Introduction
For the past 30 years, lung cancer has been the
primary cause of cancer-associated mortality in China (1). Specifically, >80% of all lung cancer
cases are non-small cell lung cancer (NSCLC), with adenocarcinoma
being the most prevalent NSCLC subtype (2). Despite increased understanding of lung
adenocarcinoma, the 5-year survival rate remains <17% (3–5). It is
therefore of great importance to develop novel therapeutic targets
and effective treatments to improve the prognoses of patients with
adenocarcinoma.
The extracellular signal-regulated kinase (ERK)
family is typically considered to been associated with the
proliferation, differentiation and migration of cells (6). However, a number of studies have
reported that activation of the Ras/Raf/ERK pathway may induce
apoptosis, autophagy and senescence in tumor cells under certain
circumstances (7,8). Several commonly used chemotherapeutic
agents, including piperlongumine (9), paclitaxel (10) and Wentilactone A (11), activate the Ras/Raf/ERK pathway to
extert an anti-proliferative effect.
Ginseng, the root of Panax ginseng C.A. Meyer
(Araliaceae), has been used as a traditional herbal medicine in
China for centuries (12). Ginseng
possesses a number of pharmacological activities, including
immunomodulatory, anti-mutagenic and anti-aging effects (13). Ginsenoside Rh2 (G-Rh2) is one of the
primary effective constituents of ginseng and has been reported to
induce apoptosis in human lung adenocarcinoma A549 cells and human
breast cancer MCF-7 cells (14,15).
In a previous study, a number of dammarane-type
derivatives were prepared and their activities were investigated
(16). Qian et al (17) at the College of Chemistry of Jilin
University designed a novel dammarane-type derivative, namely
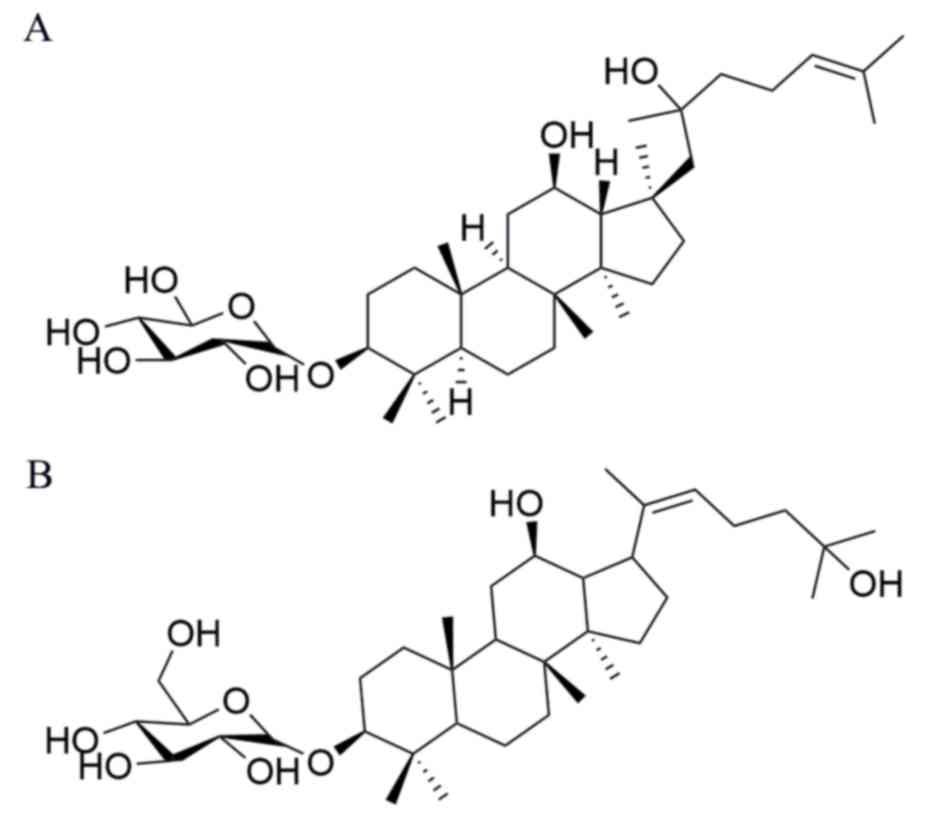
β-D-Glucopyranoside,(3b,12b,20E)-12,25-dihydroxydammar-20 (22)-en-3-yl, pseudo-G-Rh2, which possesses
a different side chain at C-17 compared with G-Rh. In a previous
study by our group, pseudo-G-Rh2 was reported to induce apoptosis
in human gastric cancer SGC-7901 cells in vitro (18). However, whether pseudo-G-Rh2 induces
apoptosis in lung adenocarcinoma A549 cells remains unclear. The
aim of the present study therefore was to elucidate the antitumor
effects of pseudo-G-Rh2 in lung adenocarcinoma cells.
Materials and methods
Chemicals
Pseudo-G-Rh2 (Fig. 1)
was provided by Professor Chen (College of Chemistry, Jilin
University, Changchun, China). The purity of pseudo-G-Rh2 used in
experiments was >95% as assessed by high-performance liquid
chromatography using Agilent 1100 with a Zoebax Extent C18 column
(250×4.6 mm, 5 µm) (both, Agilent Technologies, Inc., Santa Clara,
CA, USA) at 25°C. Methanol and water (90:10) was used as the mobile
phase, the flow rate was 1.2 ml/min and the sample quantity was 10
µl). Antibodies against procaspase-3 (cat. no. 19677-1-AP),
procaspase-9 (cat. no. 66169-1-Ig) and β-actin (cat. no.
20536-1-AP) were purchased from Wuhan Sanying Biotechnology (Wuhan,
China). Antibodies against PARP (cat. no. 9542), Bcl-2 (cat. no.
2876), Bax (cat. no. 2772), Ras (cat. no. 3965), phosphorylated
(p)-Raf (cat. no. 9427), Raf (cat. no. 9422), p53 (cat. no. 9282),
ERK (cat. no. 4695), c-Jun N-terminal kinase (JNK) (cat. no. 9258),
p38 (cat. no. 8690), p-ERK (cat. no. 4370), p-JNK (cat. no. 4668)
and p-p38 (cat. no. 4511) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The BCA protein assay reagent
kit, DAPI staining kit, reactive oxygen species (ROS) assay kit
(cat. no. S0033) and Rhodamine 123 were purchased from Beyotime
Institute of Biotechnology (Jiangsu, China). An Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit was
obtained from Tianjin Sungene Biotech Co., Ltd. (Tianjin, China).
MTT and all other reagents were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany).
Cell culture and treatment
Lung adenocarcinoma A549 cells were obtained from
the Shanghai Institute of Cell Biology, Chinese Academic of
Sciences (Shanghai, China). A549 cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Zhejiang Tianhang
Biotechnology Co., Ltd., Huzhou, China) under standard culture
conditions (37°C in an atmosphere containing 5% CO2).
