Introduction
Deafness is a serious health problem with unknown
etiology, and it is estimated that 1 in 1,000 newborns suffer from
the condition (1). It may be
associated with numerous risk factors, including the administration
of aminoglycoside antibiotics (AmAn) (2). Genetic alterations in human
mitochondrial (mt)DNA are also thought to be associated with
deafness (3). Human mtDNA is a
double-stranded, circular molecule, encoding 13 protein subunits, 2
ribosomal RNAs (rRNAs) and 22 transfer RNAs (tRNAs). In particular,
the well-known 12S rRNA A1555G mutation has been demonstrated to be
an important molecular basis of hearing loss (4,5).
Furthermore, the A to G transition at position 1555 is located in
the region of small ribosomal RNA that is highly conserved in
bacteria and mammals (6). The
corresponding region in Escherichia coli forms an essential
part of the decoding site of the ribosome (7) and is crucial for subunit association
either by RNA-protein or the RNA-RNA interaction (8). It is interesting to note that among
individuals who carry this mutation, the use of AmAn for clinical
treatment of fever is likely to result in irreversible hearing
impairment (9). However, individuals
who carry the A1555G mutation exhibit a wide range of clinical
phenotypes, from profound hearing loss to normal hearing (4). Thus, if an individual carrying the
A1555G mutation cannot use aminoglycosides, screening for the
A1555G mutation is crucial for prevention and early diagnosis of
maternally inherited deafness. In addition, lymphoblastoid cell
lines derived from an Arab-Israeli family demonstrated more severe
biochemical defects in those that were symptomatic to those that
were not (10). Therefore, it is
hypothesized that other unidentified risk factors, including
polymorphisms in mtDNA, nuclear gene mutations, AmAn and epigenetic
modification, may contribute to hearing impairment (10).
To explore the potential association between mtDNA
polymorphisms and deafness, a mutational screening was conducted
for the presence of A1555G in 300 infants with hearing impairment
and 100 healthy controls. Consequently, 5 patients with the
mutation were identified. Among these infants carrying the A1555G
mutation, only one had a family history of exposure to AmAn.
Materials and methods
Subjects and clinical evaluations
From January 2015 to January 2017, a total of 300
infants with hearing loss (180 females and 120 males), aged from
1–3 years (with an average age of 2 years), as well as 100 age- and
sex-matched control subjects (60 females and 40 males) were
recruited from Fujian Provincial Hospital (Fuzhou, China). A
physical examination was performed for each participant that was
enrolled. In addition, the level of hearing impairment was tested
using pure-tone audiometry. Furthermore, the level of hearing loss
was classified as follows: Normal, ≤25 dBHL; mild, 26–40 dBHL;
moderate, 41–70 dBHL; severe, 71–90 dBHL; profound, >90 dBHL.
Written informed consent was obtained from the patients' parents or
guardians, and the study was approved by the Ethics Committee of
Fujian Provincial Hospital. Patients were included if the infants
possessed the sole clinical phenotype. Those patients who carried
other mitochondrial disorders, including Leigh syndrome, exercise
intolerance and developmental delay were excluded. In addition,
healthy patients exhibited normal hearing, and did not carry any
diseases.
Screening for the mitochondrial A1555G
mutation
Genomic DNA was extracted from blood samples
obtained from each participant using Puregene DNA Isolation kits
(Gentra; Qiagen, Inc., Valencia, CA, USA). The mitochondrial 12S
rRNA gene was amplified using polymerase chain reaction (PCR;
Takara, Bio, Inc., Otsu, Japan), the primer sequence for
amplification the 12S rRNA gene was as follows: (5): Forward, 5′-CGATCAACCTCACCACCTCT-3′ and
reverse, 5′-TGGACAACCAGCTATCACCA-3′. The thermocycling conditions
for PCR were as follows: 95°C for 5 min, 94°C for 10 sec, 60°C for
30 sec, 30 cycles at 72°C for 60 sec and 72°C for 10 min for
extension. Following amplification, the PCR product was digested
with the restriction enzyme BsmAI to screen for the
occurrence of the A1555G mutation. Equal amounts (5 µl) of various
digested samples were then analyzed using electrophoresis with 1.5%
agarose gel. Following electrophoresis, a BandPeeper (MaestroGen
Inc., Hsinchu, Taiwan) instrument was utilized to detect the
results using the Invitrogen™ E-Gel™ Imager software (version
2.2.4; Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA,
USA).
