Introduction
Autism spectrum disorder (ASD) comprises a range
complex neurological diseases with impairments in social skills,
communication and repetitive behaviors (1). The incidence of ASD in males is four
times of that in females (2). ASD
causes great obstacles in interpersonal relationships (3), and it has become a heavy burden on
society and the families of affected individuals (4,5). Early
intervention may change the long-term prognosis of ASD patients
(6).
The pathogenesis of ASD involves a complex
interaction between heredity and environment (7). Epigenetics may provide the best bridge
between genetic and environmental factors (8). DNA methylation is an important
epigenetic modification that regulates the expression of numerous
functional genes in the human nervous system (6,9).
Aberrant DNA methylation has been reported in diseases or disorders
of the central nervous system (10,11).
These include hypermethylation of oxytocin R (12), Reelin (13), and SH3 and multiple ankyrin repeat
domains 3 (14) in ASD.
Apolipoprotein E (APOE) encodes a glycoprotein
associated with lipoproteins in the periphery and the brain
(15,16). Abnormal methylation of APOE was
reported to be associated with Alzheimer's disease (AD) (17), which may have overlapping mechanisms
with ASD (18). A genetic study
indicated that a variant of APOE is associated with ASD (19); however, it has remained elusive
whether APOE methylation is linked to ASD.
In the light of those previous results, the goal of
the present study was to explore whether APOE methylation is
associated with ASD.
Materials and methods
Patients and samples
A total of 135 pediatric subjects, including 62 ASD
patients (with no other disorders or diseases) and 73 healthy
age-matched individuals, volunteered for the study and were
recruited at Ningbo Kangning Hospital (Ningbo, China) between
September 2015 and September 2017. The ASD patients were diagnosed
according to the Diagnostic and Statistical Manual of Mental
Disorders, Fourth Edition, the Autism Diagnostic Observation
Schedule and the Childhood Autism Rating Scales (CARS) (5). Blood samples were drawn from healthy
controls and ASD cases prior to treatment. The clinical data
acquired from patients mainly included maternal pregnancy history,
family history, and disease duration. The study was approved by the
Ethics Committees in Ningbo Kangning Hospital and Ningbo University
(Ningbo, China), and written informed consent was obtained from the
parents of all patients.
DNA isolation and bisulphite
conversion
DNA was extracted from blood with the
E.Z.N.A.™ Blood DNA Kit (Omega Bio-Tek, Norcross, GA,
USA), according to the manufacturer's instructions. The
Nanodrop2000 spectrophotometer (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used to measure the DNA concentration. The EZ
DNA Methylation-Gold kit™ (Zymo Research, Irvine, CA, USA) was used
to convert unmethylated cytosines into the corresponding uracils,
while the methylated cytosines remained in their positions.
SYBR green-based quantitative
methylation-specific polymerase chain reaction (qMSP)
qMSP was used for to assess the methylation of APOE.
The reaction system contained 10 µl SYBR Green I Master Mix (Roche
Diagnostics, Basel, Switzerland), 0.5 µl forward primer (10 µm),
0.5 µl reverse primer (10 m), 1.0 µl templates and 8 µl
double-distilled (dd)H2O. The thermocycling conditions
comprised an initial denaturation at 95°C for 10 min, followed by
45 cycles of 95°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec.
Subsequently, melting curve analysis was performed at 95°C for 15
sec and 60°C for 1 min, followed by increases in the temperature by
0.11°C per sec to 95°C. Human sperm DNA (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) was methylated as the
positive control by excess SssI methyltransferase (Thermo Fisher
Scientific, Inc.), and ddH2O as a negative control. ACTB
was used as the internal reference to correct the differences in
the quality and quantity between samples. The sequences of the qMSP
primers were APOE forward, 5′-CGAGGTGTAGGTTATGTTC-3′ and reverse,
5′-TACGCAACTTACGCAAAT-3′; and β-actin (ACTB) forward,
5′-TGGTGATGGAGGAGGTTTAGTAAGT-3′ and reverse,
5′-AACCAATAAAACCTACTCCTCCCTTAA-3′. The percentage of methylation of
a reference (PMR) of APOE in each sample was calculated by the
following approach: ΔΔCq=sample DNA (CqAPOE
gene-CqACTB control)-fully methylated DNA
(CqAPOE gene-CqACTB control) (20,21).