Culture medium was replaced every 3 days with fresh complete medium
and maintained in log phase growth. Pseudo-G-Rh2 was dissolved in
dimethyl sulfoxide (DMSO) at room temperature and 104 µM was the
maximum solubility. Then Pseudo-G-Rh2 was added to the culture
media at the final concentrations. The final concentration of DMSO
was <0.1%. Cells were treated with different concentrations of
Pseudo-G-Rh2 (40, 56, 72, 88 or 104 µM) for 24, 48 and 72 h at
37°C, prior to an MTT assay. The cells were administered with 24,
48 or 96 µM Pseudo-G-Rh2 for 24 h at 37°C prior to DAPI staining,
flow cytometry and western blot analysis.
MTT assay
Cell viability was assessed using an MTT assay as
previously described (19). Briefly,
A549 cells were seeded into 96-well plates (5×104/ml)
following treatment with 40, 56, 72, 88 or 104 µM pseudo-G-Rh2.
Cells were incubated for 20 h at 37°C. A total of 10 µl of MTT
solution (5 mg/ml in PBS) was added to each well and plates were
incubated for a further 4 h at 37°C. A total of 100 µl DMSO was
added to each well and plates were agitated for 10 min. Absorbance
was measured at 570 nm using a microplate reader (SpectraMax
340PC384; Molecular Devices LLC, Sunnyvale, CA, USA). Cell
viability was calculated as a fraction of the untreated cells. The
half-maximal inhibitory concentration (IC50) was calculated using
GraphPad Prism v5 (GraphPad Software, Inc., La Jolla, CA, USA).
DAPI staining
DAPI staining was performed as previously described
(20). Briefly, A549 cells were
seeded on 6-well plates (9×104/ml) and treated with 24,
48 and 96 µM pseudo-G-Rh2 at 37°C for 24 h. Cells were collected
and washed twice with PBS, permeabilized with 0.1% Triton X-100 and
stained with 2 µg/ml DAPI for 10 min at room temperature. Cells
were subsequently observed using a fluorescence microscope
(magnification, ×100; Nikon TE-2000U; Nikon Corporation, Tokyo,
Japan).
Annexin V-FITC/propidium iodide (PI)
assay
To assess pseudo-G-Rh2-induced apoptosis in A549
cells, Annexin V-FITC/PI staining and flow cytometry were performed
as previously described with an (19). Briefly, following pseudo-G-Rh2
treatment, A549 cells were collected, washed twice with PBS and
resuspended in 300 µl of binding buffer containing PI and Annexin
V-FITC. The samples were incubated for 15 min at room temperature
in dark and subsequently detected by flow cytometry and CellQuest™
Pro (version 6.0) software (BD Biosciences, Franklin Lakes, NJ,
USA).
Caspase activity assay
The activities of caspase-3 and caspase-9 were
measured using a caspase 3 Activity Assay kit (cat. no. C1115) and
caspase 9 Activity Assay kit (cat. no. C1157) according to the
manufacturer's protocol (both Beyotime Institute of Biotechnology).
Briefly, cell lysates were incubated in a radioimmunoprecipitation
lysis buffer (cat. no. P0013B; Beyotime Institute of Biotechnology)
for 15 min on ice and centrifuged at 18,000 × g for 10 min at 4°C.
Supernatants were collected and total protein was quantified using
the Bradford method. Protein lysates was mixed with reaction buffer
(Ac-DEVD-pNA for caspase-3, Ac-LEHD-pNA for caspase-9) and
incubated at 37°C for 2 h in the dark. Cell absorbance was
subsequently measured at 405 nm using a microplate reader. Results
were reported as the percentage activity change compared with the
control.
Mitochondrial membrane potential
(ΔΨm)
The ΔΨm was measured using the cationic dye
Rhodamine 123 (cat. no. C2007; Beyotime Institute of Biotechnology)
as previously described (21).
Briefly, A549 cells were treated with 24, 48 and 96 µM pseudo-G-Rh2
for 24 h and incubated with Rhodamine 123 at 37°C for 30 min.
Fluorescence intensities were analyzed by flow cytometry and
CellQuest™ Pro software as above.
Measurement of ROS
ROS levels were assessed using the nonfluorescent
probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Beyotime
Institute of Biotechnology) as previously described (22). DCFH-DA permeates into cells and is
deacetylated by nonspecific esterase in the cell to form
2′,7′-dichlorofluorescein, which is nonfluorescent. The cellular
oxidizing agent oxidize DCFH to dichlorofluorescein, which is a
highly fluorescent compound (23).
As such, the amount of ROS produced in the cells is detected by
measuring the fluorescence intensity. Following pseudo-G-Rh2
treatment, A549 cells were collected, washed twice with PBS and
incubated with 10 µM DCFH-DA at 37°C for 20 min in the dark. Cells
were subsequently washed three times with PBS. Fluorescence was
observed under a Nikon TE-2000U fluorescence microscope
(magnification, ×100) and measured using flow cytometry as
above.
Western blotting
Western blotting was performed to assess protein
expression. Following treatment with pseudo-G-Rh2, A549 cells were
harvested and lysed in radioimmunoprecipitation assay buffer
(Beyotime Biotechnology, Jiangsu, China) for 30 min on ice. The
protein concentration was determined using a BCA protein assay kit
according to the manufacturer's protocol. Proteins (20 µl) were
separated by 12% SDS-PAGE and transferred onto a polyvinylidene
fluoride membrane, which was subsequently blocked with 5% (w/v)
non-fat milk for 1 h at room temperature. Membranes were incubated
with the appropriate primary antibodies against procaspase-3,
procaspase-9, PARP, Bcl-2, Bax, Ras, p-Raf, Raf, p53, ERK, c-JNK,
p38, p-ERK, p-JNK, p-p38 and β-actin at 1:1,000 dilution at 4°C
overnight. Primary antibody binding was detected by incubation with
a secondary antibody conjugated to horseradish peroxidase. The
goat-anti-mouse (cat. no. IH-0031) and goat-anti-rabbit (cat. no.
IH-0011) secondary antibodies (Beijing Dingguo Changsheng
Biotechnology Co., Ltd., Beijing, China) were used at 1:5,000
dilution at room temperature for 1 h. The bands were visualized
using a BeyoECL Plus enhanced chemiluminescence kit (Beyotime
Institute of Biotechnology). ImageJ software (version 1.5.0.26;
National Institutes of Health, Bethesda, MD, USA) was used for
analysis.
Statistical analysis
The results are expressed as the mean + standard
deviation of three independent experiments. Statistical differences
were evaluated using one-way analysis of variance with Tukey's post
hoc test. P<0.05 was considered to indicated a statistically
significant difference.
