Introduction
Malignant tumors are a serious threat to the health
of patients and a burden on their families and society.
Cyclophosphamide (Cyp) is one of the most widely used
chemotherapeutic drugs for the treatment of malignant tumors, but
due to its severe immunosuppressive adverse effect (1,2), it is
necessary to co-administer drugs with immunomodulatory function and
food supplements with an immune-regulating function.
In recent years, the biological activity of
polysaccharides has become a hot spot in research and development
of drugs. Certain polysaccharides are known to have anti-oxidant,
hypolipidemic, anti-hypoglycemic and anti-tumor effects, and a
large number of studies have reported on the immunomodulatory
effect of plant-derived polysaccharides (3–5).
Schisandra, a well-known traditional medicine in China, is
the dried ripe fruit of Schisandra chinensis (Turcz.) Baill;
it has been applied for thousands of years and is a representative
tonic Chinese herbal medicine (6).
The major active components of Schisandra are its lignans
and polysaccharides, and modern pharmacological studies indicate
that Schisandra polysaccharide (SCP) prevents
radiation-induced immune dysfunction (7), enhances innate immune responses and
disease resistance against Aeromonas hydrophila in fish
(8), and exerts immunomodulatory
effects through Toll-like receptor 4-mediated activation of
macrophages (9). However, the effect
of SCP on the immune function in an animal model induced by Cyp has
been rarely reported. In order to more comprehensively understand
the effect of SCP on the immune system, the effect of SCP was
investigated in mice with Cyp-induced immunosuppression. The
present study provides a basis for the research and development of
Schisandra medicines and health foods.
Materials and methods
Experimental animals and feed
preparation
Male ICR mice (age, 6 weeks; weight, 18–22 g), were
provided by the Changchun Institute of Biological Products Co.,
Ltd. [Changchun, China; certificate no. SCXK (Ji) 2016-0008]. The
animals were kept in a specific pathogen-free laboratory with ad
libitum access to food and water. The temperature was
controlled at 20–24°C, the humidity was ~50% and mice were
subjected to a 12 h light/dark cycle. The weight of mice was
recorded twice a week. The animal experiments were approved by the
Institutional Animal Care and Use Committee of Beihua University
(Jilin, China). All of the experimental procedures were performed
in accordance with the Guide for the Care and Use of Laboratory
Animals (China).
Chemicals and materials
The following drugs and reagents were used in the
present study: Cyp for injection (Jiangsu Shengdi Pharmaceutical
Co., Ltd., Jiangsu, China); India ink (Shanghai Ruiyong
Biotechnology Co., Ltd., Shanghai, China); 10% sheep red blood cell
(SRBC) suspension (Beijing Bersee Science and Technology Co., Ltd.,
Beijing, China); concanavalin A (Con A; Sigma-Aldrich, Merck KGaA,
Darmstadt, Germany); RPMI 1640 medium (Thermo Fisher Scientific,
Inc., Waltham, MA, USA); and ELISA kits for tumor necrosis factor-α
(TNF-α) and interferon-γ (IFN-γ; Shanghai Lengton Bioscience Co.,
Ltd., Shanghai, China). All of the reagents were of analytical
grade or chromatographically pure.
SCP preparation
The dried ripe fruit of Schisandra chinensis
(Turcz.) Baill was purchased from Jilin Province Jian City
Schisandra Planting Base and its identity was verified by Professor
Fengli Li at the Department of Pharmacognosy (College of Pharmacy,
Beihua University, Jilin, China).
The dried Schisandra berries (1.5 kg) were
ground into powder, which was sieved through a 60-mesh sieve, then
immersed in 10 l distilled water and soaked overnight at room
temperature. Subsequently, the Schisandra-water mixture was
boiled for 3 h to obtain the aqueous extract phase, which was
concentrated with a rotary evaporator (cat. no. R206B; Shanghai
Senco Technology Co., Ltd., Shanghai, China) at 80°C to 2 l and
then centrifuged at 4,500 × g for 15 min at 20°C. The precipitate
was discarded and the supernatant was kept; 95% ethanol was added
to the supernatant to adjust the final ethanol concentration of the
supernatant to 75%, and the mixture was left to precipitate
overnight at room temperature. Following centrifugation at 4,500 ×
g for 15 min at 20°C, the precipitate was collected, washed once
with 95% ethanol and anhydrous ethanol in turn, and then
freeze-dried routinely to obtain a powder-like SCP. Voucher
specimens were deposited at the College of Pharmacy, Beihua
University (Jilin, China; sample no. 20170520-1).
Analysis of chemical properties
The total carbohydrate content of SCP was determined
with the phenol-sulfuric acid method (10), with glucose as the standard. The
uronic acid content was determined with the m-hydroxydiphenyl
method (11), with D-galactose as
the standard. The protein content was determined using the Bradford
assay (12). The monosaccharide
composition was determined by high-performance liquid
chromatography (HPLC) (13).
Animal grouping, model establishment
and drug administration
A total of 200 mice were divided into 4 batches,
with 50 mice in each batch. The mice in each batch were randomly
divided (each, n=10) into a control group, model group, low-dose
SCP group (SCP-L), medium-dose SCP group (SCP-M) and high-dose SCP
group (SCP-H). All of the mice were allowed to acclimatize to the
laboratory environment for 3 days. Mice in the SCP-L, SCP-M and
SCP-H groups were intragastrically via oral gavage administered the
corresponding doses of SCP aqueous solution (0.4, 0.8 and 1.6 mg/10
g), and those in the control group and the model group were
intragastrically given the same volume of distilled water (14) (0.2 ml/10 g), successively for 21
days. On day 17 after the administration, the mice in the model,
SCP-L, SCP-M and SCP-H group were intraperitoneally injected with
Cyp (20 mg/kg), and those in the control group were injected the
same volume of normal saline.
The mice in the present study were divided into 4
batches. The first batch of mice was used for observation of
phagocytosis in macrophages and were injected with India ink, which
was utilized only in this batch as it would interfere the results
of other tests in this study (including histomorphology, optical
density of TNF-α and IFN-γ) (15).