Statistical analysis
All statistical analyses were performed using SPSS
software version 18.0 (SPSS, Inc., Chicago, IL, USA). The
independent-samples t-test was applied for the comparisons of
differences in APOE methylation between ASD cases and controls.
Receiver operating characteristic curves (ROC) were drawn to
evaluate the diagnostic value of APOE methylation for ASD. The
Spearman rank test was applied to assess the associations between
APOE methylation and clinical indicators in ASD. P<0.05 was
considered to indicate a statistically significant difference.
Results
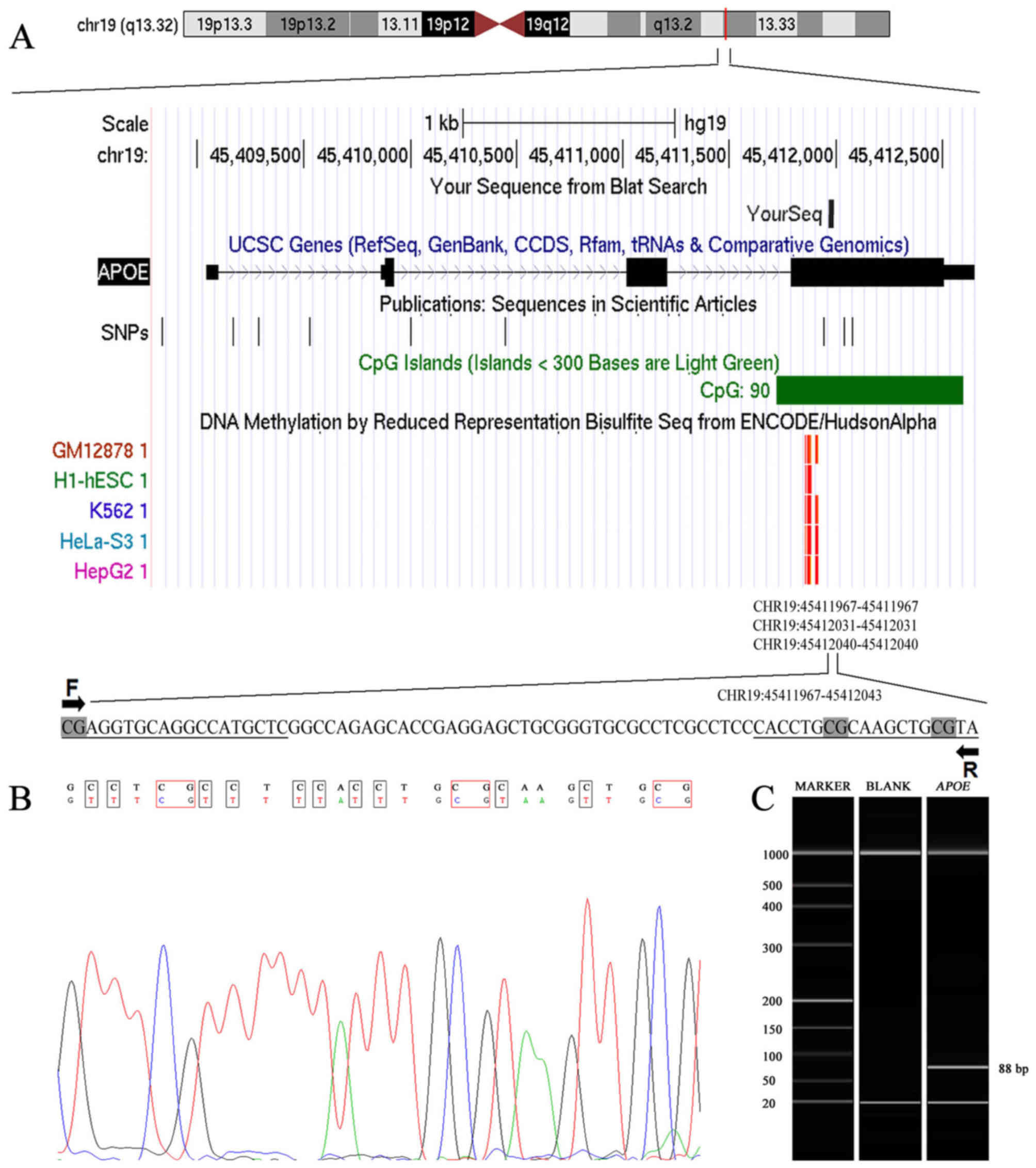
As presented in Fig.