Results
Pseudo-G-Rh2 inhibits A549 cell
proliferation
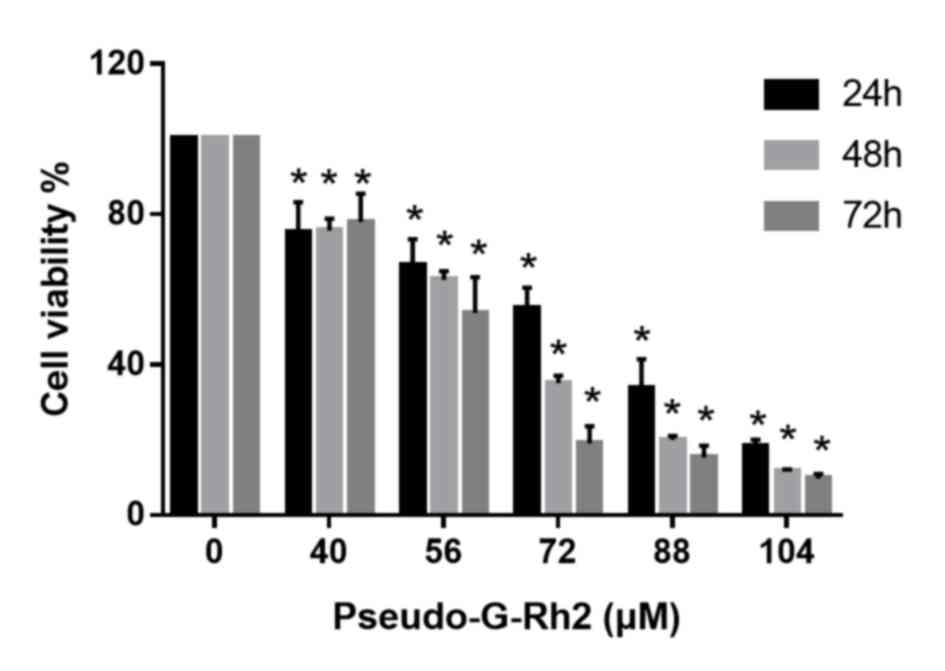
To evaluate the antiproliferative effects of
pseudo-G-Rh2 in vitro, A549 cells were treated with 40, 56,
72, 88 or 140 µM pseudo-G-Rh2 for 24, 48 or 72 h, following which
cell viability was assessed by MTT assay. The highest dose of
pseudo-G-Rh2 selected was 104 µM, as this is the maximum solubility
of this compound in DMSO. The results demonstrated that
pseudo-G-Rh2 significantly decreased the viability of A549 cells in
a concentration-dependent manner compared with the control
(Fig. 2). The IC50 value was 74.5 µM
for 24 h. Based on these results, dosages of 24, 48 and 96 µM
pseudo-G-Rh2 were selected for further experiments
Pseudo-G-Rh2 induces
mitochondria-associated apoptosis in A549 cells
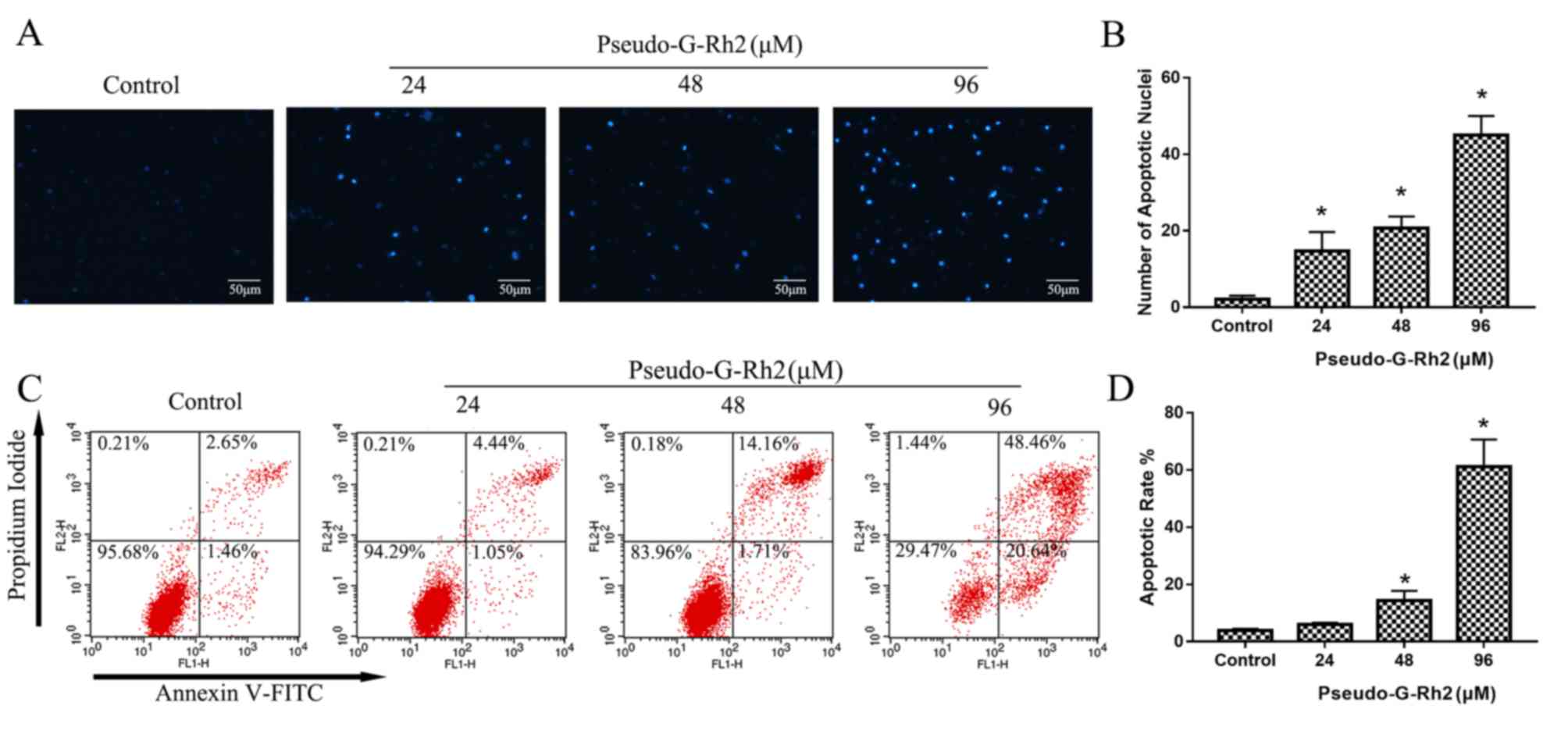
To elucidate whether pseudo-G-Rh2 induces apoptosis,
DAPI staining was performed. DAPI staining revealed an increase in
the number of apoptotic bodies containing nuclear fragmentations in
cells treated with pseudo-G-Rh2 compared with the control group
(Fig. 3A and B), which indicates
that pseudo-G-Rh2 induces apoptosis in A549 cells. The apoptotic
rate was measured using Annexin V-FITC/PI staining and the
