Introduction
Parkinson's disease is a neurodegenerative disease
that can lead to senile dementia and leads to higher morbidity and
mortality rates among elderly populations (1,2). A
number of biomarkers, including dopamine transporter and aromatic
L-amino acid decarboxylase for prodromal Parkinson's disease have
been promising but require further study, including their
application to and validation in prodromal cohorts followed
longitudinally, which suggests that accurate identification of
prodromal Parkinson's disease will likely require a multimodal
approach (3). Pathogenesis of
Parkinson's disease predominantly targets the hippocampal area,
which may lead to cognitive dysfunction pertaining to memory,
language or attention (4,5). Previous systematic reviews and
meta-analyses have demonstrated the potential diagnostic or
prognostic markers that are associated with the degree of cognitive
function in patients with Parkinson's disease (6,7).
Plasminogen activator inhibitor-1 (PAI-1) serves an
important role in the process of human cardiovascular diseases,
which thereby leads to promotion of fibrinolysis (8,9). Xu
et al (10) have recently
demonstrated that PAI-1 gene polymorphism is associated with the
development and progression of predominant proteinuria diabetes
nephropathy. In addition, high plasma levels of thrombomodulin,
PAI-1 and fibrinogen were detected in elderly, diabetic patients
with depressive symptoms and further prospective larger studies are
required to provide potential directions for future research,
treatment and prevention of co-morbid depression and diabetes
(8). Furthermore, Zhou et al
(11) have recently demonstrated
that inhibition of PAI-1 activity may prevent the formation of the
initial PAI-1t-PA complex, which further blocks PAI to bind to the
hinge region of the reactive center loop. However, the role of
PAI-1 has not been elucidated in patients with Parkinson's
disease.
At present, intraoperative measurement of
subthalamic nucleus width via microelectrode recording is a common
proxy for optimal electrode location during deep brain stimulation
(DBS) surgery for patients with Parkinson's disease (12). However, to the best of our knowledge,
the role of PAI-1 in evaluating the therapeutic effects of DBS has
not been investigated in patients with Parkinson's disease. In the
present study, plasma PAI-1 levels in patients with Parkinson's
disease was investigated prior to and following treatment with deep
brain stimulation. The association between plasma PAI-1 levels and
cognitive competence were analyzed as well as degree of Parkinson's
disease. Patients with Parkinson's disease were reported to have
higher plasma levels of PAI-1 and may contribute to the progression
of Parkinson's disease.
Materials and methods
Study design, subjects and
sampling
A total of 102 patients with Parkinson's disease
(male: n=50, female: n=52) and 85 healthy volunteers (male: n=42,
female: n=43) were recruited in the present study from the
Affiliated Huai'an Hospital of Xuzhou Medical University between
June 2013 and May 2015. The treatment period was 8 weeks and the
follow-up was 36 months. The age of patients was 64.6–83.4 years.
Patients were included following diagnosis of Parkinson's disease
using Canadian Primary Care Sentinel Surveillance Network data
(13). Staging of Parkinson's
disease was performed using Hoehn-Yahr criteria (14). Patients' Positive and Negative
Syndrome Scale (PANSS) was analyzed according to a previous study
(15). The methodology used in the
present study was approved by the Central Ethics Committee of
Huai'an Second People's Hospital (Huai'an, China). Patients with
cerebral hemorrhage, cerebral infarction or epilepsy were excluded.
All patients were required to provide written, informed consent
prior to inclusion in the present study.
Measure of blood pressure, lipids and
glucose parameters
Blood pressure in patients with Parkinson's disease
was measured using a non-invasive blood pressure gauge (Shanghai
Yuyan Instruments Co., Ltd., Shanghai, China). The pressure value
was recorded prior to and following treatment. Lipids and glucose
parameters in the blood were recorded every 2 days for a total of
14 days using Amplex™ Red Glucose/Glucose Oxidase assay kit (cat.
no. A22189; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to a methodology described in a previous study (16).
Montreal cognitive assessment (MoCA)
score
The cognitive function of patients with Parkinson's
disease was analyzed via MoCA scoring (17). Briefly, the maximal MoCA score that
may be attained during analysis is 30 and a score <26 is
considered to indicate Parkinson's disease. The maximal percent
increase in each patient was calculated using the following
formula: [(maximal MoCA score - MoCA score on admission)/MoCA score
on admission] ×100.
ELISA
Plasma levels of PAI-1 were detected prior to DBS
treatment and on day 5 following DBS in patients with Parkinson's
disease using an ELISA kit (cat. no. MAB32010; Bio-Techne,
Minneapolis, MN, USA) according to the manufacturer's protocol.