1A, a methylation assay was performed on an 88-bp fragment of
the CpG island of APOE. A successful bisulphite transformation was
evidenced by detection of transformed thymine by Sanger sequencing
(Fig. 1B). The target fragment
length of 88 bp was confirmed by capillary electrophoresis
(Fig. 1C).
The present results indicated that APOE methylation
in pediatric ASD patients was significantly higher than that in
healthy age-matched individuals (median PMR, 33.36 vs. 10.61%;
P=2.36×10−10; Fig. 2A).
APOE hypermethylation was identified in 36 out of 62 subjects with
ASD. Considering the higher prevalence of ASD in males, a breakdown
analysis by gender was performed. The results indicated that APOE
methylation levels were higher in male ASD cases than in healthy
male controls (median PMR, 32.12 vs. 11.00%;
P=8.51×10−7; Fig. 2B).
Similar results were also obtained in females (mean PMR, 43.35 vs.
7.87%; P=4.18×10−6; Fig.
2C).
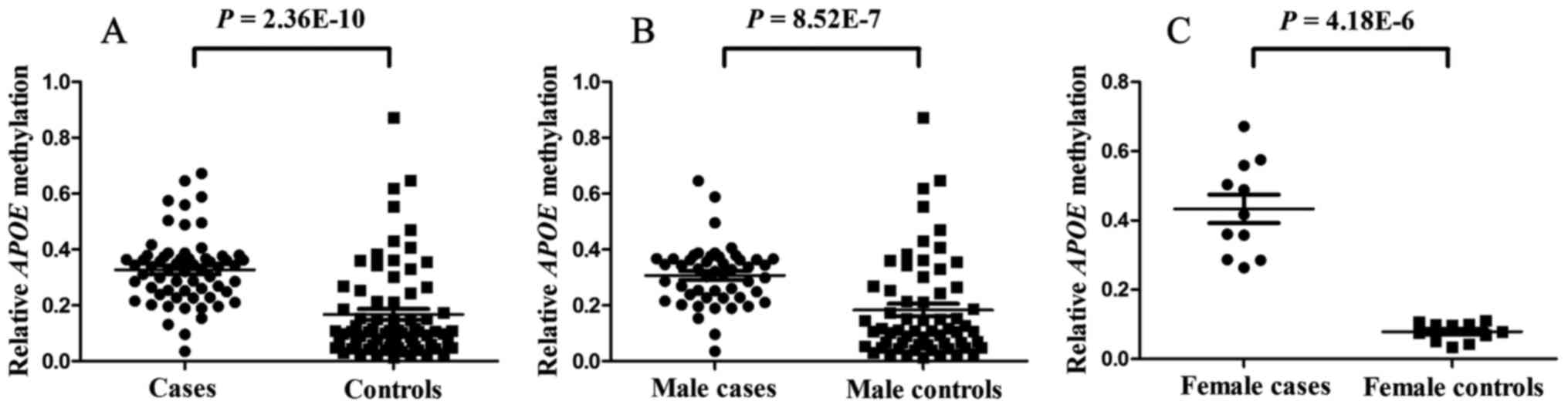 | Figure 2.Comparison of the relative APOE
methylation between ASD cases and healthy controls. (A) Comparison
between ASD cases and controls. The median PMR in cases and