Molecular features of the pedigree
harboring mitochondrial A1555G mutation
A Chinese pedigree was collected in Fujian
Provincial Hospital. The complete mitochondrial genomes of the
matrilineal individuals (I-2, II-1, II-5, II-8, II-10, III-9,
III-10 and III-12) were amplified using 24 primers, as previously
described (6). The PCR reagents (all
obtained from Takara Bio, Inc.,) were as follows: 200 µM dNTPs, 2
µl 10X PCR buffer (10X0.2 µl Taq DNA polymerase and 15 mmol/l
Mg2+). Subsequently, the PCR products were purified and
sequenced using the ABI PRISM™ 3700 machine (Applied Biosystems;
Thermo Fisher Scientific, Inc.) (11). A total of 24 PCR product sequences
were compared with the reverse Cambridge sequence, using DNA Star
software version 5.01 (DNASTAR Inc., Madison, WI, USA) to detect
variations in mitochondrial genes (GenBank accession no. NC_012920)
(12).
Phylogenetic conservation
analysis
To discern the potential pathogenic mtDNA mutations,
the phylogenetic approach was utilized to assess the conservation
index (CI) of each mtDNA mutation. A total of 17 species were used
to analysis the CIs. Notably, CI>75% was regarded as exhibiting
functional potential (13).
Detecting GJB2 mutations
In order to identify whether GJB2 mutations
were involved in hearing impairment, variants of GJB2 were
screened using PCR and direct sequencing. The primers for
amplification of the GJB2 coding region were as follows:
Forward, 5′-TATGACACTCCCCAGCACAG-3′ and reverse,
5′-GGGCAATGCTTAAACTGGC-3′ (14). The
PCR reagents (all obtained from Takara Bio, Inc.) were as follows:
200 µM dNTPs, 2 µl 10X PCR buffer (10×0.2 µl Taq DNA polymerase)
and 15 mmol/l Mg2+. PCR was performed under the
following thermocycling conditions: 95°C for 5 min, 94°C for 10
sec, 60°C for 30 sec, 30 cycles at 72°C for 60 sec, and 72°C for 10
min for extension Then, the PCR products were sequenced using the
ABI PRISM™ 3700 machine (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and compared with the wild-type GJB2
sequence using DNA Star software version 5.01 (GenBank accession
no. M86849) to detect potential pathogenic mutations.
Screening for the TRMU A10S
variant
Nuclear modified gene TRMU was previously
identified to be associated with hearing loss (15). To understand the role of TRMU
in hearing impairment, the TRMU A10S variant was screened in
the matrilineal relatives using PCR amplification of exon 1 of this
gene. The PCR reagents (all obtained from Takara Bio, Inc.,) were
as follows: 200 µM dNTPs, 2 µl 10X PCR buffer (10×0.2 µl Taq DNA
polymerase) and 15 mmol/l Mg2+. The primer sequence for
exon 1 was as described previously (15). PCR was performed under the following
thermocycling conditions: 95°C for 5 min, 94°C for 10 sec, 58°C for
30 sec, 30 cycles at 72°C for 60 sec, and 72°C for 10 min for
extension. Then, the PCR product was sequenced using the ABI PRISM™
3700 machine (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and compared with the wild-type version of TRMU and DNA Star
software version 5.01 (GenBank accession no. AF448221) (16).