percentage of apoptotic cells was revealed to be 2.65, 4.44, 14.1
and 48.56% in A549 cells treated with 0, 24, 48 and 96 µM of
pseudo-G-Rh2, respectively (Fig. 3C and
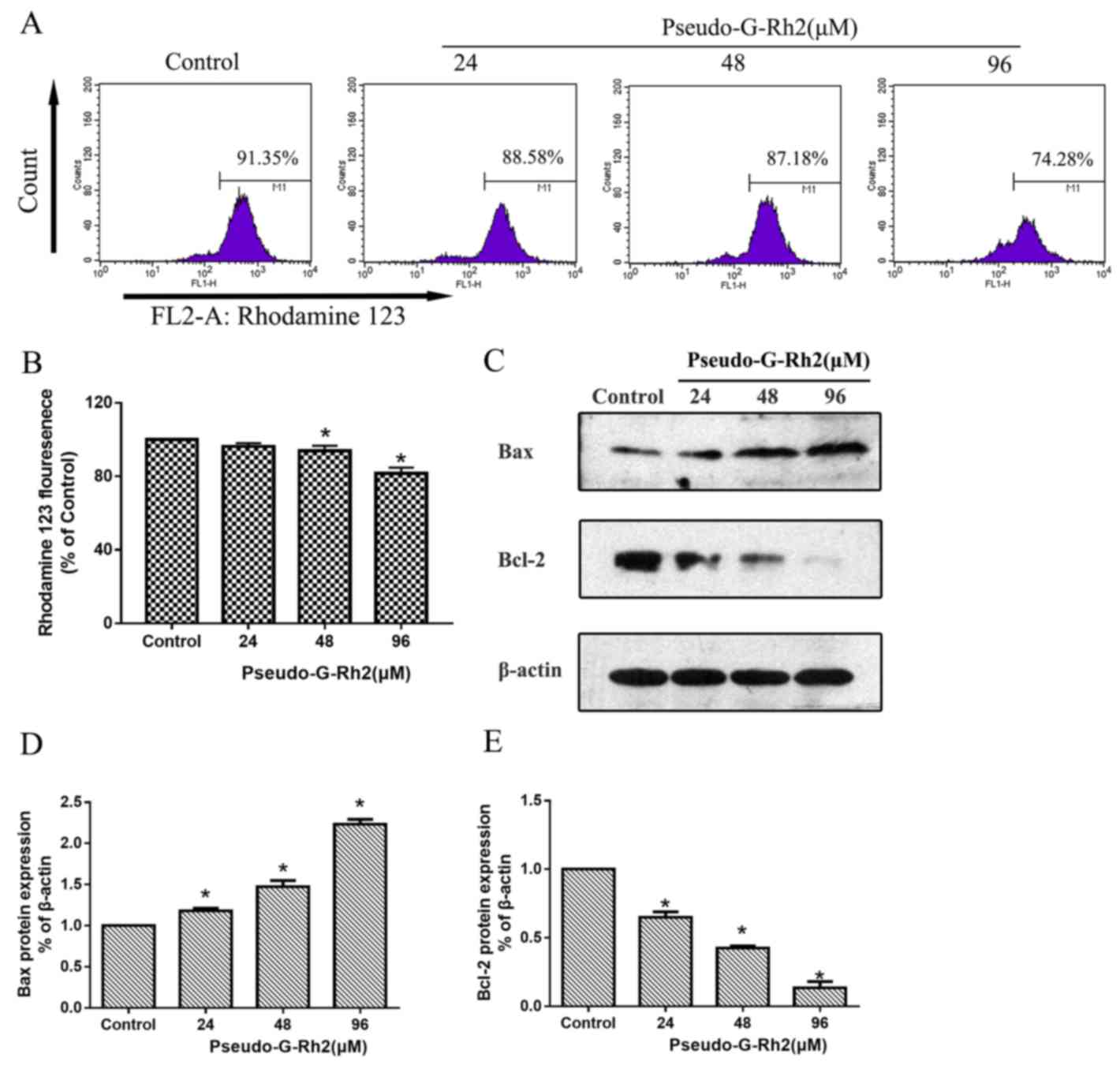
D). To further investigate the mechanism by which pseudo-G-Rh2
induces apoptosis in A549 cells, the ΔΨm was examined using
Rhodamine 123 (Fig. 4A). The
ΔΨm (percentage of strong fluorescent cells) in A549 cells
treated with 0, 15, 30 and 60 µg/ml pseudo-G-Rh2 for 24 h was
91.35, 88.85, 87.18 and 74.28%, respectively (Fig. 4A and B). In addition, western
blotting revealed that the expression of B-cell lymphoma 2 (Bcl-2)
was decreased compared with the control, whereas that of
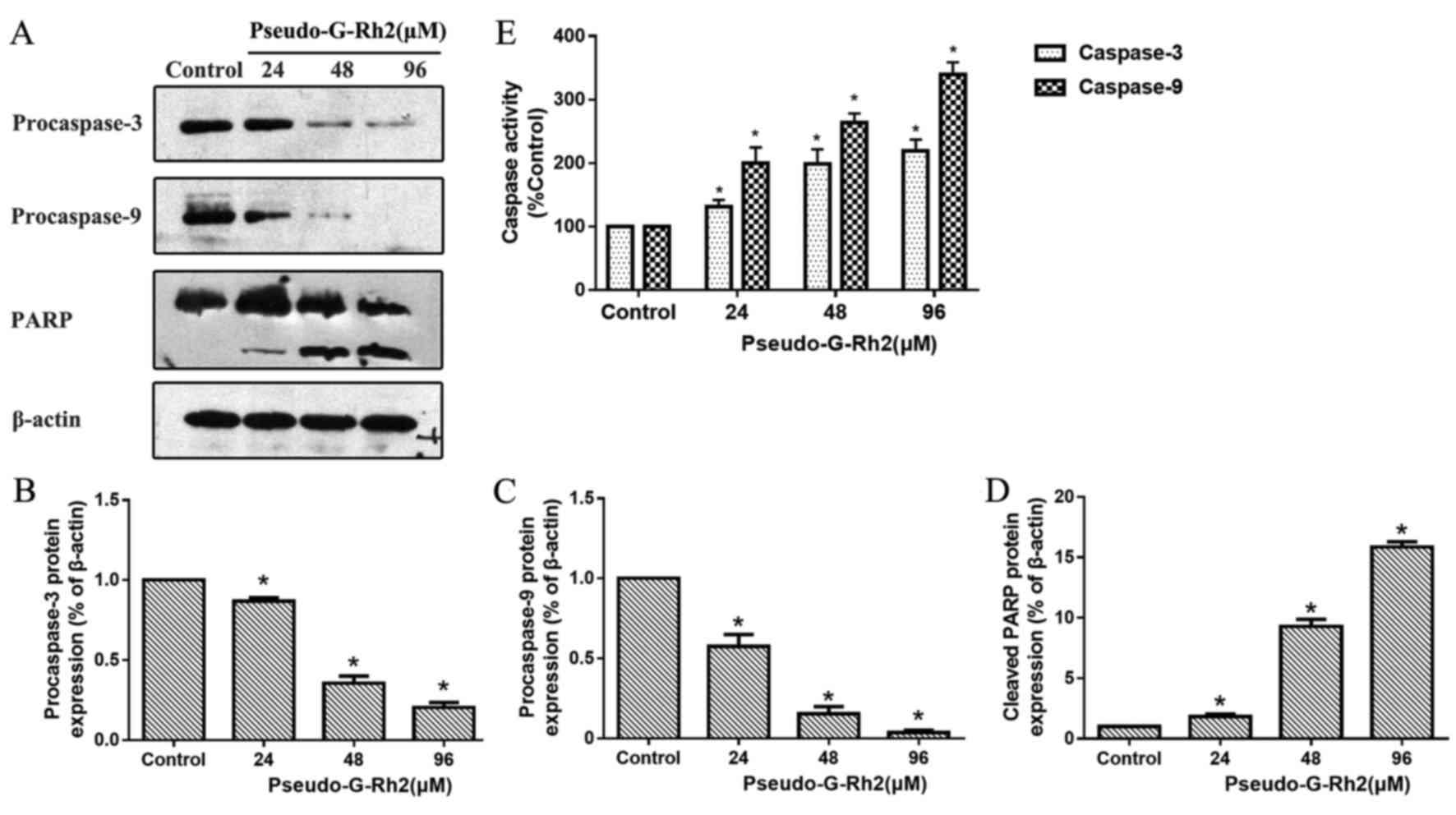
Bcl-2-associated X protein (Bax) was increased (Fig. 4C-E). Western blotting revealed that
precursors of caspase-9 and caspase-3 were decreased in A549 cells
following treatment with pseudo-G-Rh2 treatment compared with the
control (Fig. 5A-D). Upregulated
cleaved poly ADP-ribose polymerase (PARP), a product of caspase-3,
was observed following pseudo-G-Rh2 treatment compared with the
control. Furthermore, spectrophotometry results revealed that
pseudo-G-Rh2 enhanced the activity of caspase-9 and caspase-3
compared with the control (Fig. 5E).