controls was 33% (interquartile range, 25 and 37%) and 11%
(interquartile range, 6 and 23%), respectively. The P-value for the
comparison between the cases and the controls was
2.36×10−10. (B) Comparison between male ASD cases and
male controls. The median PMR values of cases and controls were 32%
(23, 37%) and 11% (6, 27%), respectively. The P-value for the
comparison between the male cases and the male controls was
8.52×10−10. (C) Comparison between female ASD cases and
female controls. The median PMR values of cases and controls were
0.42 (0.29, 0.56) and 0.08 (0.05, 0.10), respectively. The P-value
for the comparison between the female cases and the female controls
was 4.18×10−6. APOE, apolipoprotein E; ASD, autism
spectrum disorder; PMR, percentage of methylation of a
reference. |
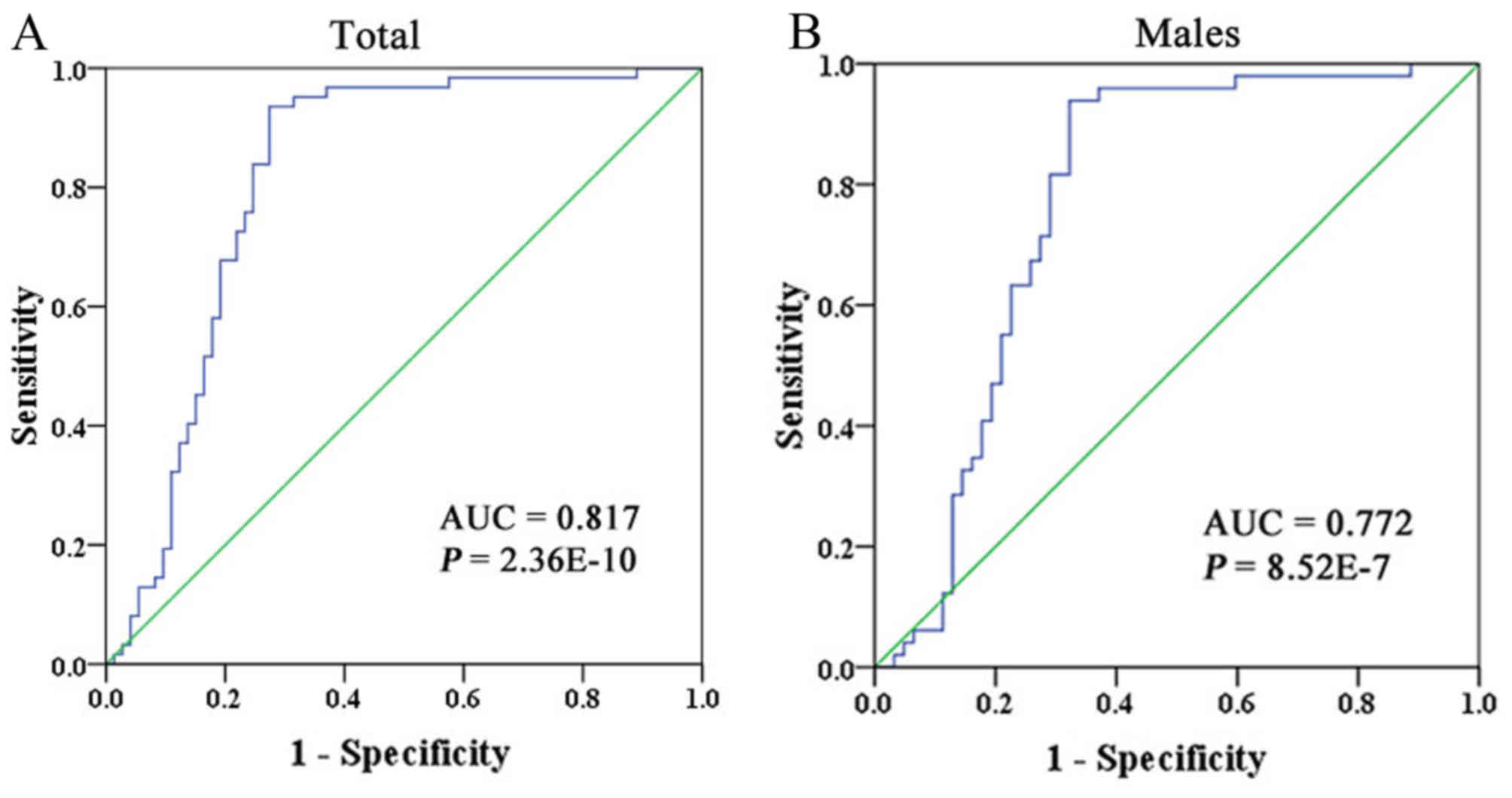
In addition, ROC curve analysis was performed to
quantitatively evaluate the diagnostic value. As presented in
Fig. 3A, the area under the curve
(AUC) was 0.817 [95% confidence interval (CI), 0.741–0.893]. The
ROC curves demonstrated that PMR of 15.4% was the optimal cut-off
for predicting ASD (sensitivity, 93.5%, specificity, 72.6%;
P=2.36×10−10; Fig. 3A).