Bioinformatics analysis
To investigate whether T15943C mutation altered the
structure of tRNAThr, the RNA Fold web server was
employed to analyze the secondary structure of tRNAThr
with and without the T15943C mutation (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi)
(17). The wild-type version of
tRNAThr gene was:
5′-GTCCTTGTAGTATAAACTAATACACCAGTCTTGTAAACCGGAGATGAAAACCTTTTTCCAAGGACA-3′.
The sequence of tRNAThr gene carrying the T15943C
mutation was:
5′-GTCCTTGTAGTATAAACTAATACACCAGTCTTGTAAACCGGAGATGAAAACCTTTCTCCAAGGACA-3′.
Results
Frequency of mitochondrial A1555G
mutation in infants with deafness
To detect the allele frequency of the A1555G
mutation, 300 infants were enrolled who were confirmed to have
non-syndromic hearing loss by a clinical physician in Fujian
Provincial Hospital. The restriction enzyme BsmAI was used
to screen the mitochondrial A1555G mutation following PCR
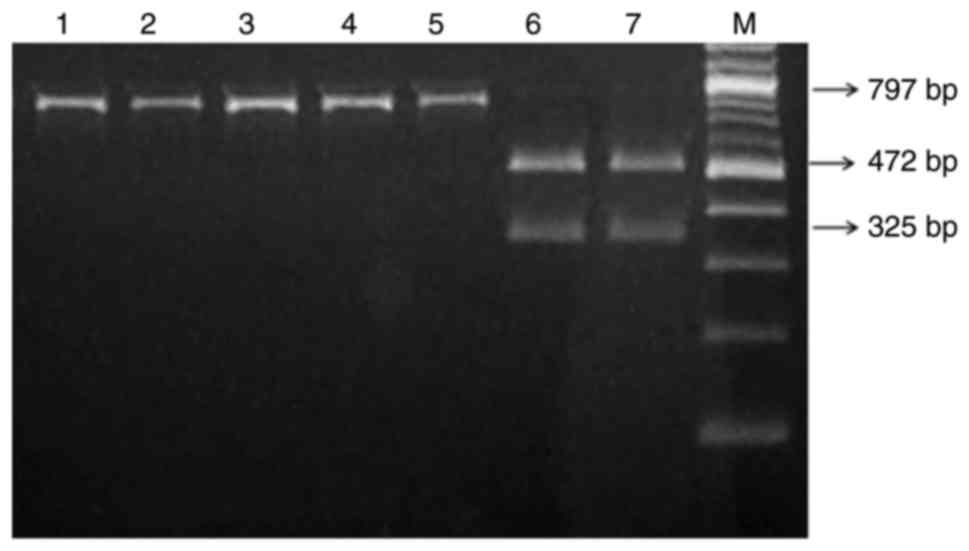
amplification of the 12S rRNA gene. As indicated in Fig. 1, following electrophoresis, it was
observed that samples carrying the A1555G mutation exhibited only
one 797 bp band, while the samples without this mutation resulted
in two specific bands: 472 and 325 bp. Consequently, 5 patients
with this mutation were identified (5/300; 1.67%). The mutation was
not detected in the 100 healthy controls. Further clinical
evaluation indicated that 1 patient with the mutation had an
obvious family history of using AmAn.