Anxiety analysis and discrimination
index
Anxiety of patients with Parkinson's disease was
determined using the Quality of Recovery Score (QoR-40) as
described previously (18). All
patients with Parkinson's disease received DBS (3.5 V; 130 Hz; Pins
Medical, Beijing, China) or placebo (control) at the subthalamic
nucleus and anxiety was analyzed prior to or following this
treatment according to a previously described method (19). Discrimination index was used to
analyze the evidence from event-related brain potentials for
patients with Parkinson's disease as described previously (20).
Regression analysis
The plasma levels of PAI-1 were subjected to
regression analysis in Parkinson's disease patients at different
clinical stages using least square convergence (21). The predicted curve that results in
the lowest sum of squares is the best fit. If the fit is robust,
the parameters of the observed curve can be inferred from those of
the predicted data. All data were analyzed using SPSS software
version 19.0 (IBM Corp., Armonk, NY, USA).
Statistical analysis
Data are presented as the means + or ± the standard
deviation of three repeated experiments. All data were analyzed
using GraphPad Prism version 6.0 software (GraphPad Software, Inc.,
La Jolla, CA, USA). Unpaired data were analyzed using Student's
t-test whereas comparisons of data between multiple groups were
analyzed via one-way analysis of variance followed by Tukey's
honest significant difference test. Kaplan-Meier analysis was used
to estimate the rate of relapse and re-treatment during the 368-day
treatment period. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of patients with
Parkinson's disease and plasma levels of PAI-1
A total of 102 patients with Parkinson's disease and
85 healthy volunteers were recruited for the present clinical
analysis. All analyses were performed in an easy and comfortable
environment for patients. The characteristics of patients with
Parkinson's disease are summarized in Table I. It was demonstrated that the mean
scores of PANSS were 82.2±10.0. Anxiety and slow locomotion were
the main external characteristics for patients with Parkinson's
disease (22). Blood pressure and
glucose parameters in patients were similar to healthy volunteers
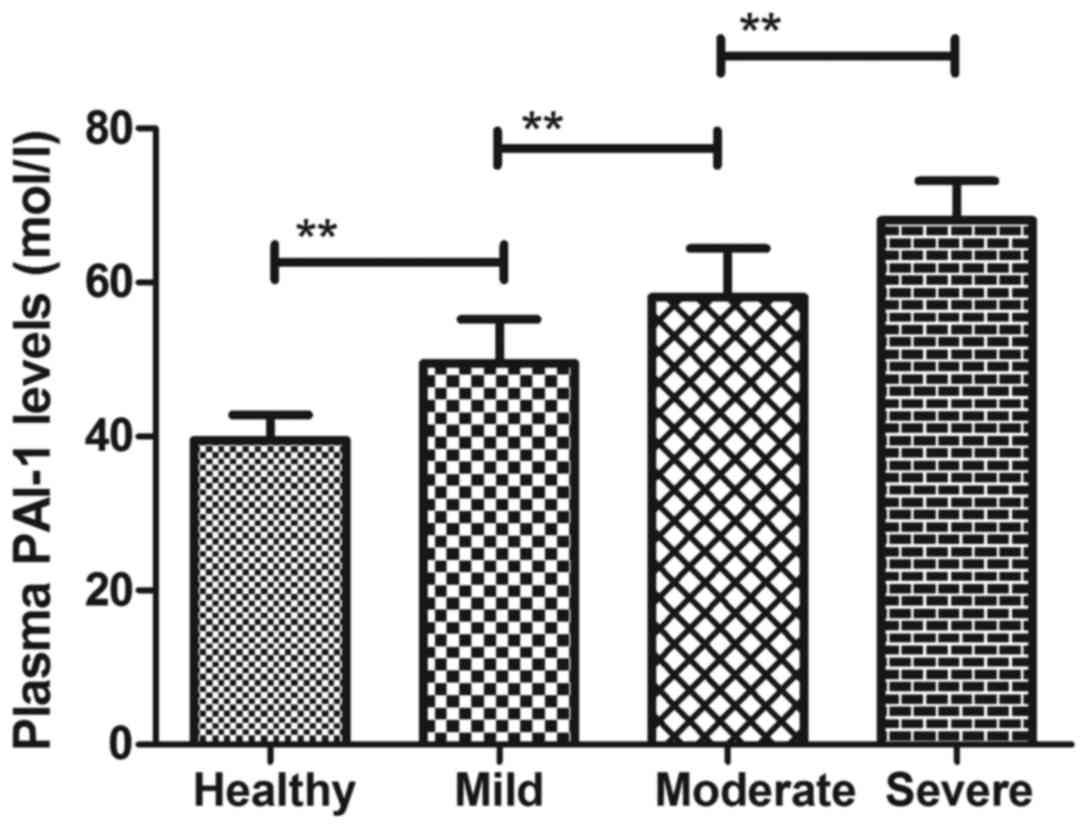
(data not shown). It was also demonstrated that plasma levels of
PAI-1 were significantly higher in patients with Parkinson's
disease compared with healthy controls (Fig. 1). Plasma levels of PAI-1 were
upregulated according to severity of Parkinson's disease (Fig. 1). These results suggest that patients
with Parkinson's disease present higher plasma PAI-1 levels than
healthy individuals.
 | Table I.Characteristics of patients with
Parkinson's disease. |
Table I.