Further subgroup analysis by gender provided a similar result in
males (AUC=0.772; 95% CI, 0.682–0.865; sensitivity, 93.9%;
specificity, 67.7%; P=8.52×10−7; Fig. 3B), PMR of 15.3% was the optimal
cut-off for predicting ASD. Due to a limited number of female
individuals, it was not possible to perform any ROC curve analysis
in females.
Discussion
APOE is a multifunctional protein in the brain, and
it is known as a carrier of cholesterol and other lipids in the
central nervous system (22–24). APOE methylation levels have been
reported to be significantly elevated in AD patients (17). Previous studies have indicated that
ASD and AD have various common mechanisms in the course of their
development (25–27). Therefore, it was speculated that APOE
is associated with the occurrence and the development of ASD. The
present results demonstrated that APOE hypermethylation is
significantly associated with ASD. Based on the ROC curve, APOE
hypermethylation may be regarded as a potential biomarker for the
diagnosis of ASD.
APOE polymorphisms (APOE ε2 and APOE ε4) are
involved in the etiological complexity of the predisposition for
ASD (19,28). A transmission distortion of the APOE
ε2 allele has been reported in families with cases of ASD. Compared
with APOE ε3 and APOE ε4, the APOE ε2 protein product displays the
lowest receptor binding affinity (28). DNA methylation is an epigenetic
modification and regulates gene expression in response to
environmental stimuli (29). Gene
methylation is generally inversely correlated with gene expression
(30–32). APOE expression has been reported to
be downregulated by APOE hypermethylation in malignant transformed
TRL 1215 cells (33). Due to
material restrictions, it was not possible to measure the
correlation between APOE expression and APOE methylation in the
present study. Thus, The Cancer Genome Atlas (TCGA) database was
used to collect the methylation and the transcription data of APOE.
This data analysis in TCGA indicated that APOE hypermethylation was
indeed inversely correlated with lower expression
(P=1.96×10−27, r=−0.604). In the present case-control
study, APOE hypermethylation was detected in pediatric patients
with ASD compared with healthy age-matched individuals. Therefore,
it was speculated that APOE hypermethylation may reduce APOE
expression, eventually leading to the onset of ASD.
Early intervention of ASD may change the long-term
prognosis of ASD patients (6). The
current diagnostic methods for ASD comprise the ASD behavior
checklist (ABC) (34), the CARS
(5) and the Social Communication
Questionnaire (SCQ) (35). The ABC
scale has a specificity of 97% and a sensitivity of 38% with an
idiomatic cutoff score at 67 (36).
In addition, the CARS has a sensitivity of 83% and a specificity of
82% (37), and the SCQ scale has a
sensitivity of 85% and a specificity of 75% (35). These tests are also substantially
used in the clinic.
