had a tendency of decrease in size
(P<0.05).
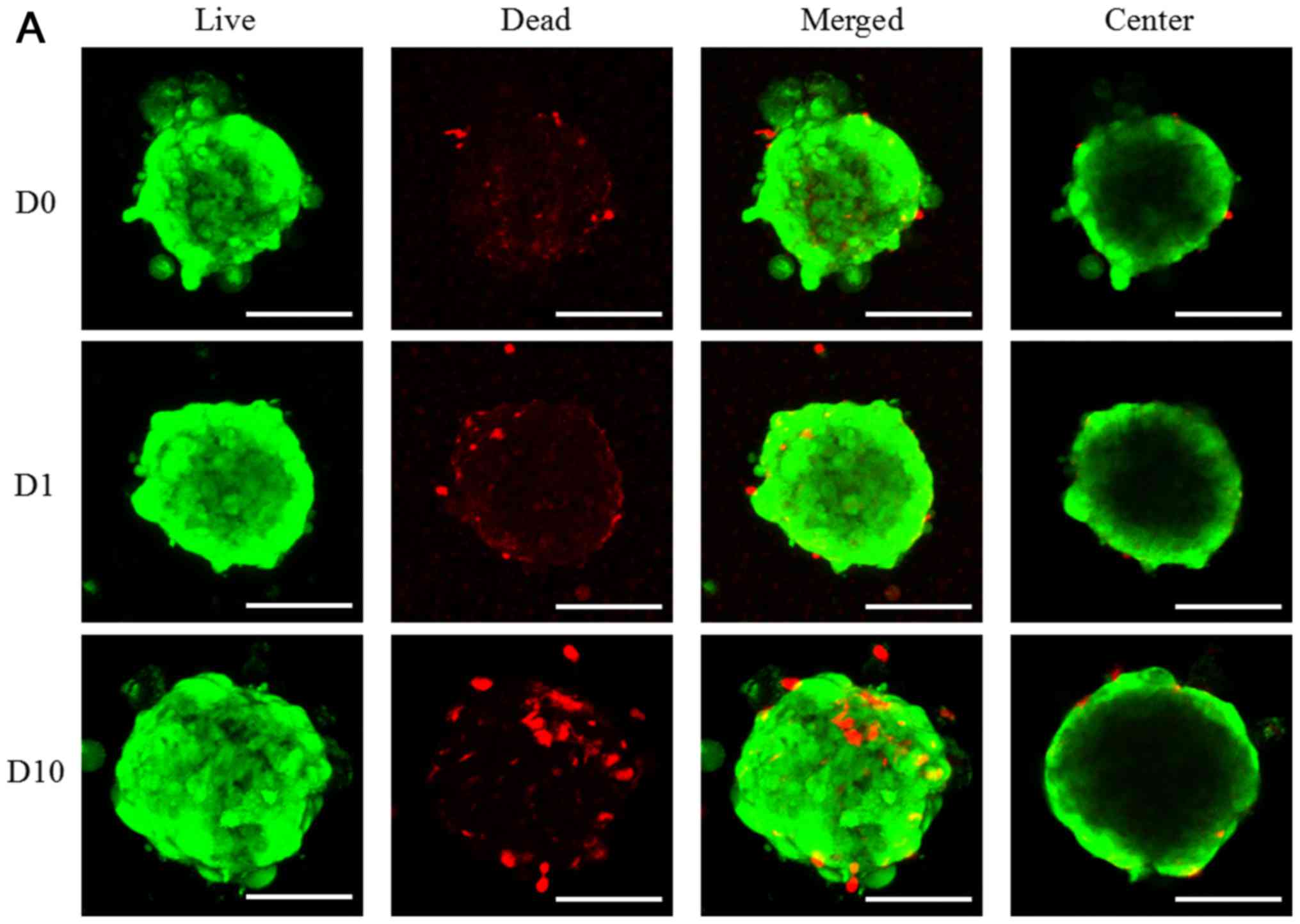
The cellular viability was determined via live and
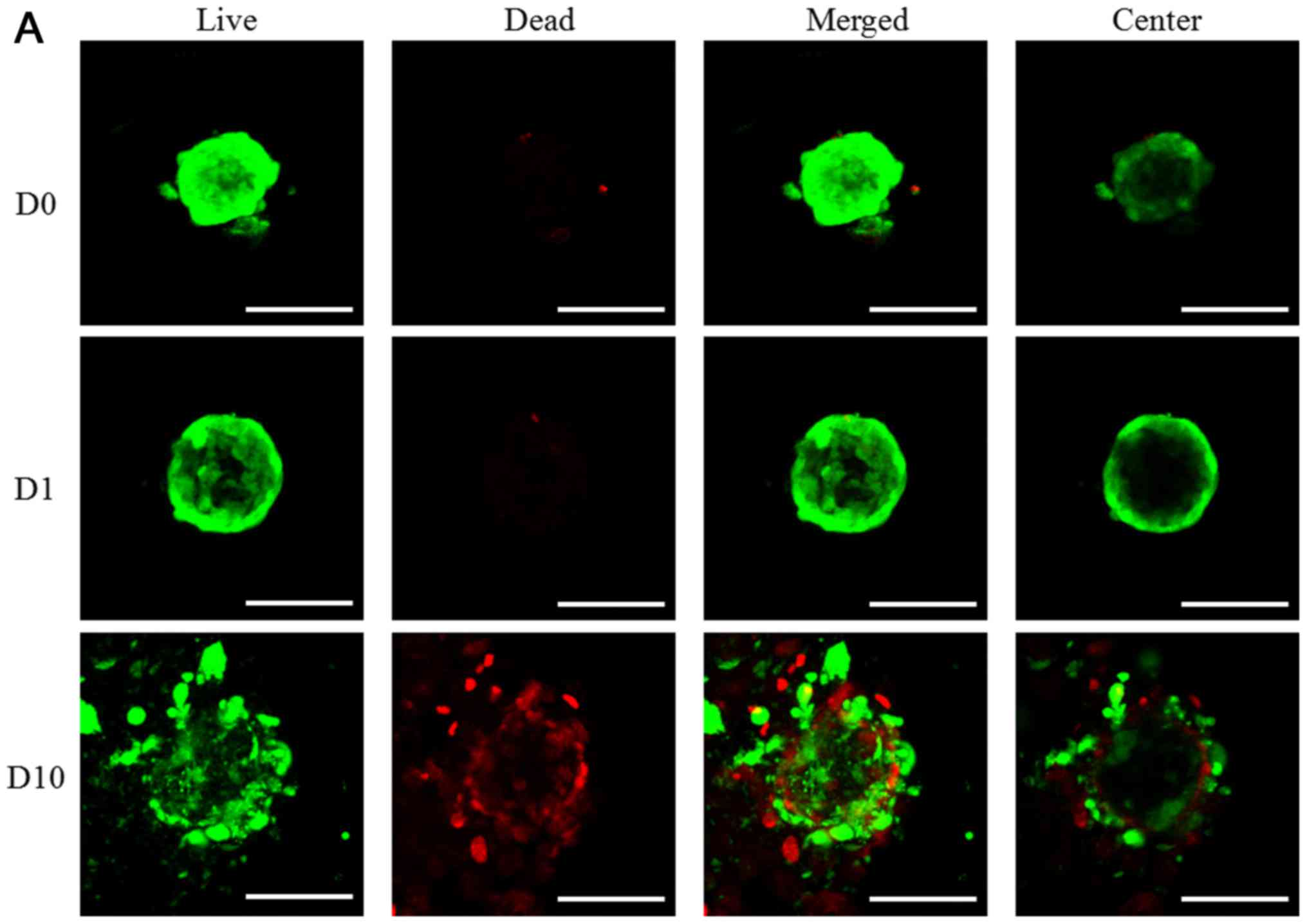
dead assay using confocal microscope as shown in Fig. 3. In the result of day 1, most of the