Characteristics of patients with
Parkinson's disease.
| Characteristic | Patients | Healthy controls |
|---|
| Total, n | 102 | 85 |
| Male, n | 50 | 42 |
| Female, n | 52 | 43 |
| Anxiety,
discrimination index (mean ± SD) | 0.73±0.08 | 0.06±0.02 |
| PANSS score (mean ±
SD) | 82.2±10.0 | 4.6±2.2 |
| Glucose, mmol/l
(range) | 5.8–7.5 | 5.4–7.4 |
| Blood pressure, mmHg
(mean ± SD) | 126±12 | 118±10 |
| Therapy, n |
|
|
| DBS | 62 | 0 |
|
Placebo | 40 | 0 |
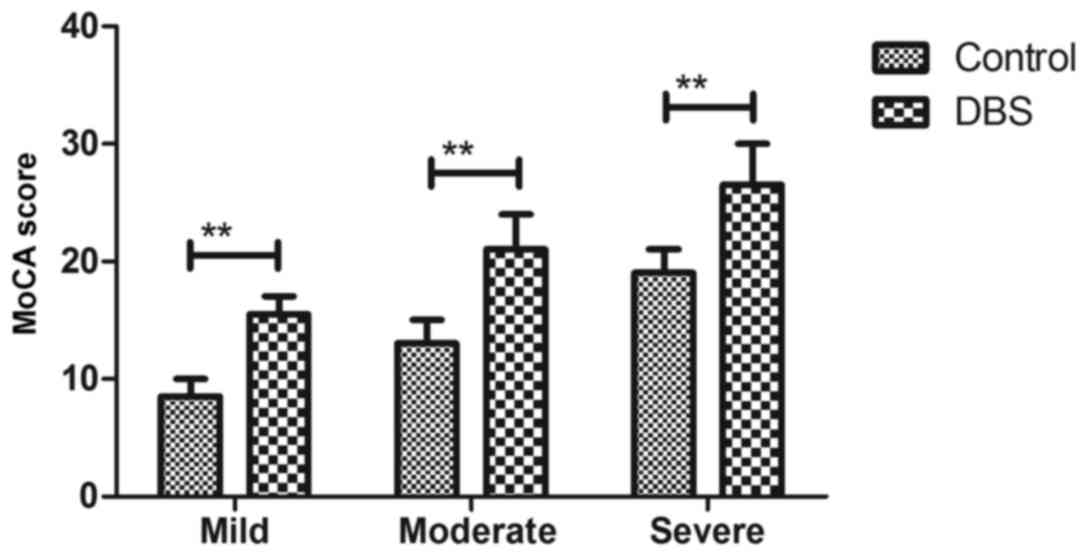
Efficacy of DBS for patients with
Parkinson's disease
As presented in Fig.
2, patients with Parkinson's disease who received DBS presented
significantly improved cognitive competence compared with controls,