The second batch were subjected to the serum hemolysin test. Mice
were immunized using an intraperitoneal injection of SRBC 5 days
prior to experimentation. This treatment caused an immune response
to the body, meaning these mice were only appropriate for use in
the serum hemolysin test (16). The
third batch of mice were used for the determination of organ
indexes, TNF-α, IFN-γ and leukocyte count as well as
histomorphology. For histomorphology, spleens were fixed using 10%
formalin solution at room temperature for 24 h and thus could not
be utilized for the preparation of the splenic lymphocyte
suspension. The fourth batch was used for the assessment of splenic
lymphocyte proliferation and apoptosis. Mice were sterilized by
placing them in alcohol and their spleens were removed under
aseptic conditions for the preparation of splenic lymphocyte
suspension, so they could not be used for the other
experiments.
Measurement of organ indexes and
histomorphology observation
At 1 h after the intragastric administration, the
mice were anesthetized with ether and sacrificed by CO2
inhalation; death was confirmed by observing apnea over 5 min. The
thymus and spleen of the mice were isolated, and the excess tissues
and fascia were stripped and then weighed. The thymus and spleen
indexes were calculated according to the following equation:
Organ index=organ mass/animal body
mass
Subsequently, the thymus and spleen were fixed with
10% formalin solution at room temperature for 24 h. The
pathological specimens were routinely sliced (5 µm), embedded in
paraffin and stained with H&E for 10 min at room temperature.
Pathological changes were observed under a light microscope
(magnification, ×100) and images were captured.
Leukocyte count in the peripheral
blood
At 1 h after the last intragastric administration,
the mice were inhalation of anesthetized with 5% ether and blood
samples were collected by removing the eyeballs. Mice were
monitored for the loss of righting reflex and were maintained at a
respiratory rate of 100–200 breaths/min to ensure that anesthesia
was effective. The mice were sacrificed by CO2
asphyxiation, the concentration of input CO2 was 100%
and the flow rate of CO2 was set at 20% of the chamber
volume/min, the apnea of mice was an indicator to determine if mice
had succumbed (the mice sacrificed in other tests used the same
method). Aliquots of blood (20 µl) were added to 0.38 ml 2% acetic
acid and mixed. The number of white blood cells in the blood was
counted with a counting plate under a microscope.
Observation of phagocytosis of
macrophages in mice
At 1 h after the last intragastric administration,
the mice were anesthetized using ether (as aforementioned) and
injected with 50% diluted India ink (0.1 ml/10 g) through their
tail veins. The time (t) of the injection was immediately recorded,
and 25 µl blood was taken from the inner canthus venous plexus at
t=3 and 11 min after the ink injection, respectively, and
immediately added to 2 ml 0.1% Na2CO3
solution. The optical density values of the two samples taken at 3
and 11 min were measured at 600 nm wavelength by a microplate
reader to obtain the absorbance values (A3 and A11, respectively),
with Na2CO3 solution used as the control. The
carbon clearance index (K) and the phagocytic index (α) were
calculated according to the following equations:
K=(logA3-logA11)/(t11-t3)
Phagocytic index α=body mass/(liver weight + spleen
weight) × K1/3
Measurement of TNF-α and IFN-γ
At 1 h after the last intragastric administration,
the mice were anesthetized with ether and the blood samples were
collected by removing the eyeballs. The blood was centrifuged at
3,000 × g for 10 min at 4°C to obtain the serum, and TNF-α and
IFN-γ levels in the serum of mice were detected according to the
instructions of TNF-α and IFN-γ kits using a microplate reader
(Infinite M200; Tecan, Maennedorf, Switzerland).
Measurement of serum hemolysin
On day 17 after the intragastric administration, the
mice were immunized by intraperitoneal injection of 0.2 ml 5% SRBC,
and after 5 days, the mice were anesthetized with ether and their
blood samples were collected by removing their eyeballs. The blood
was centrifuged at 3,000 × g for 10 min to obtain the serum, which
was diluted 100 times with normal saline; 0.5 ml of the diluted
serum (0.5 ml normal saline in the control group) was mixed with
0.5 ml 5% SRBC suspension, 0.5 ml 10% complement solution (10%
guinea pig serum; Nanjing SenBeiJia Biological Technology Co.,
Ltd., Nanjing, China) and 0.5 ml normal saline, another well was
set as 50% hemolysis (0.5 ml 5% SRBC suspension with 1.5 ml normal
saline), then left to stand at 37°C for 30 min, and then
immediately put into an ice water bath to stop the reaction. The
mixture was centrifuged at 2,000 × g for 10 min at 4°C to obtain
the supernatant, and the A value of the supernatant at the
wavelength of 540 nm was measured with a spectrophotometer. The
content of serum hemolysin was reflected by the HC50
value, which was calculated according to the following
equation:
HC50=A value of the sample/A value of
SRBC of50%hemolysis × dilution factor
Splenic lymphocyte proliferation
test
At 1 h after the last intragastric administration,
mice in the different groups were sacrificed to remove their
spleens under aseptic conditions. The spleen was ground from frozen
and placed in 4 ml lymphocyte separation liquid to prepare the
splenic lymphocyte suspension. The suspension was centrifuged at
1,500 × g for 10 min at 4°C and the supernatant was discarded.
After the centrifugation was repeated two times, 2.5 ml RPMI-1640
medium was added to the precipitate, 0.1 ml of the solution was
placed into a small centrifuge tube and trypan blue was added for
staining. After 1 min, half a drop of the solution was added to a
counting plate, the cells were counted under a microscope and the
cell density in the suspension was adjusted to 1×106/ml.