Molecular characterization of the
pedigree carrying mitochondrial A1555G mutation
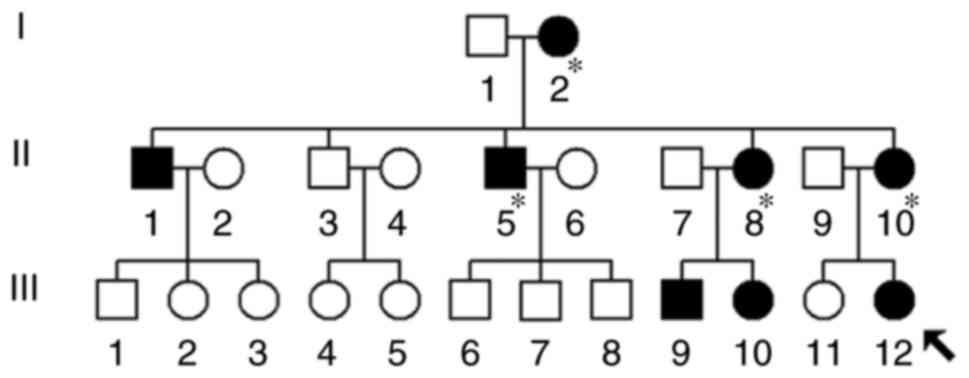
The proband's mother (II-10) was 26 years old and
came from the Fuzhou area of Fujian Province, China. She received
AmAn (gentamycin, 5 mg/kg/dose) for clinical treatment of a high
fever when she was 21 years old. Unfortunately, 2 weeks following
the antibiotic treatment, she suffered from bilateral hearing loss
and clinical examination indicated that she had severe deafness (75
dB in left ear, 88 dB in right ear; see Table I). Further physical and audiological
examinations were performed for the matrilineal relatives in this
family. It was observed that I-2, II-5 and II-8 had a history of
exposure to AmAn.
 | Table I.Summary of clinical and molecular data
for members of a Han Chinese family with AmAn-induced hearing
loss. |
Table I.
Summary of clinical and molecular data
for members of a Han Chinese family with AmAn-induced hearing
loss.
| Subject | Sex | Age at onset
(years) | Age at test
(years) | Use of AmAn | PTA (dB), left
ear | PTA (dB), right
ear | Level of hearing
loss |
|---|
| I-2 | Female | 55 | 62 | Yes | 90 | 90 | Profound |
| II-1 | Male | 18 | 30 | No | 75 | 80 | Severe |
| II-5 | Male | 25 | 32 | Yes | 35 | 40 | Moderate |
| II-8 | Female | 23 | 28 | Yes | 88 | 85 | Severe |
| II-10 | Female | 21 | 26 | Yes | 75 | 88 | Severe |
| III-9 | Male | 3 | 5 | No | 70 | 80 | Severe |
| III-10 | Female | 1 | 2 | No | 95 | 90 | Profound |
| III-12 | Female | 1 | 1 | No | 55 | 65 | Severe |
| III-1 | Male | N/A | 5 | No | 18 | 20 | Normal |
As indicated in Fig.
2, this pedigree had an obvious maternal transmission pattern.
It was noted that the pedigree manifested a high penetrance and
expressivity of deafness: The penetrance of deafness was 80 and 40%
when patients who were exposed to AmAn were included and excluded,
respectively. Furthermore, it was identified that the age at onset
of deafness varied from 1 to 55 years (Table I). Other members of this family did
not exhibit any other mitochondrial disorders, including type 2
diabetes, cardiovascular events, vision loss or neurological
diseases.
Screening for mutations in
mitochondrial genes
The maternally inherited pattern of this family
indicated that mitochondrial dysfunction may be involved in the
pathogenesis of hearing loss. In order to investigate this, the
variants in mtDNA were screened using PCR and direct sequencing.