These results suggest that pseudo-G-Rh2 induces apoptosis via the
mitochondrial pathway in A549 cells.
Pseudo-G-Rh2 increases ROS
production
It has previously been reported that ROS serve
important roles in mediating cancer initiation, promotion and
apoptosis (24,25). To determine whether pseudo-G-Rh2
affects ROS production, A549 cells were treated with 24, 48 or 96
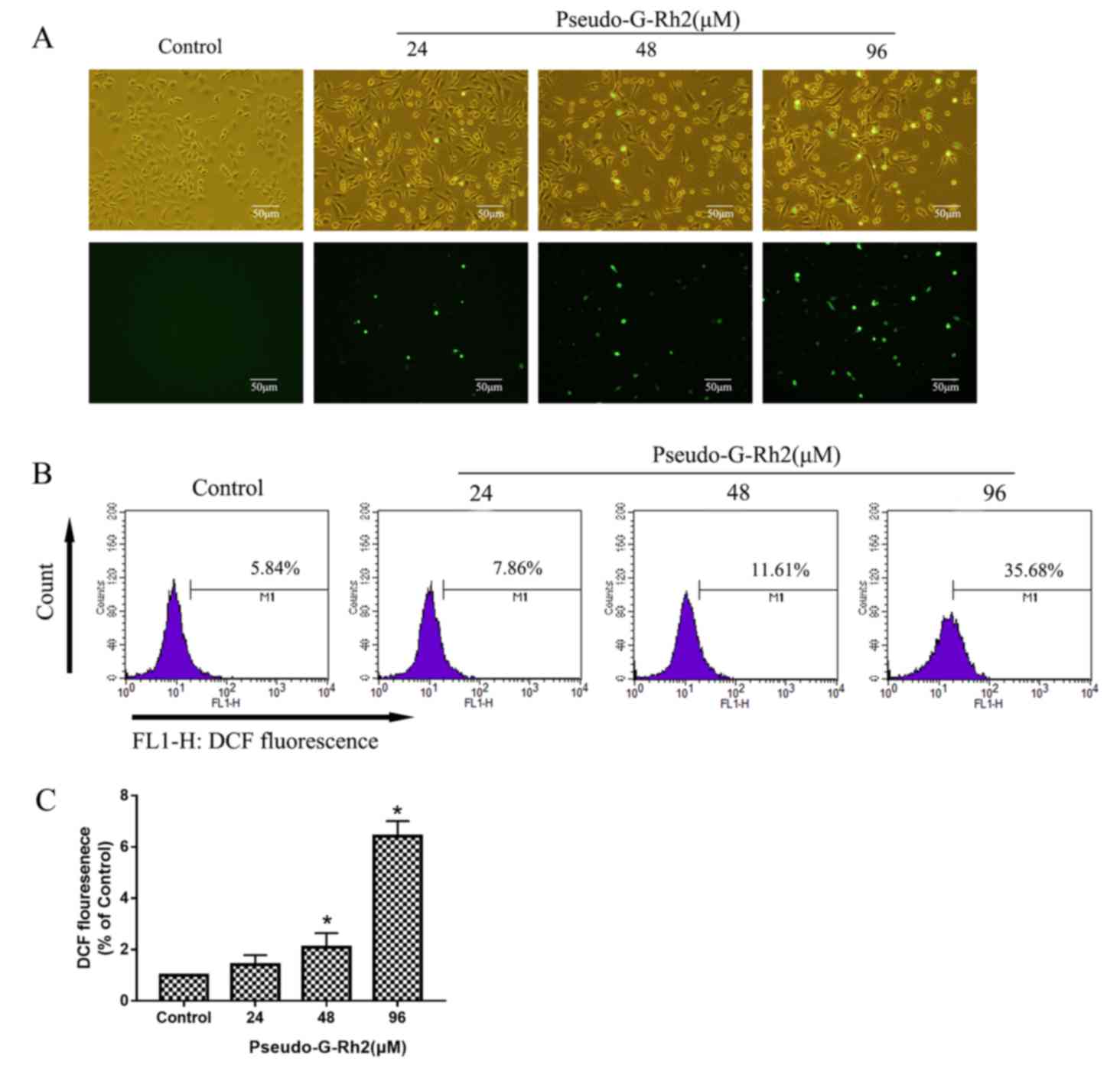
µM pseudo-G-Rh2 for 24 h and subjected to DCFH-DA fluorescence
analysis. Pseudo-G-Rh2 increased the intensity of green
fluorescence, which indicates ROS-positive cells, in a dose
dependent manner compared with the control (Fig. 6A). The results of flow cytometry
revealed that ROS expression (percentage of strong fluorescent
cells) was 5.84, 7.86, 11.61 and 35.68% in cells treated with 0,
24, 48 or 96 µM pseudo-G-Rh2 for 24 h, respectively (Fig. 6B). These results indicate that
pseudo-G-Rh2 induces ROS production in A549 cells, which may lead
to apoptosis.