A large variety of molecular biomarkers have been
studied for their diagnostic value in ASD, including paraoxonase-1
(PON-1), lipoprotein-associated phospholipase A2 (Lp-PLA2)
(38), microRNAs (39,40),
interleukin (IL)-6 and tumor necrosis factor (TNF)α (41). However, only few biomarkers are
actually used as an auxiliary in the diagnosis of ASD in the
clinic. The sensitivity of Lp-PLA2 is 70% and its specificity is
77%; furthermore, PON-1 has a relatively low AUC of 0.660
(sensitivity, 59%; specificity, 63%) (38). IL-6 and TNFα have a low sensitivity
of 84.0 and 76.0%, respectively, for the diagnosis of ASD (41). In the present study, APOE methylation
yielded a higher AUC of 0.817 (sensitivity, 93.5%; specificity,
72.6%). It is possible to accurately measure DNA methylation in a
variety of materials, including plasma and serum, and it is
considered an ideal marker for experimental determination (42,43). The
results of the present study indicated that APOE methylation has a
high diagnostic value for ASD, suggesting that APOE
hypermethylation in peripheral blood may be a biomarker for the
early diagnosis of ASD.
In conclusion, the present study indicated that APOE
hypermethylation is associated with ASD. Future studies may be
necessary to confirm the utility of APOE hypermethylation in
peripheral blood DNA as a diagnostic marker for ASD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical
Science and Technology Project of Zhejiang Province (grant no.
2017207569) and the K.C. Wong Magna Fund of Ningbo University.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
ZH, YY and SD contributed to the conception, design
and final approval of the submitted version. YZ, HY, XY, DZ, JZ,
ZZ, JL, RP, WZ and FC contributed by performing the experiments,
interpreting the data and designing the figures. All authors read
and approved the final manuscript.
Ethical approval and consent to
participate
The study was approved by the Ethics Committees in
Ningbo Kangning Hospital and Ningbo University and written informed
consent was obtained from the parents of all the children.
Consent for publication
Written informed consent was obtained for the
publication of the patient's data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shpyleva S, Ivanovsky S, de Conti A,
Melnyk S, Tryndyak V, Beland FA, James SJ and Pogribny IP:
Cerebellar oxidative DNA damage and altered DNA methylation in the
BTBR T+tf/J mouse model of autism and similarities with human post
mortem cerebellum. PLoS One. 9:e1137122014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ginsberg MR, Rubin RA, Falcone T, Ting AH
and Natowicz MR: Brain transcriptional and epigenetic associations
with autism. PLoS One. 7:e447362012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bird G and Cook R: Mixed emotions: The
contribution of alexithymia to the emotional symptoms of autism.
Transl Psychiatry. 3:e2852013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buescher AV, Cidav Z, Knapp M and Mandell
DS: Costs of autism spectrum disorders in the United Kingdom and
the United States. JAMA Pediatr. 168:721–728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howsmon DP, Kruger U, Melnyk S, James SJ
and Hahn J: Classification and adaptive behavior prediction of
children with autism spectrum disorder based upon multivariate data
analysis of markers of oxidative stress and DNA methylation. PLoS
Comput Biol. 13:e10053852017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernell E, Eriksson MA and Gillberg C:
Early diagnosis of autism and impact on prognosis: A narrative
review. Clin Epidemiol. 5:33–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen T, Giri M, Xia Z, Subedi YN and Li Y:
Genetic and epigenetic mechanisms of epilepsy: A review.
Neuropsychiatr Dis Treat. 13:1841–1859. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esteller M: Profiling aberrant DNA
methylation in hematologic neoplasms: A view from the tip of the
iceberg. Clin Immunol. 109:80–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mill J, Tang T, Kaminsky Z, Khare T,
Yazdanpanah S, Bouchard L, Jia P, Assadzadeh A, Flanagan J,
Schumacher A, et al: Epigenomic profiling reveals DNA-methylation
changes associated with major psychosis. Am J Hum Genet.
82:696–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng J, Wang Y, Zhou K, Wang L, Li J,
Zhuang Q, Xu X, Xu L, Zhang K, Dai D, et al: Male-specific
association between dopamine receptor D4 gene methylation and
schizophrenia. PLoS One. 9:e891282014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai D, Cheng J, Zhou K, Lv Y, Zhuang Q,
Zheng R, Zhang K, Jiang D, Gao S and Duan S: Significant
association between DRD3 gene body methylation and schizophrenia.