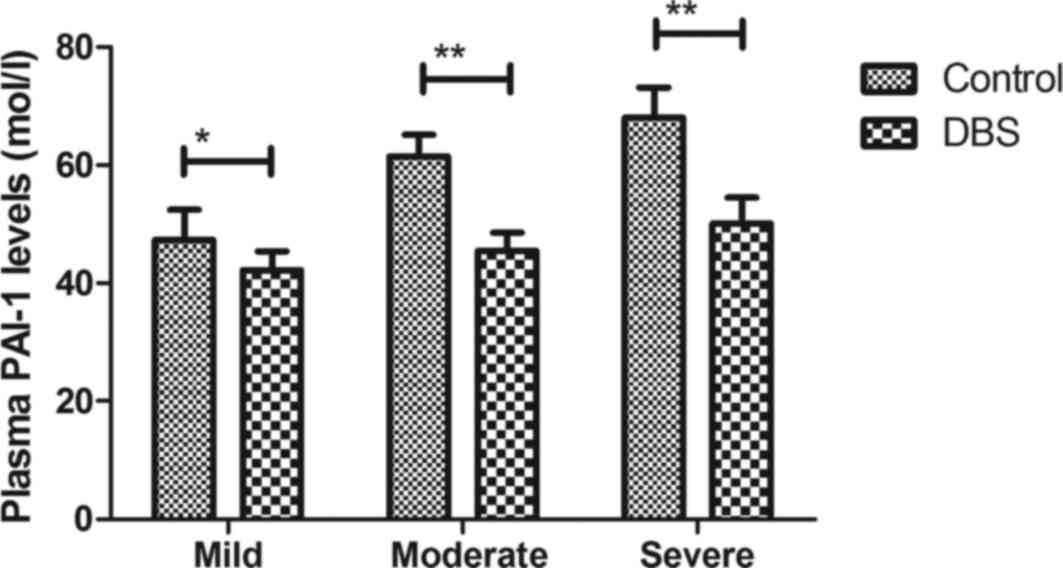
as determined by MoCA. It was observed that DBS treatment
significantly decreased plasma PAI-1 levels in patients with
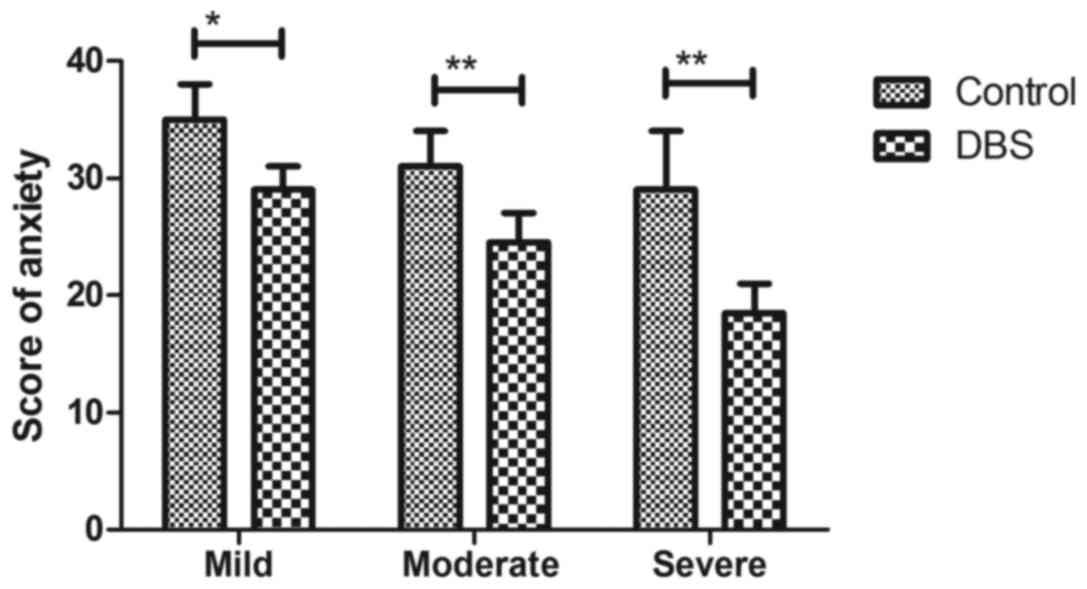
Parkinson's disease (Fig. 3). It was
also observed that DBS treatment significantly relieved the degree
of anxiety for patients with Parkinson's disease compared with the
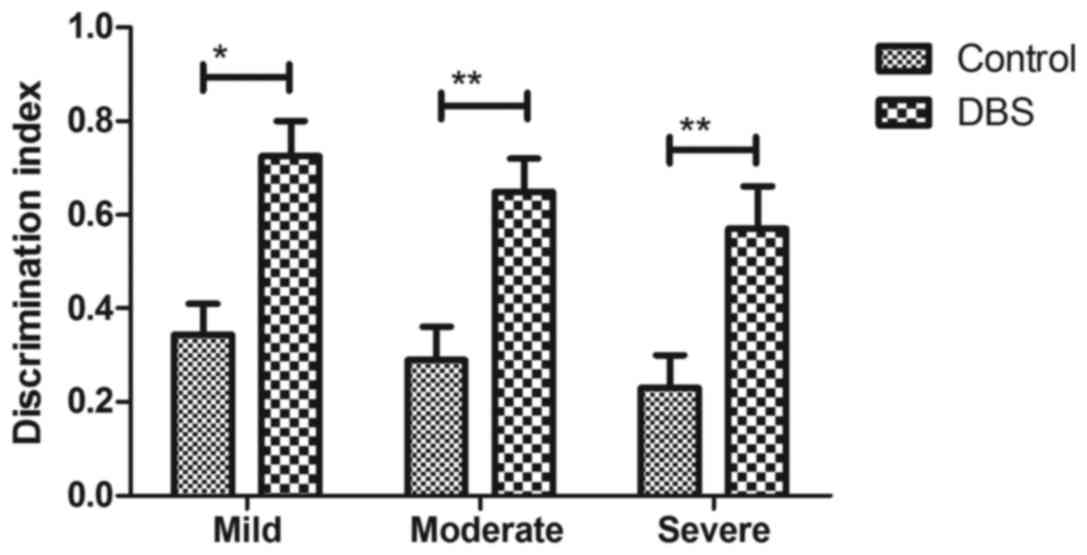
placebo group (Fig. 4). As presented
in Fig. 5, discrimination index was
significantly improved by the treatment of DBS for patients with
Parkinson's disease. These results suggest that DBS is beneficial
for the treatment of patients with Parkinson's disease.