Of each cell suspension, 1 ml each was added to 2 wells of a
24-well culture plate; to one well, 50 µl Con A was added, and the
other one was used as the control with 50 µl RPMI-1640 medium. The
plates were placed in a CO2 incubator, in which the
cells were cultured at 37°C for 72 h. At 4 h prior to the end of
the incubation, 0.7 ml culture medium and 20 µl 5 mg/ml MTT
solution was added. At the end of the incubation, the supernatant
was discarded and 1 ml acidic isopropanol (HCl: isopropanol, 1:24)
was added to each well, followed by agitation to completely
dissolve the purple crystals. The optical density (OD) values were
measured at the wavelength of 570 nm with a spectrophotometer
(UV2550; Shimadzu, Kyoto, Japan). The difference in OD values
between the samples that were incubated with and without Con A
represented the proliferation ability of splenic lymphocytes.
Splenic lymphocyte apoptosis test
The splenic lymphocyte suspension (1 ml) was
centrifuged at 1,000 × g for 5 min at 4°C, the supernatant was
discarded and the precipitate was suspended in binding buffer (0.5
M NaCl, 20 mM Tris and 5mM Imidazole) with 200 µl 10% Annexin
V-fluorescein isothiocyanate (FITC), followed by mixing. The
solution was incubated at room temperature for 10 min, then
centrifuged at 1,000 × g for 5 min at 4°C and the supernatant was
discarded. The precipitate was suspended in 195 µl Annexin V-FITC
binding buffer, 10 µl propidium iodide (PI) staining solution was
added, the solution was gently mixed under exclusion of light and
then analyzed with an Epics-XL Flow Cytometer (Beckman Coulter,
Brea, CA, USA), in which Annexin V-FITC exhibited a green
fluorescence and PI a red fluorescence.
Statistical methods
All values are expressed as the mean ± standard
deviation. The number of samples in each group was expressed as
‘n’. SPSS software (version 19.0 for Windows; IBM Corp., Armonk,
NY, USA) was used for statistical analysis. One-way analysis of
variance was used for comparison between groups followed by a
Dunnett's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Chemical properties of SCP
SCP (yield, 128.2 g; 8.55%) was obtained from 1.5 kg
Schisandra fruit by hot water boiling extraction followed by
precipitation in 75% ethanol and conventional drying. The total
carbohydrate, uronic acid, protein and monosaccharide content of
SCP are listed in Table I. The
results of the HPLC analysis indicated that SCP is composed of
glucose (38.0%), galactose (36.7%), galacturonic acid (12.0%),
arabinose (7.3%), rhamnose (4.0%), mannose (1.2%) and glucuronic
acid (0.6%).
 | Table I.Chemical properties of
Schisandra polysaccharide. |
Table I.
Chemical properties of
Schisandra polysaccharide.
|
|
|
| Monosaccharide
composition (%) |
|---|
|
|
|
|
|
|---|
| Carbohydrate
(%) | Uronic acid
(%) | Protein (%) | Glc | Gal | GalA | Ara | Rha | Man | GlcA |
|---|
| 40.60 | 24.70 | 1.51 | 38.00 | 36.70 | 12.00 | 7.30 | 4.00 | 1.20 | 0.60 |
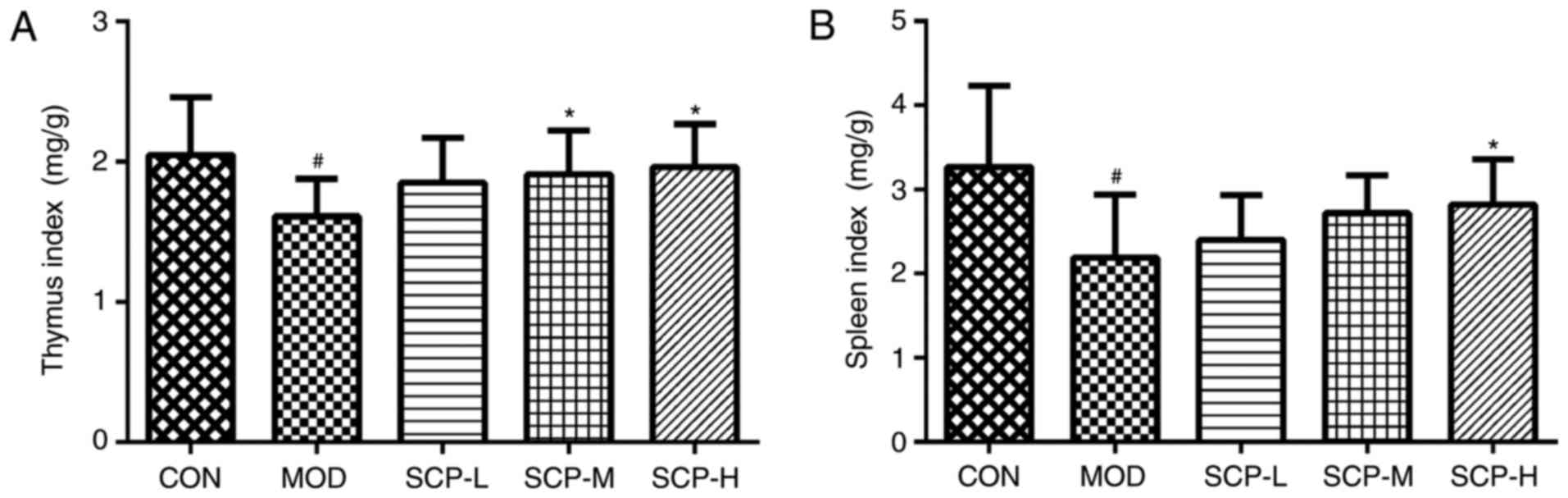
Effects of SCP on organ index and
histomorphology
As indicated in Fig.
1, the thymus and spleen index of mice in the model group was
significantly decreased compared with that in the control group
(P<0.05). Furthermore, compared with that in the model group,
the spleen index in the SCP-H group, and the thymus index in the
SCP-M and SCP-H groups, was significantly increased
(P<0.05).
As presented in Fig.
2, histomorphological examination (magnification, ×100)
indicated that the lobular beam, and the size and shape of the
thymic cortex were normal, the medulla inside each cortex was
visible, and the medullar structure was clear in the control group.