The results indicated that matrilineal relatives (I-2, II-1, II-5,
II-8, II-10, III-9, III-10 and III-12) in this family harbored 22
mtDNA polymorphisms, as well as the well-known A1555G mutation
(Table II). Of these, six variants
were identified in the D-loop, three variants in 12S rRNA, one
variant in 16S rRNA and one mutation in tRNAThr. The
other sequence alterations were primarily in oxidative
phosphorylation-associated genes. Furthermore, there were six
missense mutations, including ND2 C5178A (L237M) and A5301G
mutations (I278V), ATP6 A8860G mutation (T112A), ND3
A10398G mutation (T114A), and CytB C14766T (T7I) and A15326G
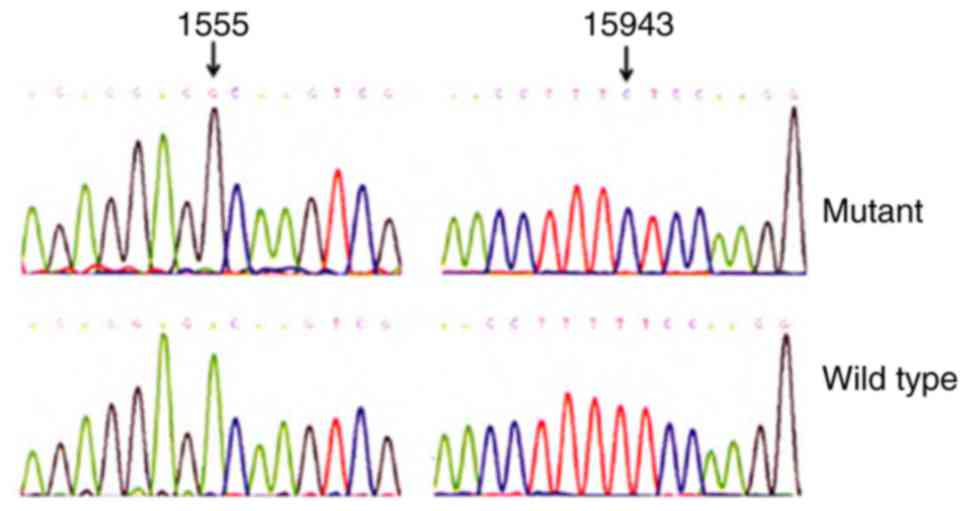
(T194A) mutations. In addition, evolutionary conservation analysis
was performed for these variants between 17 vertebrates, including
the mouse (18), bovine (19) and Xenopus laevis (20). However, it was identified that none
of these variants were conserved, with the exception of A1555G and
T15943C mutations (Fig. 3 and
Table III). In addition, the
A1555G and T15943C mutations were absent in the control subjects.
This suggested that the A1555G and T15943C mutations were involved
in the pathogenesis of deafness in this family.
 | Table II.Mitochondrial DNA sequence variants
in a Han Chinese family with hearing loss. |
Table II.
Mitochondrial DNA sequence variants
in a Han Chinese family with hearing loss.
| Gene | Position | Nucleotide
change | Conservation
(H/B/M/X)a |
|---|
| D-loop | 73 | A to G |
|
|
| 263 | A to G |
|
|
| 310 | T to CTC |
|
|
| 16189 | T to C |
|
|
| 16355 | C to T |
|
|
| 16519 | T to C |
|
| 12S rRNA | 750 | A to G | A/G/G/- |
|
| 1438 | A to G | A/A/A/G |
|
| 1555 | A to G | A/A/A/A |
| 16S rRNA | 2706 | A to G | A/G/A/A |
| ND2 | 4769 | A to G |
|
|
| 5178 | C to A (Leu to
Met) | L/T/T/T |
|
| 5301 | A to G (Ile to
Val) | I/I/M/L |
| CO1 | 7028 | C to T |
|
| ATP6 | 8860 | A to G (Thr to
Ala) | T/A/A/T |
| ND3 | 10398 | A to G (Thr to
Ala) | T/T/T/A |
| ND4 | 11719 | G to A |
|
| ND5 | 12705 | C to T |
|
|
| 13344 | A to G |
|
| CytB | 14766 | C to T (Thr to
Ile) | T/S/T/S |
|
| 15301 | G to A |
|
|
| 15326 | A to G (Thr to
Ala) | T/M/I/I |
|
tRNAThr | 15943 | T to C | T/T/T/T |
 | Table III.Sequence alignment of
tRNAThr gene from different species. |
Table III.