Association between plasma levels of
PAI-1 and degree of Parkinson's disease
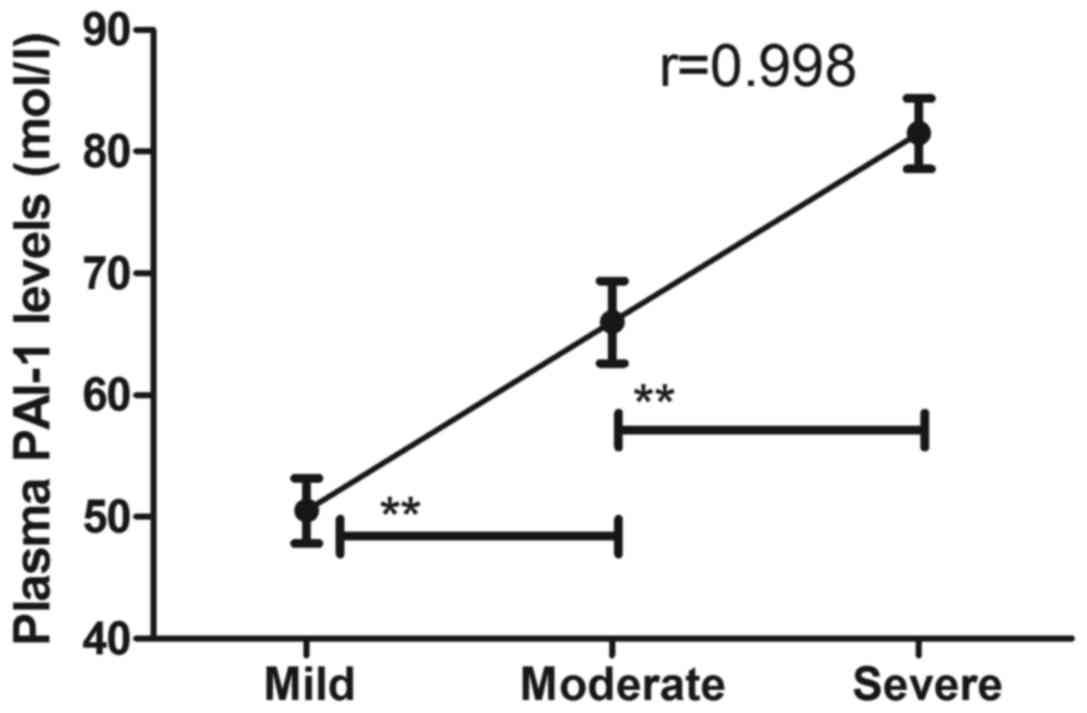
The association between plasma levels of PAI-1 and
degree of Parkinson's disease was investigated in patients with
Parkinson's disease. It was demonstrated that plasma levels of
PAI-1 were a significant predictor of the degree of clinical stage
of Parkinson's disease (Fig. 6).
These results suggest that plasma levels of PAI-1 may be a
potential biomarker for accessing the clinical stage of Parkinson's
disease.
Association between plasma PAI-1
levels and cognitive function in patients with Parkinson's
disease
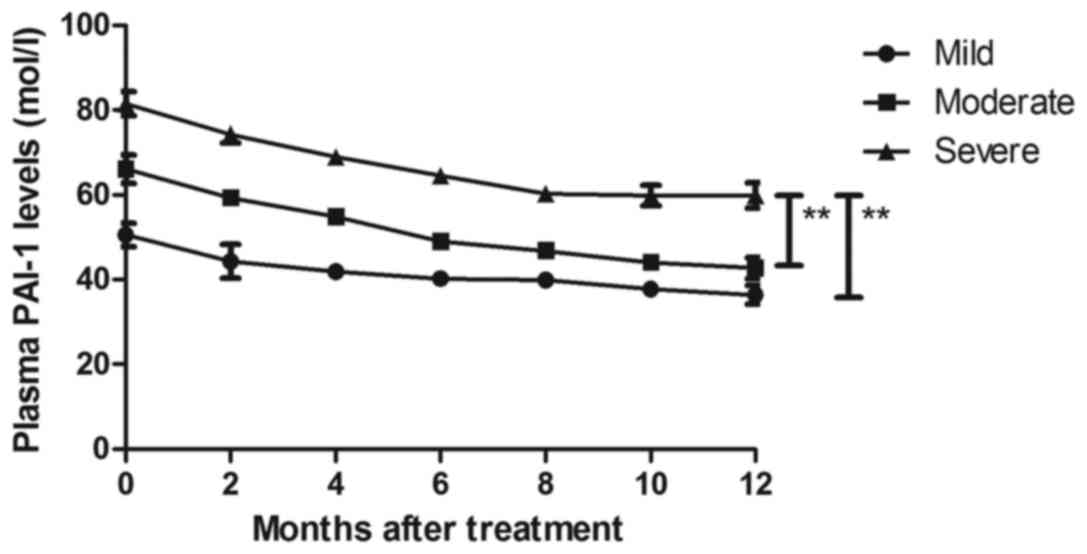
Changes in plasma PAI-1 levels were investigated in
patients with Parkinson's disease. The present results revealed
that plasma PAI-1 levels were significantly decreased after
treatment with DBS for patients with Parkinson's disease (Fig. 7). Notably, it was revealed that
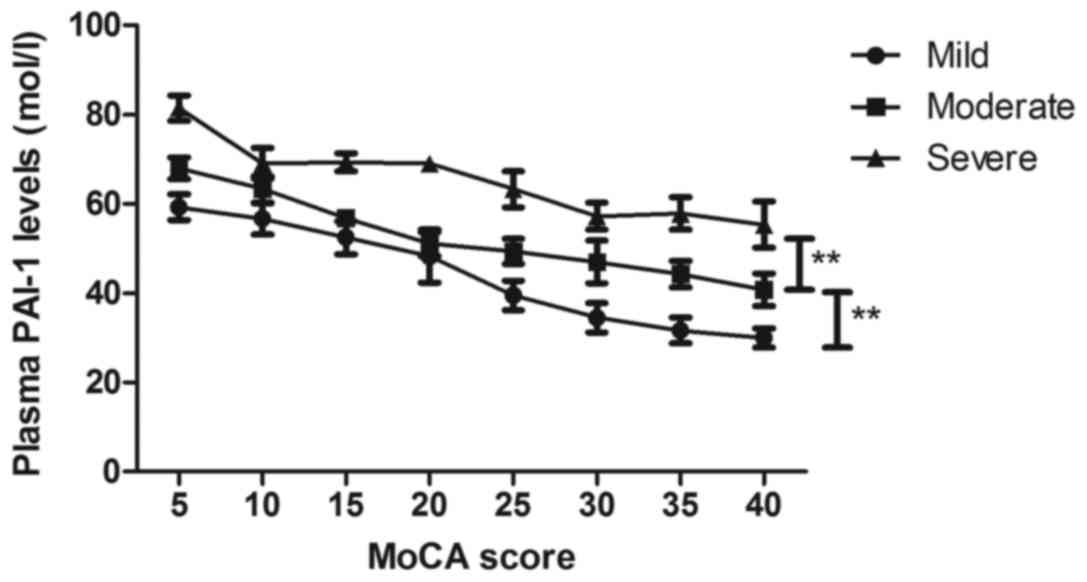
plasma PAI-1 levels were negatively c associated with cognitive
function for patients with Parkinson's disease (Fig. 8). These results indicated that plasma
levels of PAI-1 are negatively associated with cognitive function
in patients with Parkinson's disease.
Discussion
Parkinson's disease is characterized by cognitive
impairment and neurodegeneration (23,24).
Previous evidence has demonstrated that alternative treatment
procedures combining drainage and intraventricular fibrinolysis
with recombinant tissue plasminogen activator may prevent
haemorrhagic complications (25).
Notably, a previous study has reported the changes of focal and
brainstem neurological signs in patients with traumatic brain
injury and their dependence on the −675 4/5 G polymorphism in the
PAI-1 gene (26). Therefore, in the
present study it was assumed that plasma PAI-1 level may be