Psychiatry Res. 220:772–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuksel Elagoz M, Yuceturk B, Karatas OF,
Ozen M and Dogangun B: The altered promoter methylation of oxytocin
receptor gene in autism. J Neurogenet. 30:280–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lintas C, Sacco R and Persico AM:
Differential methylation at the RELN gene promoter in temporal
cortex from autistic and typically developing post-puberal
subjects. J Neurodev Disord. 8:182016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu L, Wang X, Li XL, Towers A, Cao X,
Wang P, Bowman R, Yang H, Goldstein J, Li YJ and Jiang YH:
Epigenetic dysregulation of SHANK3 in brain tissues from
individuals with autism spectrum disorders. Hum Mol Genet.
23:1563–1578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tamboli IY, Heo D and Rebeck GW:
Extracellular proteolysis of apolipoprotein E (apoE) by secreted
serine neuronal protease. PLoS One. 9:e931202014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raiford KL, Shao Y, Allen IC, Martin ER,
Menold MM, Wright HH, Abramson RK, Worley G, DeLong GR, Vance JM,
et al: No association between the APOE gene and autism. Am J Med
Genet B Neuropsychiatr Genet. 125B:57–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Foraker J, Millard SP, Leong L, Thomson Z,
Chen S, Keene CD, Bekris LM and Yu CE: The APOE gene is
differentially methylated in Alzheimer's disease. J Alzheimers Dis.
48:745–755. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Napoli E, Ross-Inta C, Wong S, Hung C,
Fujisawa Y, Sakaguchi D, Angelastro J, Omanska-Klusek A, Schoenfeld
R and Giulivi C: Mitochondrial dysfunction in Pten
haplo-insufficient mice with social deficits and repetitive
behavior: Interplay between Pten and p53. PLoS One. 7:e425042012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giunco CT, de Oliveira AB, Carvalho-Salles
AB, Souza DS, Silva AE, da Rocha SS and Fett-Conte AC: Association
between APOE polymorphisms and predisposition for autism. Psychiatr
Genet. 19:3382009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kristensen LS, Mikeska T, Krypuy M and
Dobrovic A: Sensitive melting analysis after real time-methylation
specific PCR (SMART-MSP): High-throughput and probe-free
quantitative DNA methylation detection. Nucleic Acids Res.
36:e422008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Hu H, Liu J, Yang Y, Liu G, Ying
X, Chen Y, Li B, Ye C, Wu D and Duan S: FOXF2 promoter methylation
is associated with prognosis in esophageal squamous cell carcinoma.
Tumour Biol. 39:10104283176922302017.PubMed/NCBI
|
|
22
|
Huang Y and Mahley RW: Apolipoprotein E:
Structure and function in lipid metabolism, neurobiology, and
Alzheimer's diseases. Neurobiol Dis. 72:3–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu S, Park S, Allington G, Prelli F, Sun
Y, Sun Y, Martá-Ariza M, Scholtzova H, Biswas G2, Brown B, Verghese
PB, et al: Targeting Apolipoprotein E/Amyloid β Binding by Peptoid
CPO_Aβ17-21 P Ameliorates Alzheimer's disease related pathology and
cognitive decline. Sci Rep. 7:80092017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao N, Liu CC, Qiao W and Bu G:
Apolipoprotein E, Receptors, and Modulation of Alzheimer's Disease.
Biol Psychiatry. 83:347–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Folmsbee SS, Wilcox DR, Tyberghein K, De
Bleser P, Tourtellotte WG, van Hengel J, van Roy F and Gottardi CJ:
αT-catenin in restricted brain cell types and its potential
connection to autism. J Mol Psychiatry. 4:22016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blomqvist ME, Andreasen N, Bogdanovic N,
Blennow K, Brookes AJ and Prince JA: Genetic variation in CTNNA3
encoding alpha-3 catenin and Alzheimer's disease. Neurosci Lett.