Sequence alignment of
tRNAThr gene from different species.
| Organism | Acc-stem | D-stem | D-loop | D-stem | Ac-Stem | Anticd-loop | Ac-stem | V-region | T-stem | T-loop | T-stema | Acc-stem |
|---|
| Homo
sapiens | GTCCTTGTA | GTAT | AAACTA | ATACA | CCAGT | CTTGTAA | ACCGG | AGAT | GAAAA | CCT | TTTTC | CAAGGACA |
| Mus
musculus | GTCTTGATA | GTAT | AAACA | TTACT | CTGGT | CTTGTAA | ACCTG | AAAT | GAAGA | TCTTC | TCTTC | TCAAGACA |
| Myoxus
glis | GTCCTGGTA | GTAT | AAAGTTA | TTACT | CTGGT | CTTGTAA | ACCAG | AAAT | GGAAA | TCTCAAT | TTTCC | CCAGGACA |
| Colobus
guereza | GCCCTCGTA | GTAC | AAACTA | GTATA | CCGGT | CTTGTAA | ACCGA | AGAT | GGAGA | CT | TCTCC | CTAGGACA |
| Pan
paniscus | GCCCTTGTA | GTAT | AAGCTA | ATACA | CCGGT | CTTGTAA | ACCGG | AAAC | GAAAA | CTT | TATTC | CAAGGACA |
| Ceratotherium
simum | GTCTTTGTA | GTAT | ATGAA | TTACT | CTGGT | CTTGTAA | ACCAG | AAAA | GGAAA | GCACCAC | TTTCC | CCAAGACT |
| Arctocephalus
forsteri | GTCTTCGTA | GTAT | AACAA | TTACC | TTGGT | CTTGTAA | ACCAA | AAAT | GGAGA | ACACCACGAC | TCTCC | CTAAGACT |
| Elephas
maximus | ACCCCTATA | GTAT | AAGACA | TTACA | ATGGT | CTTGTAA | GCCAT | AAAT | GAAAA | CCA | CTTTC | TAAGGGTA |
| Bradypus
tridactylus | GCCCTCGTA | GTAT | AACAAA | TTACA | CTAGC | CTTGTAA | ACCAG | AAAT | GAAAT | ACGC | ATTTC | CAAGGGCA |
| Gorilla
gorilla | GCCCTTGTA | GTAC | AGACCA | ATACA | CCAGT | CTTGTAA | ACCGG | AAAC | GAAGA | CCT | CCTTC | CAAGGGCA |
Genotyping analysis of GJB2 gene
To evaluate the contribution of GJB2 gene
variants to deafness expression, GJB2 mutations were
analyzed in the matrilineal relatives (I-2, II-1, II-5, II-8,
II-10, III-9, III-10 and III-12). However, no functional variants
were observed in the GJB2 gene.
Mutational analysis of TRMU gene
A previous study indicated that the TRMU A10S
variant may contribute to the clinical expression of hearing loss
associated with mitochondrial A1555G mutation (15). Therefore, the TRMU A10S
variant was screened in matrilineal members from the current
pedigree (I-2, II-1, II-5, II-8, II-10, III-9, III-10 and III-12).
However, the variant was not detected in these subjects.