Compared with that in the control group, the number of lymphocytes
in the thymus cortex was reduced, the boundary between the medulla
and cortex was not clear, and the size of the medulla was decreased
in the model group. Compared with that in the model group, the
boundary between the cortex and medulla was clear in the SCP-H and
SCP-M groups, and the boundary between the cortex and medulla was
not clear in the SCP-L group, but slightly better than that in the
model group.
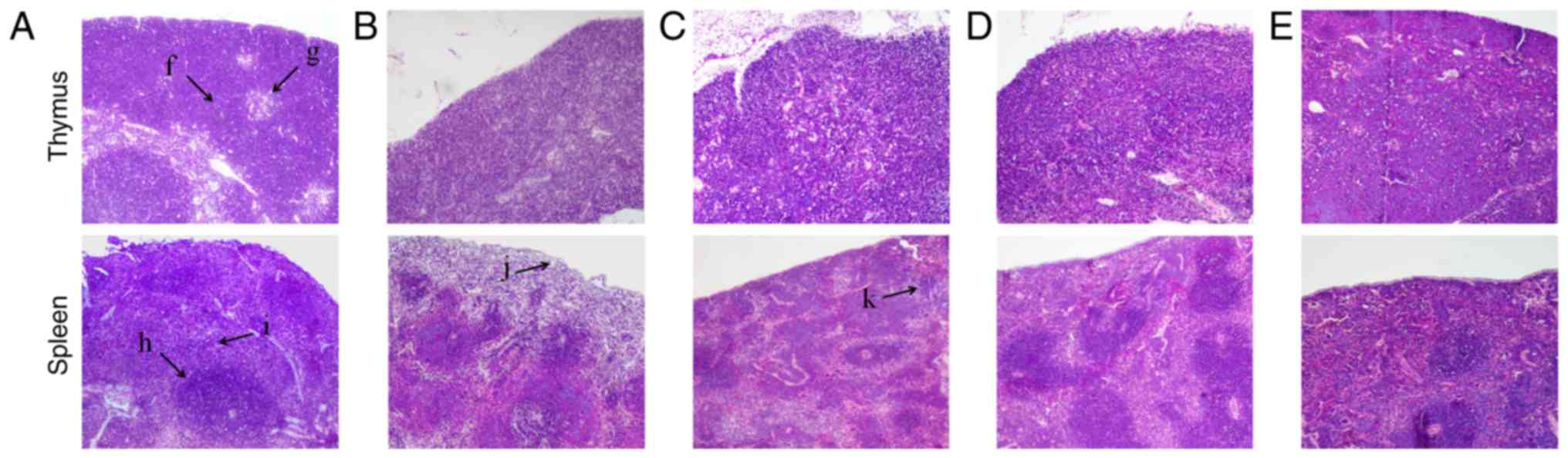 | Figure 2.Effects of SCP on histomorphological
changes in thymus and spleen of mice (hematoxylin and eosin
staining; magnification, ×100). (A) CON group; (B) MOD group; (C)
SCP-L group; (D) SCP-M group; (E) SCP-H group. The arrows indicate
the following features: f, cortex; g, medulla; h, white medulla; i,
red medulla; j, capsule; k, splenic corpuscle. Groups: CON, control
group; MOD, model group; SCP-L, low-dose SCP group (0.4 mg/10 g);
SCP-M, medium-dose SCP group (0.8 mg/10 g); SCP-H, high-dose SCP
group (1.6 mg/10 g); SCP, Schisandra polysaccharide. |
The spleen was also histomorphologically examined
(Fig. 2). The morphology and the
number of splenic corpuscles were normal, and the boundary between
the red medulla and white medulla was clear in the control group.
Compared with that in the control group, the number of splenic
corpuscles was obviously reduced and their volume was obviously
smaller in the spleen cortex near the capsule, and the volume of
splenic corpuscles was obviously reduced in the area near the red
medulla in the model group. The number of splenic corpuscles near
the capsule was slightly reduced in the SCP-L group, the number in
the SCP-H and SCP-M groups was close to that in the control group,
and the demarcation of splenic corpuscles and red medulla was clear
in the SCP-H and SCP-M groups.
Effects of SCP on leukocyte count in
peripheral blood and phagocytosis of macrophages
As presented in Fig. 3A
and B, compared with that in the control group, the number of
leukocytes and the phagocytic index in the model group were
significantly decreased (P<0.01). Compared with that in the
model group, the number of leukocytes in the SCP-treated groups was
significantly elevated (P<0.01), and the phagocytosis of
macrophages in the SCP-H group was also significantly increased
(P<0.05).