associated with the degree of Parkinson's disease. The aim of the
present study was to analyze the association between plasma PAI-1
level and cognitive function in patients with Parkinson's disease,
and it was observed that plasma PAI-1 was upregulated in patients
with Parkinson's disease and was positively associated with the
degree of Parkinson's disease severity.
In the present study, patients with Parkinson's
disease exhibited higher plasma PAI-1 levels than healthy
volunteers. Although DBS of the subthalamic nucleus therapy is an
effective treatment for motor impairments in Parkinson's disease
(27–29), the association between PAI-1 and the
efficacy of DBS have not been investigated in patients with
Parkinson's disease, to the best of our knowledge. The present
results support that DBS is beneficial for the treatment of
patients with Parkinson's disease and revealed that plasma levels
of PAI-1 are positively associated with degree of Parkinson's
disease severity and negatively associated with cognitive function
in patients with Parkinson's disease.
PAI-1 is abundantly expressed in infarcted
myocardium, but the pathogenic role of plasma PAI-1 plasma remains
unknown in patients with Parkinson's disease (30). Cho et al (31) have previously demonstrated that
valproic acid may induce astrocyte-dependent neurite outgrowth from
cultured rat primary cortical neuron via modulation of tissue
plasmogen activator PAI-1 activity. Previous studies have reported
changes in the plasminogen activator system and the inhibitors
PAI-1 and PAI-2 in posttraumatic lesions in the central nervous
system and brain injuries (32,33). In
the present study, it was observed that plasma PAI-1 levels are
upregulated in patients with Parkinson's disease and DBS therapy
decreased plasma PAI-1 levels, which is associated with cognitive
function in patients with Parkinson's disease.