Pseudo-G-Rh2 induces apoptosis via the
Ras/Raf/ERK/p53 pathway
ROS is known to be a mediator of mitogen-activated
protein kinases (MAPKs) and a number of studies have demonstrated
that ROS is able to maintain the activation of Ras, Raf and ERK,
resulting in apoptosis and autophagy (9,26,27). In
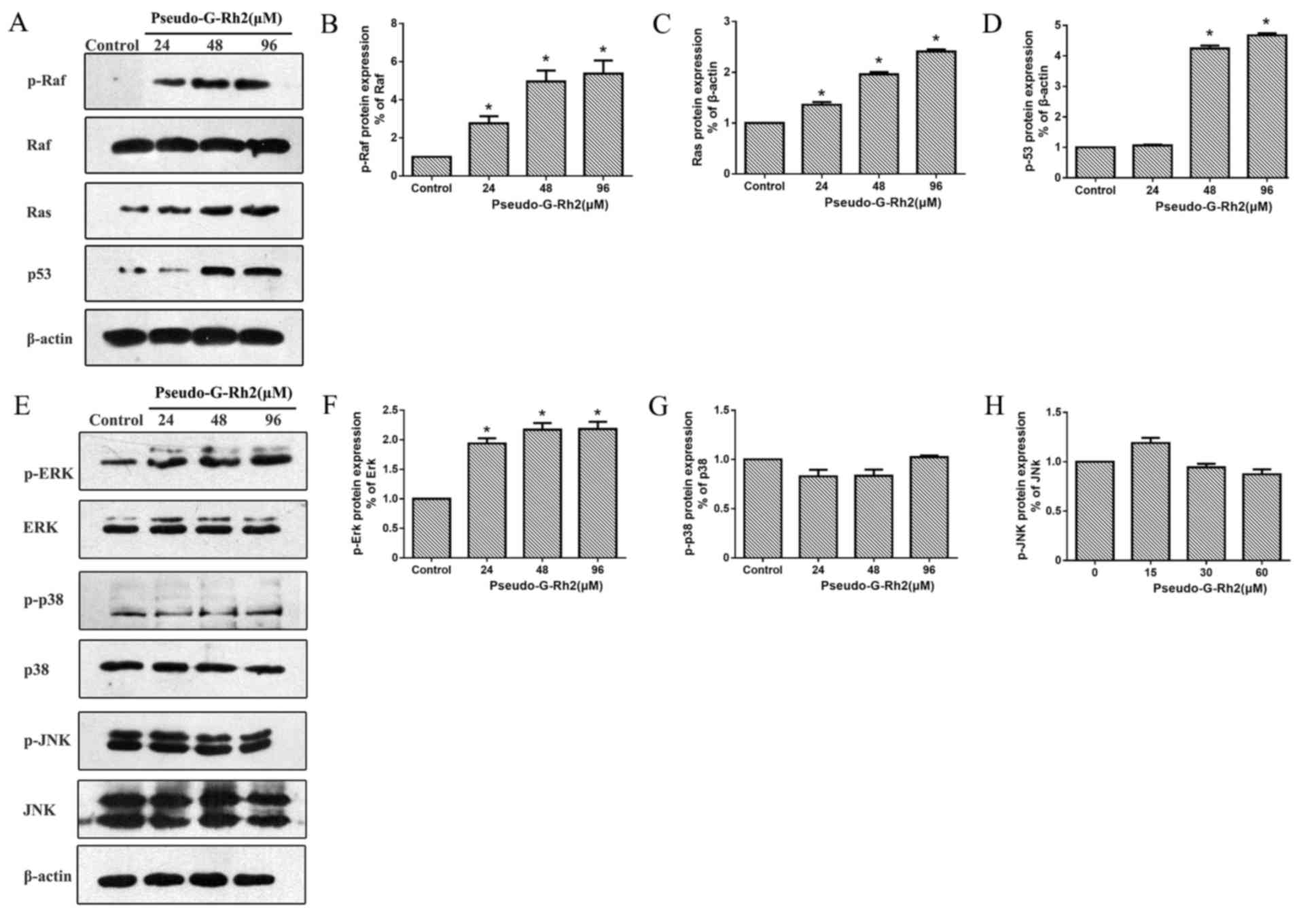
the present study, the expression of Ras, p-Raf and Raf proteins
were assessed using western blotting (Fig. 7A). Pseudo-G-Rh2 increased the
expression of p-Raf (Fig. 7B), Ras
(Fig. 7C) and p53 (Fig. 7D) compared with the control in a
dose-dependent manner; meanwhile, the ratio of p-Raf and p-ERK was
significantly increased. The expression of MAPK family proteins was
also assessed (Fig. 7E) and the
results demonstrated that p-ERK (Fig.
7F) was upregulated by pseudo-G-Rh2 in a dose-dependent manner
compared with the control. However, pseudo-G-Rh2 had no significant
effect on the ratio of p-p38/p38 or p-JNK/JNK. Increased activation
of the Ras/Raf/ERK pathway signaling is able to enhance the
stability and activity of tumor suppressor p53, which is known to
serve an important role in mediating apoptosis (11,28).
Discussion
Pseudo-G-Rh2, a novel derivative of G-Rh2, has
previously been demonstrated to have antitumor activity in human
gastric cancer SGC-7901 cells (18).
In the present study, it was revealed that pseudo-G-Rh2
significantly suppresses cell growth and induces apoptosis in A549
cells in vitro. MTT results demonstrated that pseudo-G-Rh2
is cytotoxic in A549 cells, with an IC50 of 74.5 µM. The IC50
obtained in the present study is lower than that reported for
SGC-7901 human gastric cancer cells, suggesting that A549 cells are
more sensitive to pseudo-G-Rh2 compared with SGC-7901 cells
(18). DAPI staining revealed an
increase in apoptotic bodies containing nuclear fragments following
treatment with pseudo-G-Rh2. An Annexin V-FITC/PI double-staining
assay further verified the results of DAPI staining, as the
apoptosis percentage increased. Together, these results indicate
that pseudo-G-Rh2 exerts its anticancer activity via inducing
apoptosis.
Apoptosis comprises a series of complicated and
sequential cascade reactions (29).
Apoptosis occurs via three pathways: The cell death
receptor-mediated extrinsic pathway, the endoplasmic reticulum
stress signaling pathway and the mitochondria-mediated intrinsic
pathway (30). In the present study,
a ΔΨm assay revealed that treatment with pseudo-G-Rh2
decreased the ΔΨm of A549 cells compared with the control.
The loss of ΔΨm may result in an increase in mitochondrial
cytochrome C release, which in turn promotes activation of the
caspase-9 precursor, ultimately leading to apoptosis. Western
blotting results revealed that pseudo-G-Rh2 downregulates Bcl-2,
procaspase-9 and procaspase-3, whereas Bax and cleaved PARP were
upregulated. These results indicate that pseudo-G-Rh2 induces
apoptosis in A549 cells via the mitochondrial mediated intrinsic
pathway.
The exact mechanisms of pseudo-G-Rh2 are not fully
understood. It has been reported that the p38, JNK and ERK
pathways, which are the primary pathways of the MAPK family, serve
important roles in the proliferation, invasiveness, angiogenesis
and cell cycle regulation of a number of cancers, including lung
cancer (31,32). The p38 and JNK pathways are typically
associated with cell death and the inhibition of cell
proliferation, whereas ERK is associated with cell proliferation
(33). However, a number of studies
have reported that the ERK pathway may function as tumor suppressor
(9,11,34,35). Lv
et al (11) revealed that
Wentilactone A induces apoptosis and G2/M arrest of human lung
carcinoma cells via excessive activation of the Ras/Raf/ERK/p53-p21
pathway. Furthermore, Randhawa et al (9) reported that the activation of ERK
signaling serves a role in the induction of colon cancer apoptosis
by piperlongumine. These inconsistencies may be due to variations
in the strength of the cascade signal and external stimuli,
including ROS (8). During
early-stage tumor development, ROS affects gene expression and
genome stability, resulting in loss-of-function mutations in p53
(36). Furthermore, ROS induces
metabolic adaptations (37), which
are crucial for the pathogenesis of cancer. However, during the
later stages of cancer, ROS accumulation induces p53 expression,
which in turn causes cell cycle arrest at the G2/M phase, DNA
fragmentation and apoptosis (38).