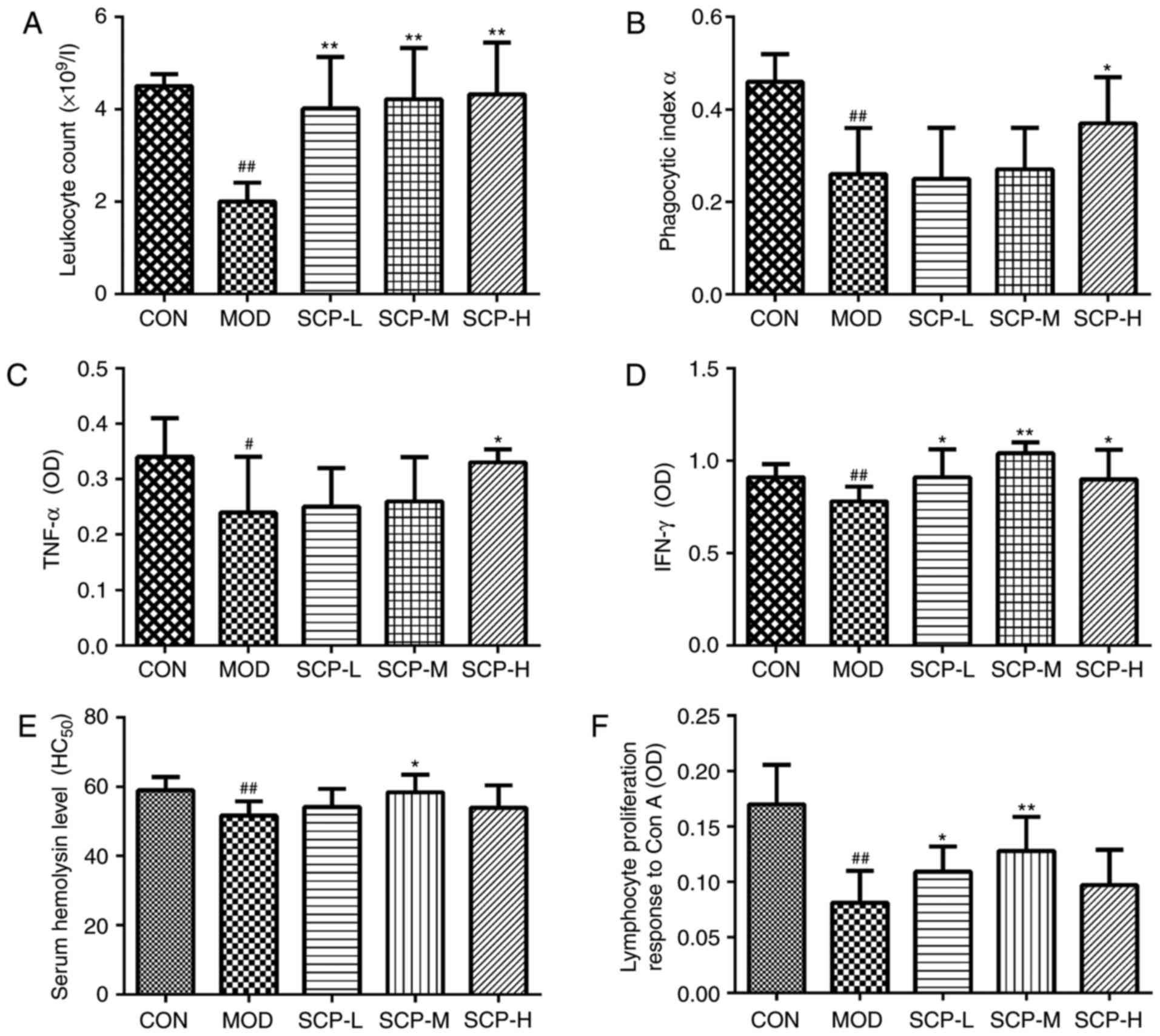 | Figure 3.Effects of SCP on (A) leukocyte count
in peripheral blood, (B) phagocytosis of macrophages, (C) TNF-α,
(D) IFN-γ, (E) serum hemolysin level and (F) splenic lymphocyte
proliferation. Values are expressed as the mean ± standard
deviation. #P<0.05, ##P<0.01 vs. CON;
*P<0.05, **P<0.01 vs. MOD. Groups: CON, control group; MOD,
model group; SCP-L, low-dose SCP group (0.4 mg/10 g); SCP-M,
medium-dose SCP group (0.8 mg/10 g); SCP-H, high-dose SCP group
(1.6 mg/10 g); SCP, Schisandra polysaccharide; TNF, tumor
necrosis factor; IFN, interferon; OD, optical density; Con A,
concanavalin A. |
Effects of SCP on TNF-α and IFN-γ
levels
Compared with those in the control group, TNF-α and
IFN-γ levels in the model group were significantly decreased
(P<0.05 and P<0.01; Fig. 3C and
D, respectively). Furthermore, compared with those in the model
group, the TNF-α levels in the SCP-H group were significantly
elevated (P<0.05), and IFN-γ levels in the SCP-L, SCP-M and
SCP-H groups were also significantly increased (P<0.05,
P<0.01 and P<0.05, respectively).
Effects of SCP on serum hemolysin and
spleen lymphocyte proliferation
As indicated in Fig.
3E, compared with that in the control group, the
HC50 value in the model group was significantly
decreased (P<0.01), indicating that Cyp significantly reduces
the level of serum hemolysin to inhibit the humoral immune function
in mice. Compared with those in the model group, the
HC50 values in the SCP-treated groups exhibited an
increasing trend, and the increase in the SCP-M group was
statistically significant (P<0.05).
Compared with that in the control group, the
proliferation of lymphocytes in the model group was significantly
decreased (P<0.01). Compared with that in the model group, the
proliferation of lymphocytes in the SCP-L and SCP-M groups was
significantly increased (P<0.05 and P<0.01, respectively),
and that in the SCP-H group was slightly but insignificantly
increased (Fig. 3F).
Effect of SCP on splenic lymphocyte
apoptosis
As presented in Table
II and Fig. 4, compared with
those in the control group, the early, late and total apoptotic
rates of lymphocytes were significantly increased in the model
group (P<0.01). In addition, compared with those in the model
group, the early and late apoptotic rates of lymphocytes were
significantly decreased in the SCP-H group (P<0.01), and the
total apoptotic rate was significantly decreased in all SCP-treated
groups (P<0.01).