cells in the spheroids emitted green fluorescence and the
morphology of the spheriods was round (Fig. 3). The increase of red fluorescence
was noted with higher doses of doxorubicin. The quantitative
results for D0, D1, D10, A0, A1, A10, C0, C1, C10, N0, and N1 on
day 1 were shown in Fig. 3E. The
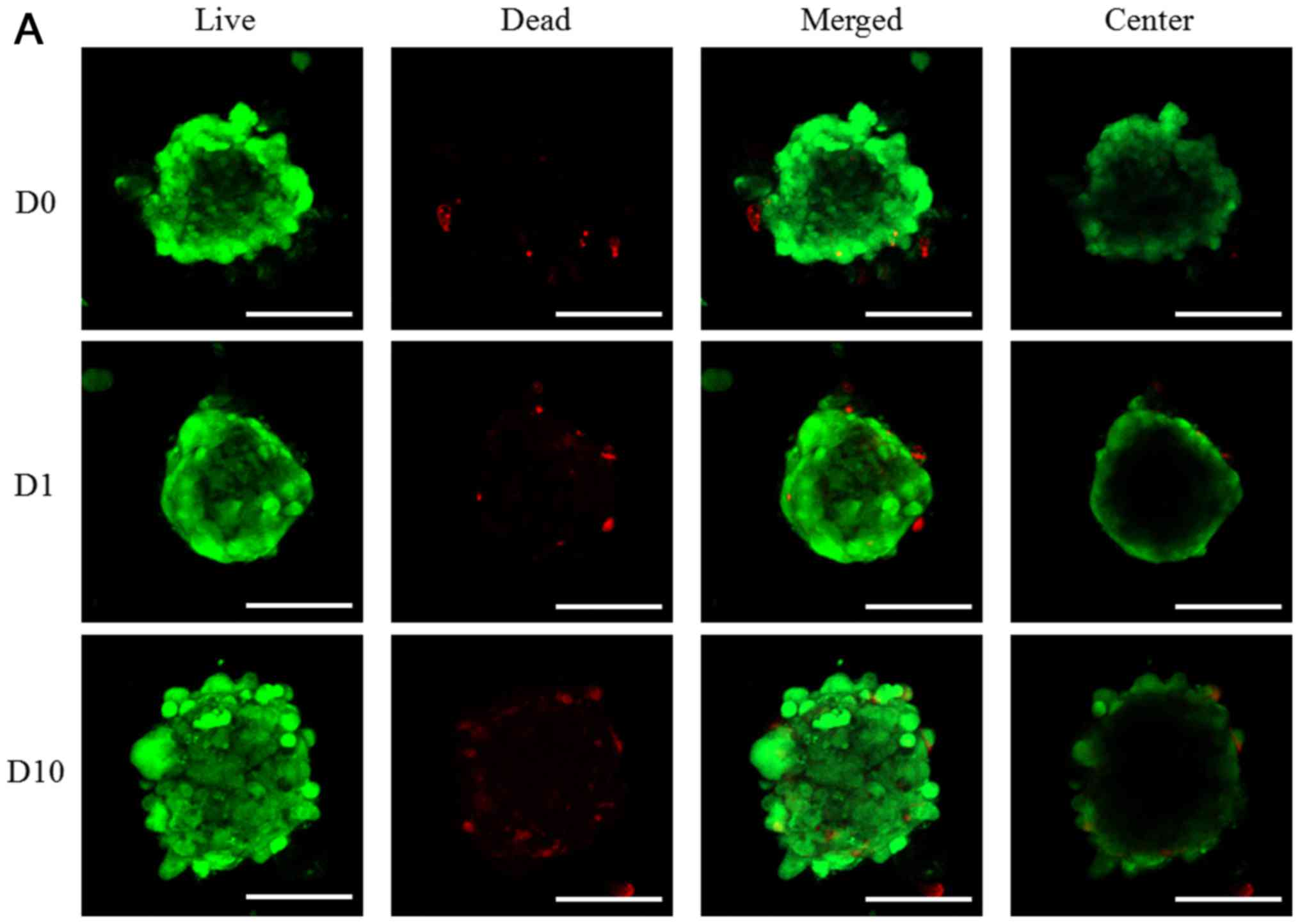
results of day 3 and day 5 are shown in Figs. 4 and 5, respectively. The quantitative results
for D0, D1, D10, A0, A1, A10, C0, C1, C10, N0, and N1 on day 3 and
day 5 were shown in Figs. 4E and
5E, respectively. The CCK-8 results