In conclusion, the present study study demonstrated
that plasma PAI-1 level serves an important role in the progression
of Parkinson's disease. The results indicate that DBS therapy was
associated with a decrease in plasma PAI-1 levels and relieved
anxiety and discrimination index in patients with Parkinson's
disease. Additionally, it was observed that plasma PAI-1 levels may
be a potential biomarker for assessing the clinical stage of
Parkinson's disease, which is negatively associated with cognitive
function in patients with Parkinson's disease. Taken together,
these findings suggest that PAI-1 is a potential diagnostic or
prognostic marker for patients with Parkinson's disease. However,
further study is required to identify the role of plasminogen
activator inhibitor-1 in the diagnosis and prognosis of patients
with Parkinson's disease in a large population size.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HP, YZ, ZZ, JZ and YZ analyzed and interpreted the
patient data regarding the hematological disease and the
transplant. HP, QZ, XC, JT and LZ performed the histological
examination of the kidney, and HP was a major contributor in
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The methodology used in the present study was
approved by the Central Ethics Committee of Huai'an Second People's
Hospital (Huai'an, China). All patients were required to provide
written, informed consent prior to inclusion in the present
study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mano T, Britton Z and Britton T:
Anatomo-functional basis of nonmotor symptoms in Parkinson disease.
Neurology. 87:2506–2507. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meles SK, Teune LK, de Jong BM, Dierckx RA
and Leenders KL: Metabolic Imaging in Parkinson disease. J Nuclear
Med. 58:23–28. 2017. View Article : Google Scholar
|
|
3
|
Cooper CA and Chahine LM: Biomarkers in
prodromal Parkinson disease: A qualitative review. J Int
Neuropsychol Soc. 22:956–967. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiou SM, Lin YC, Lu MK and Tsai CH:
Bilateral subthalamic stimulation for advanced Parkinson disease:
Early experience at an Eastern center. Neurol Sci. 36:515–520.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowacka B, Lubinski W, Honczarenko K,
Potemkowski A and Safranow K: Ophthalmological features of
Parkinson disease. Med Sci Monit. 20:2243–2249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elstein D, Alcalay R and Zimran A: The
emergence of Parkinson disease among patients with Gaucher disease.
Best Pract Res Clin Endocrinol Metab. 29:249–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang HW, Huang YP and Pan SL: Parkinson
disease and risk of acute myocardial infarction: A
population-based, propensity score-matched, longitudinal follow-up
study. Am Heart J. 169:508–514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gorska-Ciebiada M, Saryusz-Wolska M,
Borkowska A, Ciebiada M and Loba J: Plasma levels of
thrombomodulin, plasminogen activator inhibitor-1 and fibrinogen in
elderly, diabetic patients with depressive symptoms. Aging Clin Exp
Res. 28:843–851. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gettins PG and Dolmer K: The high affinity
binding site on plasminogen activator Inhibitor-1 (PAI-1) for the
low density lipoprotein receptor-related protein (LRP1) is composed
of four basic residues. J Biol Chem. 291:800–812. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu F, Liu H and Sun Y: Association of
plasminogen activator inhibitor-1 gene polymorphism and type 2
diabetic nephropathy. Ren Fail. 38:157–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou X, Hendrickx ML,
Hassanzadeh-Ghassabeh G, Muyldermans S and Declerck PJ: Generation
and in vitro characterisation of inhibitory nanobodies towards
plasminogen activator inhibitor 1. Thromb Haemost. 116:1032–1040.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shenai MB, Patel DM, Romeo A, Whisenhunt
JD, Walker HC, Guthrie S and Guthrie BL: The relationship of
electrophysiologic subthalamic nucleus length as a predictor of
outcomes in deep brain stimulation for parkinson disease.
Stereotact Funct Neurosurg. 95:341–347. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Correction: Using canadian primary care
sentinel Surveillance Network data to examine depression in
patients with a diagnosis of Parkinson disease: A retrospective
cohort study. CMAJ Open. 4:E7192016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kataoka H, Tanaka N, Eng M, Saeki K,
Kiriyama T, Eura N, Ikeda M, Izumi T, Kitauti T, Furiya Y, et al:
Risk of falling in Parkinson's disease at the Hoehn-Yahr stage III.
Eur Neurol. 66:298–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yehya A, Ghuloum S, Mahfoud Z, Opler M,
Khan A, Hammoudeh S, Abdulhakam A, Al-Mujalli A, Hani Y, Elsherbiny
R and Al-Amin H: Validity and reliability of the arabic version of
the positive and negative syndrome scale. Psychopathology.