358:220–222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin ER, Bronson PG, Li YJ, Wall N,
Chung RH, Schmechel DE, Small G, Xu PT, Bartlett J, Schnetz-Boutaud
N, et al: Interaction between the alpha-T catenin gene (VR22) and
APOE in Alzheimer's disease. J Med Genet. 42:787–792. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Persico AM, D'Agruma L, Zelante L,
Militerni R, Bravaccio C, Schneider C, Melmed R, Trillo S,
Montecchi F, Elia M, et al: Enhanced APOE2 transmission rates in
families with autistic probands. Psychiatr Genet. 14:73–82. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jaenisch R and Bird A: Epigenetic
regulation of gene expression: How the genome integrates intrinsic
and environmental signals. Nat Genet. 33 Suppl:S245–S254. 2003.
View Article : Google Scholar
|
|
30
|
Tamura Y, Kunugi H, Ohashi J and Hohjoh H:
Epigenetic aberration of the human REELIN gene in psychiatric
disorders. Mol Psychiatry. 12(519): 593–600. 2007. View Article : Google Scholar
|
|
31
|
Zilberman D, Gehring M, Tran RK, Ballinger
T and Henikoff S: Genome-wide analysis of Arabidopsis thaliana DNA
methylation uncovers an interdependence between methylation and
transcription. Nat Genet. 39:61–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang X, Yazaki J, Sundaresan A, Cokus S,
Chan SW, Chen H, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE
and Ecker JR: Genome-wide high-resolution mapping and functional
analysis of DNA methylation in arabidopsis. Cell. 126:1189–1201.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suzuki M, Takeda S, Teraoka-Nishitani N,
Yamagata A, Tanaka T, Sasaki M, Yasuda N, Oda M, Okano T, Yamahira
K, et al: Cadmium-induced malignant transformation of rat liver
cells: Potential key role and regulatory mechanism of altered
apolipoprotein E expression in enhanced invasiveness. Toxicology.
382:16–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Juneja M, Sharma S and Mukherjee SB:
Sensitivity of the autism behavior checklist in Indian autistic
children. J Dev Behav Pediatr. 31:48–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dykens EM, Roof E, Hunt-Hawkins H, Dankner
N, Lee EB, Shivers CM, Daniell C and Kim SJ: Diagnoses and
characteristics of autism spectrum disorders in children with
Prader-Willi syndrome. J Neurodev Disord. 9:182017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nordin V and Gillberg C: Autism spectrum
disorders in children with physical or mental disability or both.
II: Screening aspects. Dev Med Child Neurol. 38:314–324. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
García-López C and Narbona J: Clinical
usefulness of IDEA and CARS: Concordance with DSM-IV-TR in children
and adolescents with suspicion of PDD. An Pediatr (Barc). 80:71–76.
2014.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hayek J, Cervellati C, Crivellari I,
Pecorelli A and Valacchi G: Lactonase activity and
lipoprotein-phospholipase A2 as possible novel serum biomarkers for
the differential diagnosis of autism spectrum disorders and rett
syndrome: Results from a pilot study. Oxid Med Cell Longev.
2017:56940582017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mundalil Vasu M, Anitha A, Thanseem I,
Suzuki K, Yamada K, Takahashi T, Wakuda T, Iwata K, Tsujii M,
Sugiyama T and Mori N: Serum microRNA profiles in children with
autism. Mol Autism. 5:402014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hicks SD, Ignacio C, Gentile K and
Middleton FA: Salivary miRNA profiles identify children with autism
spectrum disorder, correlate with adaptive behavior, and implicate
ASD candidate genes involved in neurodevelopment. BMC Pediatr.
16:522016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
El-Ansary AK, Ben Bacha AG and Al-Ayadhi
LY: Proinflammatory and proapoptotic markers in relation to mono
and di-cations in plasma of autistic patients from Saudi Arabia. J
Neuroinflammation. 8:1422011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu P, Cao Z and Wu S: New progress of
epigenetic biomarkers in urological cancer. Dis Markers.
2016:98640472016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsui NB, Ng EK and Lo YM: Stability of
endogenous and added RNA in blood specimens, serum, and plasma.
Clin Chem. 48:1647–1653. 2002.PubMed/NCBI
|