It has been reported that G may induce cell cycle arrest or cell
death by increasing ROS release in cancer cells (39,40). ROS
has also been reported to be a mediator of ERK-induced cell death
(41,42); specifically, elevated ROS results in
sustained ERK activity, which in turn causes enhanced p53
expression (43). p53 protein is
associated with Bcl-2 family regulation and is believed to serve a
critical role in mediating apoptosis (43,44). ERK
activity has been reported to directly affect mitochondrial
function by decreasing mitochondrial respiration and ΔΨm,
which may lead to mitochondrial membrane disruption and cytochrome
c release (45,46). In addition, Ras/Raf/ERK activity has
been reported to be associated with the upregulation of
proapoptotic members of the Bcl-2 family, including Bax and p53
upregulated modulator of apoptosis, as well as the downregulation
of Bcl-2 and Bcl-extra large (44,47,48).
In the present study, the effect of pseudo-G-Rh2 on
the Ras/Raf/ERK pathway was assessed by measuring the expression of
total Ras/Raf/ERK and p-Ras/Raf/ERK family members. The results
demonstrated that pseudo-G-Rh2 significantly increased the ratio of
p-Raf and p-ERK in A549 cells in vitro, whereas no
significant changes were observed in the ratio of p-p38/p38 and
p-JNK/JNK. These results suggest that pseudo-G-Rh2 induces
apoptosis in A549 cells via the Ras/Raf/ERK pathway, which was
further confirmed by the increase in ROS levels and upregulation of
p53.
In conclusion, the results of the present study
demonstrated that pseudo-G-Rh2 induced apoptosis in lung
adenocarcinoma A549 cells via the mitochondrial mediated intrinsic
pathway. During this process, pseudo-G-Rh2 increased ROS generation
and caused the subsequent activation of the Ras/Raf/ERK/p53
signaling pathway. These results provide an insight into the
mechanism of pseudo-G-Rh2-induced adenocarcinoma cell apoptosis,
suggesting that pseudo-G-Rh2 may have potential as a
chemotherapeutic agent for the treatment of lung adenocarcinoma.
However, the present study is with limitations. It was confirmed
that the activation of the Ras/Raf/ERK/p53 signaling pathway was
involved in the apoptosis induced by pseudo-G-Rh2, however whether
the Ras/Raf/ERK/p53 signaling pathway is the only target of
pseudo-G-Rh2 remains unclear. Further investigation is required to
confirm the mechanisms of pseudo-G-Rh2 by employing specific
inhibitors of the Ras-ERK signaling pathway. In addition, the
authors intend to utilize multi time point treatments of
pseudo-G-Rh2 and other lung cancer cell lines in the future to
confirm the effect of pseudo-G-Rh2 in NSCLC.
Acknowledgements
The authors would like to thank Dr Huiping Du,
Department of Health Informatics, Georgia State University
(Atlanta, GA, USA), for her critical reading and language
editing.
Funding
This study was supported by Jilin Province
Department of Education (grant no. JLE20130105).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DS and HX conceived and designed the study. CL, YT,
XY, YW and ZL performed the experiments. YW and HX wrote the paper.
CL, YT, XY and YW reviewed and edited the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Zeng H and Zhang S:
Epidemiology of lung cancer in China. Thorac Cancer. 6:209–215.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burstein HJ and Schwartz RS: Molecular
origins of cancer. N Engl J Med. 358:5272008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uramoto H and Tanaka F: Recurrence after
surgery in patients with NSCLC. Transl Lung Cancer Res. 3:242–249.
2014.PubMed/NCBI
|
|
4
|
Rittmeyer A: Quality of life in patients
with NSCLC receiving maintenance therapy. Cancers (Basel).
7:950–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leidinger P, Brefort T, Backes C, Krapp M,
Galata V, Beier M, Kohlhaas J, Huwer H, Meese E and Keller A:
High-throughput qRT-PCR validation of blood microRNAs in non-small
cell lung cancer. Oncotarget. 7:4611–4623. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balmanno K and Cook SJ: Tumour cell
survival signalling by the ERK1/2 pathway. Cell Death Differ.
16:368–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cox AD and Der CJ: The dark side of Ras:
Regulation of apoptosis. Oncogene. 22:8999–9006. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cagnol S and Chambard JC: ERK and cell
death: Mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Randhawa H, Kibble K, Zeng H, Moyer MP and
Reindl KM: Activation of ERK signaling and induction of colon
cancer cell death by piperlongumine. Toxicol In Vitro.
27:1626–1633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bacus SS, Gudkov AV, Lowe M, Lyass L, Yung
Y, Komarov AP, Keyomarsi K, Yarden Y and Seger R: Taxol-induced
apoptosis depends on MAP kinase pathways (ERK and p38) and is
independent of p53. Oncogene. 20:147–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv C, Hong Y, Miao L, Li C, Xu G, Wei S,
Wang B, Huang C and Jiao B: Wentilactone A as a novel potential
antitumor agent induces apoptosis and G2/M arrest of human lung
carcinoma cells, and is mediated by HRas-GTP accumulation to
excessively activate the Ras/Raf/ERK/p53-p21 pathway. Cell Death
Dis. 4:e9522013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu T, Zhao L, Zhang Y, Chen W, Liu D, Hou
H, Ding L and Li X: Ginsenoside 20(S)-Rg3 targets HIF-1α to block
hypoxia-induced epithelial-mesenchymal transition in ovarian cancer
cells. PLoS One. 9:e1038872014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kiefer D and Pantuso T: Panax ginseng. Am
Fam Physician. 68:1539–1542. 2003.PubMed/NCBI
|
|
14
|
Zhang C, Yu H and Hou J: Effects of 20
(S)-ginsenoside Rh2 and 20 (R)-ginsenoside Rh2 on proliferation and
apoptosis of human lung adenocarcinoma A549 cells. Zhongguo Zhong
Yao Za Zhi. 36:1670–1674. 2011.(In Chinese). PubMed/NCBI
|
|
15
|
Oh M, Choi YH, Choi S, Chung H, Kim K, Kim
SI, Kim DK and Kim ND: Anti-proliferating effects of ginsenoside
Rh2 on MCF-7 human breast cancer cells. Int J Oncol. 14:869–875.
1999.PubMed/NCBI
|
|
16
|
Liu YF, Yuan HN, Bi XL, Piao HR, Cao JQ,
Li W, Wang P and Zhao YQ: 25-Methoxylprotopanaxadiol derivatives
and their anti-proliferative activities. Steroids. 78:1305–1311.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qian G, Wang Z, Zhao J, Li D, Gao W, Wang
B, Sui D, Qu X and Chen Y: Synthesis and anti-cancer cell activity
of pseudo-ginsenoside Rh2. Steroids. 92:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qu X, Qu S, Yu X, Xu H, Chen Y, Ma X and
Sui D: Pseudo-G-Rh2 induces mitochondrial-mediated apoptosis in
SGC-7901 human gastric cancer cells. Oncol Rep. 26:1441–1446.