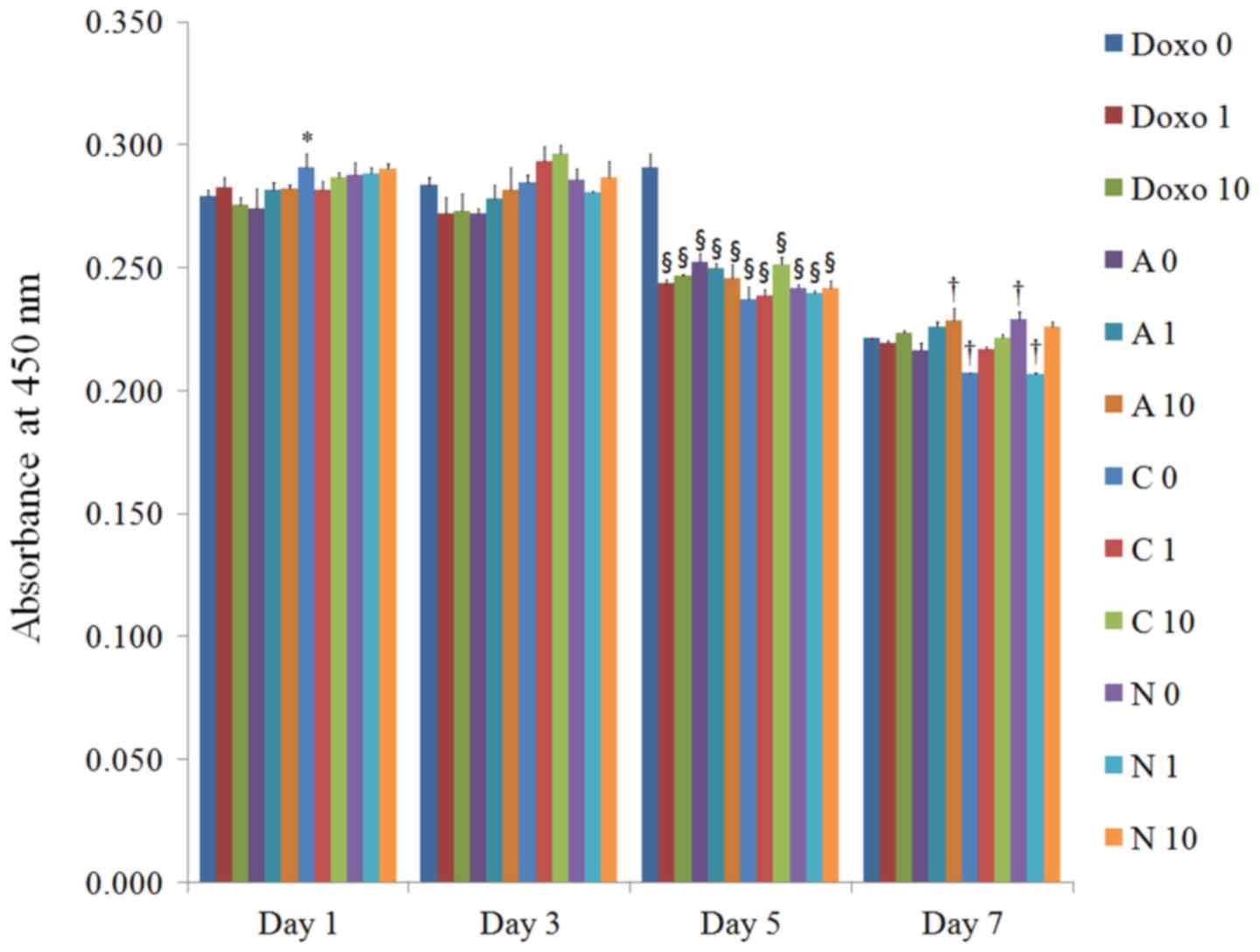
on days 1, 3, 5, and 7 are shown in Fig.
6. No significant changes in cellular viability were noted with
the addition of doxorubicin on day 1. However, significant
decreases in cellular viability were noted with the application of
doxorubicin on day 5.