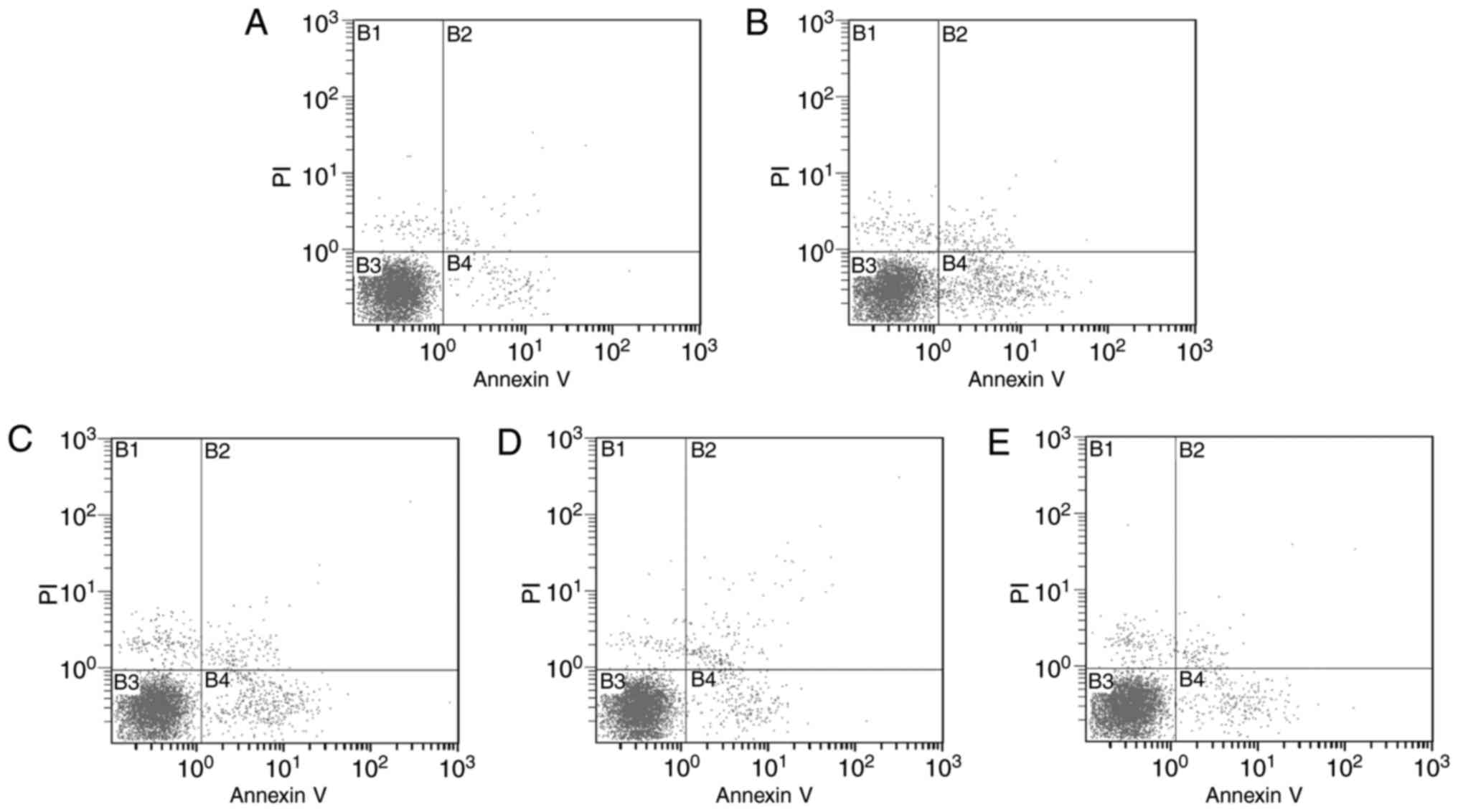 | Figure 4.Effects of SCP on splenic lymphocyte
apoptosis. (A) CON group; (B) MOD group; (C) SCP-L group; (D) SCP-M
group; (E) SCP-H group. Quadrants: B1, necrotic cells; B2, late
apoptotic cells; B3, normal cells; B4, early apoptotic cells.
Groups: CON, control group; MOD, model group; SCP-L, low-dose SCP
group (0.4 mg/10 g); SCP-M, medium-dose SCP group (0.8 mg/10 g);
SCP-H, high-dose SCP group (1.6 mg/10 g); SCP, Schisandra
polysaccharide; PI, propidium iodide. |
 | Table II.Effects of SCP on the apoptotic rate
of splenic lymphocytes. |
Table II.
Effects of SCP on the apoptotic rate
of splenic lymphocytes.
| Group | Early (%) | Late (%) | Total (%) |
|---|
| CON | 2.18±0.27 | 0.94±0.17 | 3.12±0.36 |
| MOD |
11.38±2.28a |
2.56±0.74a |
13.94±1.80a |
| SCP-L |
8.22±1.06b | 2.81±0.66 |
11.03±1.57b |
| SCP-M |
4.35±1.08b |
3.62±0.90b |
7.97±1.30b |
| SCP-H |
4.59±0.96b |
1.69±0.54b |
6.28±0.79b |
Discussion
The thymus is the central immune organ, and the site
where T lymphocytes develop, differentiate and mature; and thymus
index reflects the weight of the thymus (17). The spleen is a peripheral immune
organ and the site where mature T and B lymphocytes settle and are
involved in the immune response, and in the process of immune
activation, immune cell differentiation and proliferation leads to
an increase of its weight (18);
therefore, a reduced thymus and spleen weight signifies a reduced
immune cell number and declined immune function. The present
results indicated that Cyp affects the differentiation and
maturation of T lymphocytes by interfering with the cell cycle and
proliferation of lymphocytes of T lymphocytes to cause a reduction
of thymus and spleen weight, which was significantly inhibited by
SCP in immunocompromised mice induced by Cyp, thereby improving the
immune status that was impaired by Cyp and enhancing the
immunogenic capacity of the mice.
The thymus is an important lymphoid organ in the
body; its surface is covered by a connective tissue membrane, and
the connective tissue stretches into the essence of the thymus to
divide it into numerous incompletely separated lobules (19). The spleen is the largest lymphoid
organ in the body, and its structure is similar to that of lymph
nodes. The spleen is covered by a layer of connective tissue, and
is divided into red medulla and white medulla. Tissue inflammation
may be detected by histomorphological observation of thymus and
spleen, and an abnormal morphology of these tissues may reflect
lesions of immune organs, likely accompanied by a decrease of
immune function. The histomorphological observation performed in
the present study revealed that Cyp caused damage to thymus and
spleen tissues due to their abnormal morphology, which was
significantly inhibited by SCP.
Leukocytes are an important part of the body's
defense system and counting the number of leukocytes is a valid
method to evaluate immune function (20). A decrease in the number of leukocytes
causes disorders of certain immune factors, leading to the decrease
of immune function. Studies have reported that Cyp inhibits
hematopoietic function of bone marrow to reduce the number of
leukocytes (21). The results of the
present study indicated that SCP significantly inhibits the
decrease in leukocytes in mice immunocompromised with Cyp.