2011.PubMed/NCBI
|
|
19
|
Xu HL, Yu XF, Qu SC, Zhang R, Qu XR, Chen
YP, Ma XY and Sui DY: Anti-proliferative effect of Juglone from
Juglans mandshurica Maxim on human leukemia cell HL-60 by inducing
apoptosis through the mitochondria-dependent pathway. Eur J
Pharmacol. 645:14–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matin MM, Nakhaeizadeh H, Bahrami AR,
Iranshahi M, Arghiani N and Rassouli FB: Ferutinin, an apoptosis
inducing terpenoid from Ferula ovina. Asian Pac J Cancer Prev.
15:2123–2128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen HY, Zhang X, Chen SF, Zhang YX, Liu
YH, Ma LL and Wang LX: The protective effect of 17β-estradiol
against hydrogen peroxide-induced apoptosis on mesenchymal stem
cell. Biomed Pharmacother. 66:57–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deeb D, Gao X, Jiang H, Janic B, Arbab AS,
Rojanasakul Y, Dulchavsky SA and Gautam SC: Oleanane triterpenoid
CDDO-Me inhibits growth and induces apoptosis in prostate cancer
cells through a ROS-dependent mechanism. Biochem Pharmacol.
79:350–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H and Joseph JA: Quantifying cellular
oxidative stress by dichlorofluorescein assay using microplate
reader. Free Radic Biol Med. 27:612–616. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng X, Yu W, Zhou F, Chen J and Shen P: A
novel small molecule compound diaporine inhibits breast cancer cell
proliferation via promoting ROS generation. Biomed Pharmacother.
83:1038–1047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YJ, Cho HN, Soh JW, Jhon GJ, Cho CK,
Chung HY, Bae S, Lee SJ and Lee YS: Oxidative stress-induced
apoptosis is mediated by ERK1/2 phosphorylation. Exp Cell Res.
291:251–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang P, Wang YZ, Kagan E and Bonner JC:
Peroxynitrite targets the epidermal growth factor receptor, Raf-1,
and MEK independently to activate MAPK. J Biol Chem.
275:22479–22486. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ringer L, Sirajuddin P, Tricoli L, Waye S,
Choudhry MU, Parasido E, Sivakumar A, Heckler M, Naeem A,
Abdelgawad I, et al: The induction of the p53 tumor suppressor
protein bridges the apoptotic and autophagic signaling pathways to
regulate cell death in prostate cancer cells. Oncotarget.
5:10678–10691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsuruo T, Naito M, Tomida A, Fujita N,
Mashima T, Sakamoto H and Haga N: Molecular targeting therapy of
cancer: Drug resistance, apoptosis and survival signal. Cancer Sci.
94:15–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abraham MC and Shaham S: Death without
caspases, caspases without death. Trends Cell Biol. 14:184–193.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi C, Zheng DD, Fang L, Wu F, Kwong WH
and Xu J: Ginsenoside Rg1 promotes nonamyloidgenic cleavage of APP
via estrogen receptor signaling to MAPK/ERK and PI3K/Akt. Biochim
Biophys Acta. 1820:453–460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi YJ, Yoon JH, Cha SW and Lee SG:
Ginsenoside Rh1 inhibits the invasion and migration of THP-1 acute
monocytic leukemia cells via inactivation of the MAPK signaling
pathway. Fitoterapia. 82:911–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang C, Zhu H, Yang X, Lou J, Zhu D, Lu
W, He Q and Yang B: P53 and p38 MAPK pathways are involved in
MONCPT-induced cell cycle G2/M arrest in human non-small cell lung
cancer A549. J Cancer Res Clin Oncol. 136:437–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shih A, Davis FB, Lin HY and Davis PJ:
Resveratrol induces apoptosis in thyroid cancer cell lines via a
MAPK- and p53-dependent mechanism. J Clin Endocrinol Metab.
87:1223–1232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X, Martindale JL and Holbrook NJ:
Requirement for ERK activation in cisplatin-induced apoptosis. J
Biol Chem. 275:39435–39443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Waris G and Ahsan H: Reactive oxygen
species: Role in the development of cancer and various chronic
conditions. J Carcinog. 5:142006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weinberg F, Hamanaka R, Wheaton WW,
Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger
GR and Chandel NS: Mitochondrial metabolism and ROS generation are
essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA.
107:8788–8793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu CL, Huang AC, Yang JS, Liao CL, Lu HF,
Chou ST, Ma CY, Hsia TC, Ko YC and Chung JG: Benzyl isothiocyanate
(BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of
reactive oxygen species causes cell cycle arrest and induces
apoptosis via activation of caspase-3, mitochondria dysfunction and
nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J
Orthop Res. 29:1199–1209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ge G, Yan Y and Cai H: Ginsenoside Rh2
inhibited proliferation by inducing ROS mediated ER stress
dependent apoptosis in lung cancer cells. Biol Pharm Bull.
40:2117–2124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Singh DV, Agarwal S, Singh P, Godbole MM
and Misra K: Curcumin conjugates induce apoptosis via a
mitochondrion dependent pathway in MCF-7 and MDA-MB-231 cell lines.
Asian Pac J Cancer Prev. 14:5797–5804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Matsunaga Y, Kawai Y, Kohda Y and Gemba M:
Involvement of activation of NADPH oxidase and extracellular
signal-regulated kinase (ERK) in renal cell injury induced by zinc.
J Toxicol Sci. 30:135–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ramachandiran S, Huang Q, Dong J, Lau SS
and Monks TJ: Mitogen-activated protein kinases contribute to
reactive oxygen species-induced cell death in renal proximal tubule
epithelial cells. Chem Res Toxicol. 15:1635–1642. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Woessmann W, Chen X and Borkhardt A:
Ras-mediated activation of ERK by cisplatin induces cell death
independently of p53 in osteosarcoma and neuroblastoma cell lines.
Cancer Chemother Pharmacol. 50:397–404. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
DeHaan RD, Yazlovitskaya EM and Persons
DL: Regulation of p53 target gene expression by cisplatin-induced
extracellular signal-regulated kinase. Cancer Chemother Pharmacol.
48:383–388. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim GS, Hong JS, Kim SW, Koh JM, An CS,
Choi JY and Cheng SL: Leptin induces apoptosis via
ERK/cPLA2/cytochrome c pathway in human bone marrow stromal cells.
J Biol Chem. 278:21920–21929. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li DW, Liu JP, Mao YW, Xiang H, Wang J, Ma
WY, Dong Z, Pike HM, Brown RE and Reed JC: Calcium-activated
RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and
is abrogated by alpha B-crystallin through inhibition of RAS
activation. Mol Biol Cell. 16:4437–4453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu J, Mao W, Ding B and Liang CS:
ERKs/p53 signal transduction pathway is involved in
doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am
J Physiol Heart Circ Physiol. 295:H1956–H1965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu Z, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Phosphorylated extracellular signal-regulated kinase
up-regulated p53 expression in shikonin-induced HeLa cell
apoptosis. Chin Med J (Engl). 118:671–677. 2005.PubMed/NCBI
|