Maintenance of stemness and secretion
of human vascular endothelial growth factor from spheroids
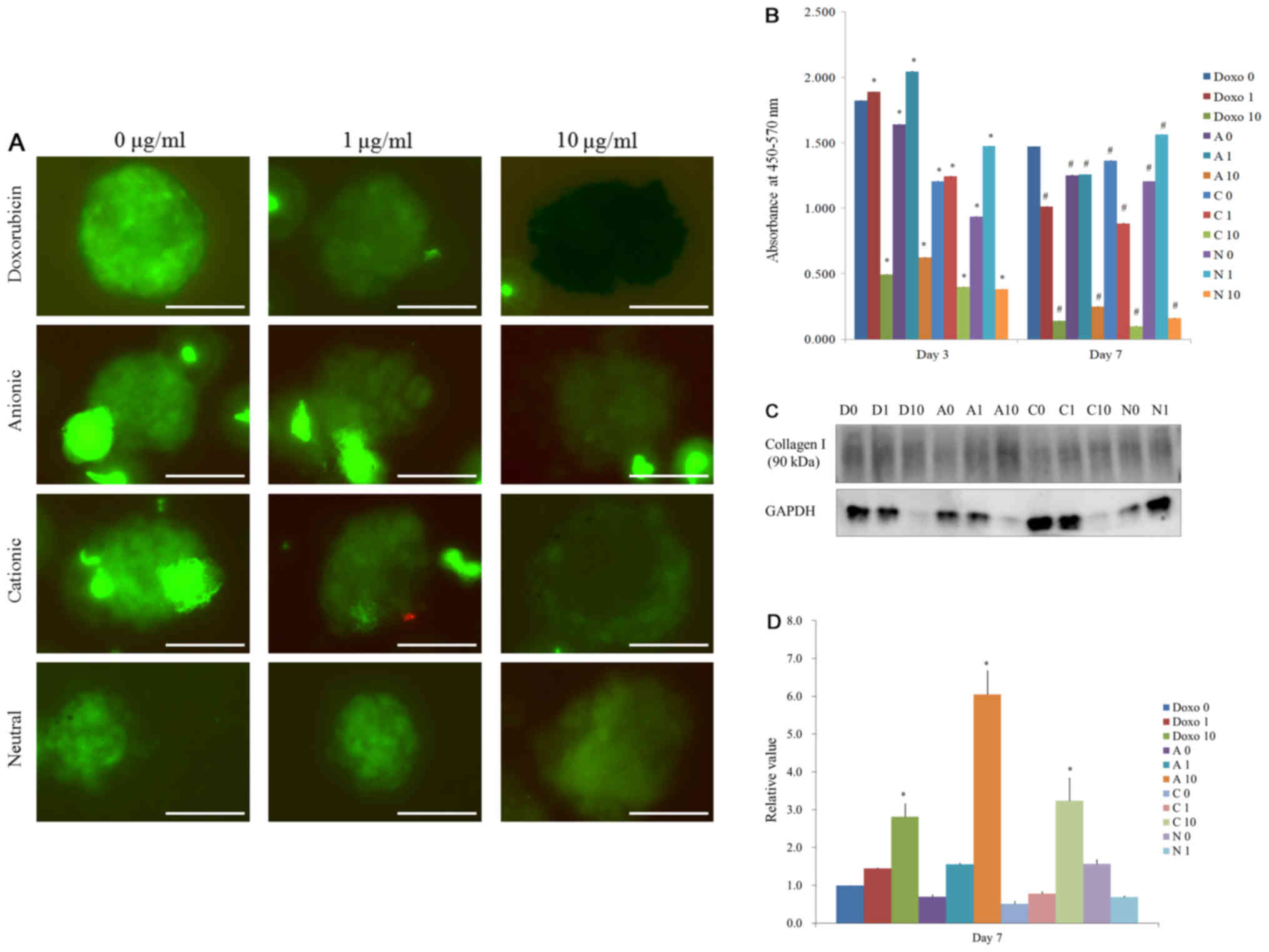
Spheroids were stained with NL493-conjugated SSEA-4
(green) antibodies on day 7 (Fig.
7A). The spheroids were positive for the stem cell markers
SSEA-4, which suggests that these spheroids primarily contained
undifferentiated human stem cells until the incubation time of 7
days. The secretions of vascular endothelial growth factors from
the spheroids were noted in all groups for day 3 and day 7
(Fig. 7B). The results clearly
showed that the secretion of the vascular endothelial growth factor
was observable on the third and seventh day and a stable secretion
of vascular endothelial growth factor during the culture period.
More severe decrease of secretions was noted with higher
concentrations of doxorubicin. The lowest secretion of vascular
endothelial growth factor was seen in C10 group on day 7.