49:181–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nunes PM, Wright AJ, Veltien A, van Asten
JJ, Tack CJ, Jones JG and Heerschap A: Dietary lipids do not
contribute to the higher hepatic triglyceride levels of
fructose-compared to glucose-fed mice. FASEB J. 28:1988–1997. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pelletier S, Nalpas B, Alarcon R, Rigole H
and Perney P: Investigation of cognitive improvement in
alcohol-dependent inpatients using the montreal cognitive
assessment (MoCA) score. J Addict. 2016:15390962016.PubMed/NCBI
|
|
18
|
McIntosh S and Adams J: Anxiety and
quality of recovery in day surgery: A questionnaire study using
Hospital Anxiety and depression scale and quality of recovery
score. Int J Nurs Pract. 17:85–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guimaraes-Pereira L, Costa M, Sousa G and
Abelha F: Quality of recovery after anaesthesia measured with
QoR-40: A prospective observational study. Braz J Anesthesiol.
66:369–375. 2016.PubMed/NCBI
|
|
20
|
Wieser MJ, Klupp E, Weyers P, Pauli P,
Weise D, Zeller D, Classen J and Mühlberger A: Reduced early visual
emotion discrimination as an index of diminished emotion processing
in Parkinson's disease?-Evidence from event-related brain
potentials. Cortex. 48:1207–1217. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayes AF and Rockwood NJ: Regression-based
statistical mediation and moderation analysis in clinical research:
Observations, recommendations, and implementation. Behav Res Ther.
98:39–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kovacs M, Makkos A, Weintraut R, Karadi K,
Janszky J and Kovacs N: Prevalence of anxiety among hungarian
subjects with Parkinson's disease. Behav Neurol. 2017:14701492017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tholfsen LK, Larsen JP, Schulz J, Tysnes
OB and Gjerstad MD: Changes in insomnia subtypes in early Parkinson
disease. Neurology. 88:352–358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wyman-Chick KA, Martin PK, Minár M and
Schroeder RW: Cognition in patients with a clinical diagnosis of
Parkinson disease and scans without evidence of dopaminergic
deficit (SWEDD): 2-year follow-up. Cogn Behav Neurol. 29:190–196.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leroux P and Marret S: PAI-1/t-PA ratio in
cord blood: A potential index of brain haemorrhage risk in extreme
preterms. Arch Dis Child Fetal Neonatal Ed. 98:F281–F282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Potapov O and Kmyta O: Changes of focal
and brainstem neurologic signs in patients with traumatic brain
injury and their dependence on the-675 4G/5G polymorphism in the
PAI-1 gene. Georgian Med News. 45–51. 2014.(In Russian). PubMed/NCBI
|
|
27
|
Knight EJ, Testini P, Min HK, Gibson WS,
Gorny KR, Favazza CP, Felmlee JP, Kim I, Welker KM, Clayton DA, et
al: Motor and nonmotor circuitry activation induced by subthalamic
nucleus deep brain stimulation in patients with parkinson disease:
Intraoperative functional magnetic resonance imaging for deep brain
stimulation. Mayo Clin Proc. 90:773–785. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pallavaram S, D'Haese PF, Lake W, Konrad
PE, Dawant BM and Neimat JS: Fully automated targeting using
nonrigid image registration matches accuracy and exceeds precision
of best manual approaches to subthalamic deep brain stimulation
targeting in Parkinson disease. Neurosurgery. 76:756–765. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wagenbreth C, Zaehle T, Galazky I, Voges
J, Guitart-Masip M, Heinze HJ and Düzel E: Deep brain stimulation
of the subthalamic nucleus modulates reward processing and action
selection in Parkinson patients. J Neurol. 262:1541–1547. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Minami Y, Horikawa K, Akiyama M and
Shibata S: Restricted feeding induces daily expression of clock
genes and Pai-1 mRNA in the heart of Clock mutant mice. FEBS Lett.
526:115–118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho KS, Kwon KJ, Choi CS, Jeon SJ, Kim KC,
Park JH, Ko HM, Lee SH, Cheong JH, Ryu JH, et al: Valproic acid
induces astrocyte-dependent neurite outgrowth from cultured rat
primary cortical neuron via modulation of tPA/PAI-1 activity. Glia.
61:694–709. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dietzmann K, von Bossanyi P, Krause D,
Wittig H, Mawrin C and Kirches E: Expression of the plasminogen
activator system and the inhibitors PAI-1 and PAI-2 in
posttraumatic lesions of the CNS and brain injuries following
dramatic circulatory arrests: An immunohistochemical study. Pathol
Res Pract. 196:15–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zervos IA, Nikolaidis E, Lavrentiadou SN,
Tsantarliotou MP, Eleftheriadou EK, Papapanagiotou EP, Fletouris
DJ, Georgiadis M and Taitzoglou IA: Endosulfan-induced lipid
peroxidation in rat brain and its effect on t-PA and PAI-1:
Ameliorating effect of vitamins C and E. J Toxicol Sci. 36:423–433.
2011. View Article : Google Scholar : PubMed/NCBI
|