Macrophages, an important type of immune cell, have
a key role in the immune response and host defense, and mediate
inflammation in non-specific immunity by phagocytosis to kill and
clear away pathogens and foreign bodies (22,23). It
is known that Cyp significantly lowers the phagocytosis of mouse
macrophages (2). The present study
indicated that SCP significantly preserves the function of
macrophages of Cyp-induced immunocompromised mice. The study by
Zhao et al (9) also indicated
that Schisandra significantly enhanced the phagocytic
function of macrophages in the reticuloendothelial system of
mice.
Cytokines are small-molecule soluble proteins
synthesized by stimulating immune cells and certain non-immune
cells, of which TNF-α, a cytokine naturally generated by the
response of macrophages to bacterial infections or other
immunogens, directly causes death of cancer cells (24), and IFN-γ, a multifunctional protein,
is produced by monocytes and lymphocytes (25). TNF-α and IFN-γ have an important role
in the differentiation and regulation of immune cells, and the
decrease in the content of TNF-α and IFN-γ may cause disorders of
immune factors, leading to a decrease of immune function. It has
also been reported that Cyp significantly reduces TNF-α and IFN-γ
levels in mice, resulting in the inhibition of immune function
(26). The present results
indicating that SCP inhibited the Cyp-induced reduction the levels
of TNF-α and IFN-γ to improve the immune function of mice.
Serum hemolysin, a specific antibody produced by B
lymphocytes in touch with red cell antigens, hemolyzes red blood
cells. The level of serum hemolysin reflects the proliferation and
differentiation of B cells, and their specific antibody secretion
into the blood after contact with the specific antigen SRBC in the
body. The determination of serum hemolysin levels of SRBC-immunized
animals may be used to evaluate the function of humoral immunity
(27). The present results suggested
that SCP significantly increases the serum hemolysin levels to
enhance the humoral immune function in Cyp-induced
immunocompromised mice.
The proliferation ability of lymphocytes is an
important index to evaluate the function of T lymphocytes in the
body. T lymphocytes induce lymphoblast cells to proliferate after
stimulation with mitogen or specific antigens, and the
proliferative rate reflects the activity of T lymphocytes. When a
blast cell proliferation response occurrs after T lymphocytes are
stimulated by Con A, the proliferating cells metabolize MTT through
mitochondrial hydrolytic enzymes to produce violet formazan
crystals, whose OD value reflects the proliferation of lymphocytes
(28). The present study indicated
that Cyp significantly lowers the proliferation ability of
lymphocytes cells and that SCP significantly enhances the immune
function of splenic lymphocytes in immunocompromised mice induced
by Cyp.
Lymphocyte apoptosis is associated with the
autoimmune system itself, and the occurrence and treatment of
immunity-associated diseases, so that the regulation of lymphocyte
apoptosis has become a hot topic in current immunology research
(29,30). Therefore, splenic lymphocyte
apoptosis was measured in the present study to evaluate changes in
immune function. An increase in the number of lymphocyte apoptosis
causes immune dysfunctions, including immunodeficiency. The present
results indicated that Cyp significantly promotes apoptosis of
splenic T lymphocytes in mice and that different doses of SCP had
anti-apoptotic effects on these T lymphocytes induced by Cyp.
Combined with the results of the splenic index and histological
examination, it is suggested that the spleen atrophy and
morphological changes observed may be due to apoptosis of
lymphocytes induced by Cyp, and SCP may improve the immune function
of the spleen by inhibiting the apoptosis of splenic
lymphocytes.
In conclusion, the results of the present study
suggest that SCP enhances the immune function, improves atrophy of
the lymphoid organs thymus and spleen, reduces histopathological
changes of thymus and spleen of immunocompromised mice, increases
the number of leukocytes, promotes the formation of hemolysin and
the transformation of lymphocytes, increases the phagocytic
function of macrophages, elevates the levels of the immune factors
TNF-α and IFN-γ and inhibits apoptosis of splenic lymphocytes. The
present study used a mouse model to demonstrate that the daily
intake of a certain amount of SCP may be an effective way to
inhibit Cyp-induced decreases in immune function, which may provide
a basis for the research and development of SCP as an effective
auxiliary immune enhancing agent. Furthermore, the mechanisms
underlying the immunomodulatory effects of SCP should be
investigated in future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Jilin Province (grant nos. 20170309006YY and
20170307016YY).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JSand JC conceived and designed the experiments; JY
and LC erformed the animal experiments; CW and HL performed the
data detection; CZ and XG contributed to the Schisandra
polysaccharides preparation; PL and YX analysed the data. The
final version of the manuscript has been read and approved by all
authors, and each author believes that the manuscript represents
honest work..
Ethical approval and consent to
participate
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Beihua University
(Jilin, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Althouse R, Huff J, Tomatis L and Wilbourn
J: Chemicals and industrial processes associated with cancer in
humans. IARC Monographs Volumes 1 to 20. IARC Monogr Eval Carcinog
Risk Chem Hum Suppl. 20:1–71. 1979.
|
|
2
|
Cheng D, Wan Z, Zhang X, Li J, Li H and
Wang C: Dietary Chlorella vulgaris ameliorates altered
immunomodulatory functions in cyclophosphamide-induced
immunosuppressive mice. Nutrients. 9:pii: E708. 2017. View Article : Google Scholar
|
|
3
|
Pan G, Xie Z, Huang S, Tai Y, Cai Q, Jiang
W, Sun J and Yuan Y: Immune-enhancing effects of polysaccharides
extracted from Lilium lancifolium Thunb. Int
Immunopharmacol. 52:119–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bo R, Sun Y, Zhou S, Ou N, Gu P, Liu Z, Hu
Y, Liu J and Wang D: Simple nanoliposomes encapsulating Lycium
barbarum polysaccharides as adjuvants improve humoral and
cellular immunity in mice. Int J Nanomedicine. 173:6289–6301. 2017.