Western blot
A Western blot analysis was performed to detect the
protein expression of collagen I, and GAPDH on day 7 (Fig. 7C). A limited quantity of protein
could only be obtained for the D10, A10, C10 and N10 groups, and
cells in the N10 group appeared to have reduced viability. There
were statistically significant differences were noted in D10, A10,
and C10 groups (P<0.05). Highest expressions were seen in A10
group.
Discussion
This report was intended to evaluate the effects of
anionic, cationic, and neutral liposomes containing doxorubicin on
the cellular viability, the secretion of vascular endothelial
growth factor and stem cell marker expression of three-dimensional
stem cell spheroids. This study clearly showed that
doxorubicin-loaded anionic liposomes produced the most sustained
release profile and cationic liposome produced the highest uptake
of the examined stem cell spheroids.
The fabrication of anionic, cationic, and neutral
liposomes were done using traditional film-hydration methods
(12). The release profile showed
that anionic liposomes had the slowest release rate compared with
cationic and neutral liposomes. This phenomenon possibly originated
from the charge-charge interaction between the anionic charge of
1,2-dipalmitoyl-sn-glycero-3-phosphoserine and amine group of
doxorubicin (1). The fluorescent
properties of doxorubicin make it feasible to monitor the release
profile of doxorubicin (13). In a
previous report, doxorubicin was chemically conjugated to a
terminal end group of poly (D, L-lactic-co-glycolic acid) by an
ester linkage, and the doxorubicin-poly (D, L-lactic-co-glycolic
acid) conjugate was formulated and a sustained release of the
doxorubicin was achieved at the injected site (14). If needed, these kinds of chemical
linkage will result in slower release of doxorubicin compared to
our liposomes. The viability of the stem cells were most
significantly affected by cationic liposomes compared with anionic
and neutral liposomes. It showed that the surface charge of the
liposomes may affect their binding and uptake to the stem cells,
similar to the cases of tumor cells (15). That application of stem cell
spheroids should meticulously be performed to obtain optimal
results for the individuals with anticancer therapy.
Previous studies showed that doxorubicin induces
decreases of cell survival and differentiation (16). The morphology of higher
concentrations of doxorubicin group seemed more dispersed and this
may be due to a loss of cell-to-cell and cell-matrix interactions
(9,17). The cellular microenvironment is
reported to affect maintenance of stemmenss (17), and the stem cell spheroids maintained
the expression of stem cell marker during the experimental periods.
However, the treatment of doxorubicin seemed to produce a reduced
expression of stem cell markers.
Various methods can be used for the fabrication of
three-dimensional tissue culture model (17). Hanging drop methods and rotary cell
culture systems can be used to generate spheroids (18,19). In
this report, the silicon elastomer-based concave microwells were
used for the fabrication of a three-dimensional system. Various
materials can be used for the surface of microwells, including
polyethylene glycol, polydimethylsiloxane, and polyurethane
(20,21). The morphology can be modified by the
shape of the microwells (10), and
the size of the spheroids can also be controlled by the number of
applied cells (5). Three-dimensional
cultures can be made with the aid of scaffolds including alginate
(6). In this study, a scaffold-free
culture system was used for the fabrication of spheroids and a
higher number of cells can be applied for same amount of space
(22).
The fabricated cell spheroids can be analyzed with
various methods. A protein assay can be used for the measurements
because the assay measures the protein content of the cells, making
it an indirect method (23).
Dye-exclusion tests, including the trypan blue test, can also be
used based on the principle that live cells with intact membranes
are not stained (24). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay,
which measures mitochondrial dehydrogenase activity of the tested
cells, can be considered a more sensitive test (25). In this test, the qualitative analysis
of cell viability was done with Live/Dead assay with confocal
microscopy, which is based on the fluorescence of thin-sectioned
images without complex fixation (26). A quantitative analysis was conducted
with a CCK-8 assay (27). This assay
does not require the solubilization process and the agent used is
reported to be less toxic with a higher sensitivity (28,29).