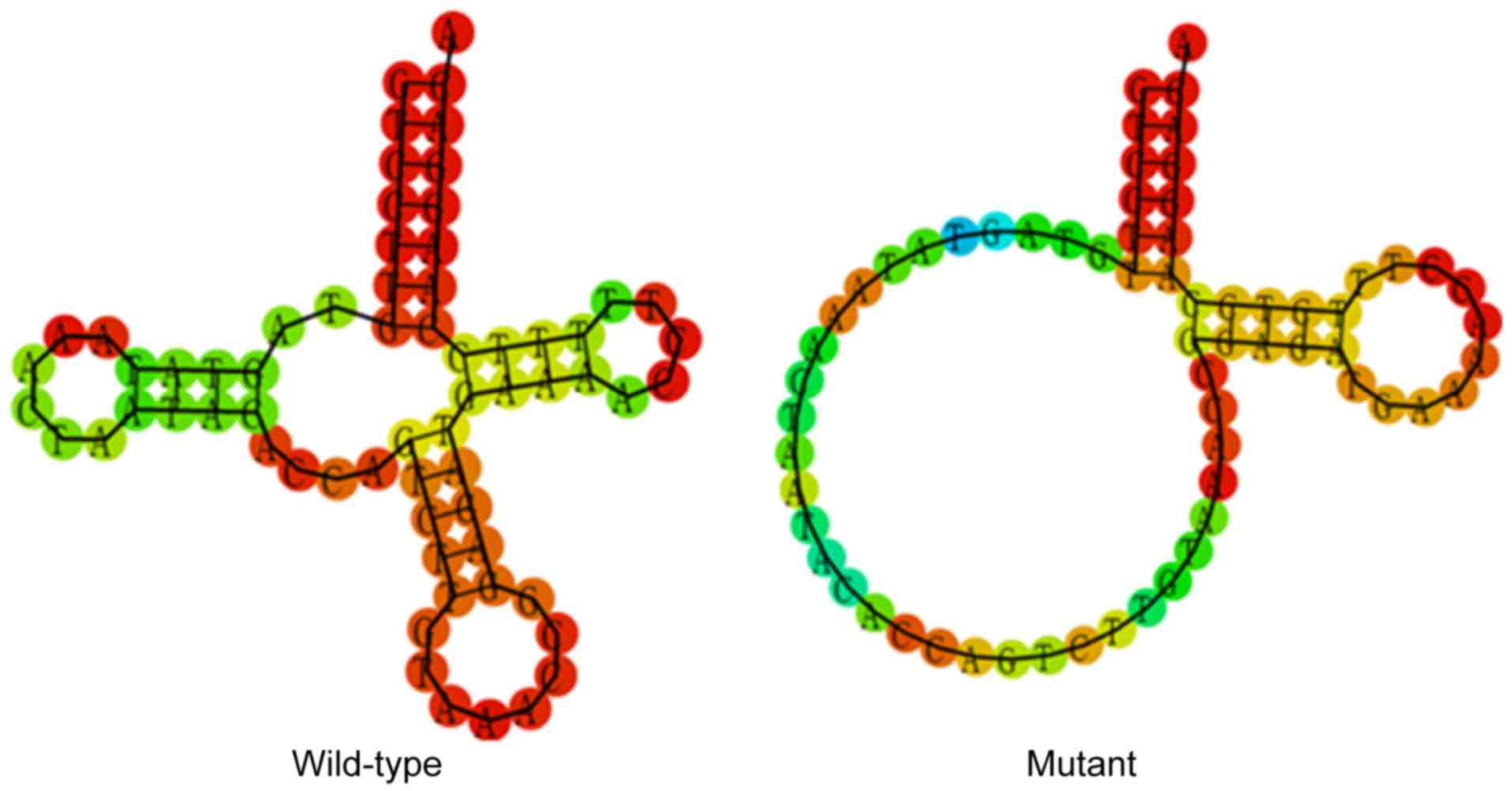
T15943C mutation affects the secondary
structure of tRNAThr
To evaluate the effect of T15943C mutation on
tRNAThr, a bioinformatics tool was used to predict the
tRNAThr secondary structure with and without this
mutation (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi)
(17). As indicated in Fig. 4, the T15943C mutation notably altered
the tRNAThr secondary structure, strongly indicating
that this mutation was pathogenic.
Discussion
The current study screened the occurrence of
mitochondrial A1555G mutation in pediatric subjects with hearing
loss. As early as 1993, the A1555G mutation was reported in an
Arab-Israeli family with non-syndromic hearing impairment (4). In fact, this mutation occurred in the
A-site of 12S rRNA gene, which was strongly conserved between
multiple species and was critical for the binding of AmAn (21,22).
Therefore, patients carrying A1555G mutation were more vulnerable
to developing hearing loss when using AmAn.