View Article : Google Scholar
|
|
5
|
Sheng X, Yan J, Meng Y, Kang Y, Han Z, Tai
G, Zhou Y and Cheng H: Immunomodulatory effects of Hericium
erinaceus derived polysaccharides are mediated by intestinal
immunology. Food Funct. 8:1020–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panossian A and Wikman G: Pharmacology of
Schisandra chinensis Bail: An overview of Russian research
and uses in medicine. J Ethnopharmacol. 118:183–212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao LM, Jia YL, Ma M, Duan YQ and Liu LH:
Prevention effects of Schisandra polysaccharide on
radiation-induced immune system dysfunction. Int J Biol Macromol.
76:63–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang E, Chen X, Wang K, Wang J, Chen D,
Geng Y, Lai W and Wei X: Plant polysaccharides used as
immunostimulants enhance innate immune response and disease
resistance against Aeromonas hydrophila infection in fish.
Fish Shellfish Immunol. 59:196–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao T, Feng Y, Li J, Mao R, Zou Y, Feng
W, Zheng D, Wang W, Chen Y, Yang L and Wu X: Schisandra
polysaccharide evokes immunomodulatory activity through TLR
4-mediated activation of macrophages. Int J Biol Macromol.
65:33–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nowotny A: Carbohydrate determination by
phenol-sulfuric acidBasic Exercises in Immunochemistry. Springer;
Berlin: pp. 171–173. 1979, View Article : Google Scholar
|
|
11
|
Murado MA, Vázquez JA, Montemayor MI, Cabo
ML and del Pilar González M: Two mathematical models for the
correction of carbohydrate and protein interference in the
determination of uronic acids by the m-hydroxydiphenyl method.
Biotechnol Appl Biochem. 41:209–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X, Yu L, Bi H, Li X, Ni W, Han H, Li
N, Wang B, Zhou Y and Tai G: Total fractionation and
characterization of the water-soluble polysaccharides isolated from
Panax ginseng C. A. Meyer. Carbohyd Polym. 77:544–552. 2009.
View Article : Google Scholar
|
|
14
|
Xu SY, Bian RL and Chen X: Methodology of
pharmacological experiment. 3rd edition. Beijing People's Medical
Publishing House; pp. 1792002
|
|
15
|
Jayathirtha MG and Mishra SH: Preliminary
immunomodulatory activities of methanol extracts of Eclipta
alba and Centella asiatica. Phytomedicine. 11:361–365.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duan BW, Li Y, Liu X and Yang YJ: Effect
of polysaccharides in processed Sibiraea on immunologic function of
immunosuppression mice. Zhongguo Zhong Yao Za Zhi. 35:1466–1469.
2010.(In Chinese). PubMed/NCBI
|
|
17
|
Zdrojewicz Z, Pachura E and Pachura P: The
thymus: A forgotten, but very important organ. Adv Clin Exp Med.
25:369–375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kraus MD: Splenic histology and
histopathology: An update. Semin Diagn Pathol. 20:84–93. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Flomerfelt FA, El Kassar N, Gurunathan C,
Chua KS, League SC, Schmitz S, Gershon TR, Kapoor V, Yan XY,
Schwartz RH and Gress RE: Tbata modulates thymic stromal cell
proliferation and thymus function. J Exp Med. 207:2521–2532. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lämmermann T and Germain RN: The multiple
faces of leukocyte interstitial migration. Semin Immunopathol.
36:227–251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang PP, Meng ZT, Wang LC, Guo LM and Li
K: Astragalus polysaccharide promotes the release of mature
granulocytes through the L-selectin signaling pathway. Chin Med.
10:172015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wynn TA and Vannella KM: Macrophages in
tissue repair, regeneration, and fibrosis. Immunity. 44:450–462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu Q, Nie SP, Wang JQ, Yin PF, Huang DF,
Li WJ and Xie MY: Toll-like receptor 4-mediated ROS signaling
pathway involved in Ganoderma atrum polysaccharide-induced
tumor necrosis factor-α secretion during macrophage activation.
Food Chem Toxicol. 66:14–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kouakou K, Schepetkin IA, Jun S,
Kirpotinal LN, Yapi A, Khramova DS, Pascual DW, Ovodov YS, Jutila
MA and Quinn MT: Immunomodulatory activity of polysaccharides
isolated from Clerodendrum splendens: Beneficial effects in
experimental autoimmune encephalomyelitis. BMC Complement Altern
Med. 13:1492013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang LC, Lu TJ, Hsieh CC and Lin WC:
Characterization and immunomodulatory activity of polysaccharides
derived from Dendrobium tosaense. Carbohydr Polym.
111:856–863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang C, Song K, Ma W, Ding J, Chen Z and
Zhang M: Immunomodulatory mechanism of Bushen Huoxue Recipe
alleviates cyclophosphamide-induced diminished ovarian reserve in
mouse model. J Ethnopharmacol. 208:44–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheng X, Yan J, Meng Y, Kang Y, Han Z, Tai
G, Zhou Y and Cheng H: Immunomodulatory effects of Hericium
erinaceus derived polysaccharides are mediated by intestinal
immunology. Food Funct. 8:1020–1027. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xing J, Xiao Y, Tang X, Sheng X and Zhan
W: Inhibition of Cyclosporine A or rapamycin on T lymphocyte counts
and the influence on the immune responses of B lymphocytes in
flounder (Paralichthys olivaceus). Fish Shellfish Immunol.
66:78–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu C, Sun Z, Xu Z, Liu T, Pan T and Li S:
Down-regulation of microRNA-155 promotes selenium
deficiency-induced apoptosis by tumor necrosis factor receptor
superfamily member 1B in the broiler spleen. Oncotarget.
8:58513–58525. 2017.PubMed/NCBI
|
|
30
|
Pinhu L, Qin Y, Xiong B, You Y, Li J and
Sooranna SR: Overexpression of Fas and FasL is associated with
infectious complications and severity of experimental severe acute
pancreatitis by promoting apoptosis of lymphocytes. Inflammation.
37:1202–1212. 2014. View Article : Google Scholar : PubMed/NCBI
|