This study demonstrated that doxorubicin-loaded
anionic liposomes produced the most sustained release profile, and
cationic liposomes produced the highest uptake of the stem cell
spheroids. Higher concentrations of doxorubicin-loaded liposomes
affected cellular viability, the secretion of vascular endothelial
growth factor and stem cell marker expression. These overall
results showed that the formulation of liposomes influenced in
different outcome in viability, the secretion of vascular
endothelial growth factor and stem cell marker expression of
three-dimensional stem cell spheroids.
Acknowledgements
The Catholic MASTER Cells supplied by Catholic
Institute of Cell Therapy (CIC, Seoul, Korea) were derived from
human bone marrow donated by healthy donors after informed
consent.
Funding
This study was supported by Basic Science Research
Program by the Ministry of Education (2016R1C1B3013951) through the
National Research Foundation of Korea and the financial support of
the Catholic Medical Center Research Foundation made in the program
year of 2016. This research was also supported by Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, Information and
Communication Technology & Future Planning
(NRF-2017R1A1A1A05001307) and by Research Fund of Seoul St. Mary's
Hospital, The Catholic University of Korea.
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
HL, JS, CN, GY, HK and JP collaborated to design the
study. HL, JS, CN, GY, HK and JP performed the experiments and data
analysis. HL, JS, CN, GY, HK and JP collaborated to write the
manuscript. All the authors reviewed the final manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of Seoul St. Mary's
Hospital, College of Medicine, Catholic University of Korea (Seoul,
Republic of Korea) approved the present study (KC17SESI0290 and
KC11SISI0348) and informed consent from the study participants was
obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Abraham SA, Waterhouse DN, Mayer LD,
Cullis PR, Madden TD and Bally MB: The liposomal formulation of
doxorubicin. Methods Enzymol. 391:71–97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soliman A N, Abd-Allah SH, Hussein S and
Eldeen Alaa M: Factors enhancing the migration and the homing of
mesenchymal stem cells in experimentally induced cardiotoxicity in
rats. IUBMB Life. 69:162–169. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Csikos Z, Kerekes K, Fazekas E, Kun S and
Borbely J: Biopolymer based nanosystem for doxorubicin targeted
delivery. Am J Cancer Res. 7:715–726. 2017.PubMed/NCBI
|
|
4
|
Fite BZ, Kheirolomoom A, Foiret JL, Seo
JW, Mahakian LM, Ingham ES, Tam SM, Borowsky AD, Curry FE and
Ferrara KW: Dynamic contrast enhanced MRI detects changes in
vascular transport rate constants following treatment with
thermally-sensitive liposomal doxorubicin. J Control Release.
256:203–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SI, Yeo SI, Kim BB, Ko Y and Park JB:
Formation of size-controllable spheroids using gingiva-derived stem
cells and concave microwells: Morphology and viability tests.
Biomed Rep. 4:97–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang WZ, Yao XD, Huang XJ, Li JQ and Xu H:
Effects of TGF-β1 and alginate on the differentiation of rabbit
bone marrow-derived mesenchymal stem cells into a chondrocyte cell
lineage. Exp Ther Med. 10:995–1002. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Dal-Pra S, Mirotsou M, Jayawardena
TM, Hodgkinson CP, Bursac N and Dzau VJ: Tissue-engineered
3-dimensional (3D) microenvironment enhances the direct
reprogramming of fibroblasts into cardiomyocytes by microRNAs. Sci
Rep. 6:388152016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lo YP, Liu YS, Rimando MG, Ho JH, Lin KH
and Lee OK: Three-dimensional spherical spatial boundary conditions
differentially regulate osteogenic differentiation of mesenchymal
stromal cells. Sci Rep. 6:212532016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsiao C, Tomai M, Glynn J and Palecek SP:
Effects of 3D microwell culture on initial fate specification in
human embryonic stem cells. AIChE J. 60:1225–1235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SI, Ko Y and Park JB: Evaluation of
the maintenance of stemness, viability and differentiation
potential of gingiva-derived stem-cell spheroids. Exp Ther Med.
13:1757–1764. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhinge KN, Gupta V, Hosain SB,
Satyanarayanajois SD, Meyer SA, Blaylock B, Zhang QJ and Liu YY:
The opposite effects of doxorubicin on bone marrow stem cells
versus breast cancer stem cells depend on glucosylceramide
synthase. Int J Biochem Cell Biol. 44:1770–1778. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lowery A, Onishko H, Hallahan DE and Han
Z: Tumor-targeted delivery of liposome-encapsulated doxorubicin by
use of a peptide that selectively binds to irradiated tumors. J
Control Release. 150:117–124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shah S, Chandra A, Kaur A, Sabnis N, Lacko
A, Gryczynski Z, Fudala R and Gryczynski I: Fluorescence properties
of doxorubicin in PBS buffer and PVA films. J Photochem Photobiol
B. 170:65–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoo HS, Lee KH, Oh JE and Park TG: In
vitro and in vivo anti-tumor activities of nanoparticles based on
doxorubicin-PLGA conjugates. J Control Release. 68:419–431. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee S, Lee SY, Park S, Ryu JH, Na JH, Koo
H, Lee KE, Jeon H, Kwon IC, Kim K and Jeong SY: In vivo NIRF
imaging of tumor targetability of nanosized liposomes in
tumor-bearing mice. Macromol Biosci. 12:849–856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rana T, Chakrabarti A, Freeman M and
Biswas S: Doxorubicin-mediated bone loss in breast cancer bone
metastases is driven by an interplay between oxidative stress and
induction of TGFβ. PLoS One. 8:e780432013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang S, Liu P, Chen L, Wang Y, Wang Z and
Zhang B: The effects of spheroid formation of adipose-derived stem
cells in a microgravity bioreactor on stemness properties and
therapeutic potential. Biomaterials. 41:15–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Y, Xu Y, Xiao Z, Zhao Y, Li J, Han S,
Chen L, Dai B, Wang L, Chen B and Wang H: The combination of
three-dimensional and rotary cell culture system promotes the
proliferation and maintains the differentiation potential of rat
BMSCs. Sci Rep. 7:1922017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ylostalo JH, Bazhanov N, Mohammadipoor A
and Bartosh TJ: Production and administration of therapeutic
mesenchymal stem/stromal cell (MSC) spheroids primed in 3-D
cultures under Xeno-free conditions. J Vis Exp. Mar 18–2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mohr JC, de Pablo JJ and Palecek SP: 3-D
microwell culture of human embryonic stem cells. Biomaterials.
27:6032–6042. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khademhosseini A, Ferreira L, Blumling J
III, Yeh J, Karp JM, Fukuda J and Langer R: Co-culture of human
embryonic stem cells with murine embryonic fibroblasts on
microwell-patterned substrates. Biomaterials. 27:5968–5977. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rogozhnikov D, O'Brien PJ, Elahipanah S
and Yousaf MN: Scaffold free bio-orthogonal assembly of
3-dimensional cardiac tissue via cell surface engineering. Sci Rep.
6:398062016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JB, Zhang H, Lin CY, Chung CP, Byun
Y, Park YS and Yang VC: Simvastatin maintains osteoblastic
viability while promoting differentiation by partially regulating
the expressions of estrogen receptors α. J Surg Res. 174:278–283.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park JB: Effects of 17-α ethynyl estradiol
on proliferation, differentiation & mineralization of
osteoprecursor cells. Indian J Med Res. 136:466–470.
2012.PubMed/NCBI
|
|
25
|
Park JB: Low dose of doxycyline promotes
early differentiation of preosteoblasts by partially regulating the
expression of estrogen receptors. J Surg Res. 178:737–742. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barker LP, George KM, Falkow S and Small
PL: Differential trafficking of live and dead Mycobacterium marinum
organisms in macrophages. Infect Immun. 65:1497–1504.
1997.PubMed/NCBI
|
|
27
|
Ha DH, Pathak S, Yong CS, Kim JO, Jeong JH
and Park JB: Potential differentiation ability of gingiva
originated human mesenchymal stem cell in the presence of
tacrolimus. Sci Rep. 6:349102016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Almazin SM, Dziak R, Andreana S and
Ciancio SG: The effect of doxycycline hyclate, chlorhexidine
gluconate and minocycline hydrochloride on osteoblastic
proliferation and differentiation in vitro. J Periodontol.
80:999–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jue SS, Lee WY, Kwon YD, Kim YR, Pae A and
Lee B: The effects of enamel matrix derivative on the proliferation
and differentiation of human mesenchymal stem cells. Clin Oral
Implants Res. 21:741–746. 2010. View Article : Google Scholar : PubMed/NCBI
|