Using PCR and direct sequencing, 5 infants with
hearing loss were identified to be carrying the A1555G mutation in
the current study. Its frequency was 1.67% in patients, but the
mutation was not detected in the control subjects. The frequency of
the A1555G mutation varies worldwide. For patients with hearing
loss who used AmAn, the frequency of the A1555G mutation was
reported to be 33% in a Japanese population (23), whereas the incidence was reported to
be 13, 10.4 and 5% in three Han Chinese populations (24–26).
These data are important, since children who carry this mutation
should not be exposed to AmAn. Thus, screening for the occurrence
of the A1555G mutation is advisable, particularly for those who
have a family history of using AmAn.
In the current study, 5 infants with hearing loss
were identified to be carrying the homoplasmic A1555G mutation.
However, only 1 of these patients had an obvious family history of
using AmAn. Following comprehensive genetic counseling, it was
identified that the family members only exhibited the deafness
phenotype, without other common diseases, including type 2 diabetes
mellitus, cardiovascular events and neurological disorders. In
addition, the pedigree manifested a high expressivity of deafness.
PCR-Sanger sequencing was performed to identify the occurrence of
the homoplasmic A1555G mutation, together with a further 22 genetic
variations belonging to the East Asian haplogroup C (20). Among these, the T15943C mutation in
tRNAThr was of particular interest. This mutation was
identified in this Chinese family with hearing loss (Patients: I-2,
II-1, II-5, II-8, II-10, III-9, III-10 and III-12) but absent in
100 healthy subjects. In addition, this mutation occurred at an
evolutionary conserved position of tRNAThr (conventional
position 63), which could disrupt 63T-55A base pairing and may
result in failures in tRNA metabolism. In fact, RNA Fold results
also indicated that this mutation caused a structural change of
tRNAThr. Thus, it was speculated that the T15943C
mutation affects tRNAThr function via the alteration of
its secondary structure.
The phenotypic variability of matrilineal relatives
within the pedigree suggested that nuclear genes or mtDNA genetic
modifiers may serve key functions in deafness expression. For
example, the TRMU A10S variant has previously been reported
to increase the penetrance and expressivity of deafness-associated
mitochondrial A1555G mutation (15).
Mutations in the GJB2 gene have been regarded as important
causes for non-syndromic hearing loss (14). However, an absence of functional
sequence variants in TRMU and GJB2 genes in the
current study indicated that these genes may not play active roles
in deafness expression. Hence, other unidentified nuclear genes may
be involved in the clinical manifestation of deafness in this
pedigree.
In conclusion, the tRNAThr T15943C may be
a secondary mutation for the phenotypic expression of mitochondrial
deafness, and should be regarded as a risk factor for hearing
impairment. Furthermore, screening for the presence of
mitochondrial A1555G mutation is advisable, particularly for those
who have a family history of using AmAn. The primary limitation of
this study was the small sample size; further studies including
larger cohorts of patients with hearing impairment should be
performed in the future.
Acknowledgements
The authors would like to thank the patients and
control subjects for participating in the present study. They would
also like to thank the members in our otolaryngology department for
discussing the project and responding to reviewer's comments.
Funding
The present study was supported by Fujian Provincial
Clinical Priority Specialty Construction Project [Fujian Medical
Health (2015) 593].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW, RL and JC designed the project, YC, MY and QW
performed the experiments and LW wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics of
Fujian Provincial Hospital (Fuzhou, China) and all
parents/guardians of participants provided written informed consent
to participate.
Consent for publication
The patients' parents provided written informed
consent for the publication of any associated data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morton ME: Genetic epidemiology of hearing
impairment. Ann NY Acad Sci. 630:16–31. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bitner-Glindzicz M, Rahman S, Chant K and
Marlow N: Gentamicin, genetic variation and deafness in preterm
children. BMC Pediatr. 14:662014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fischel-Ghodsian N: Genetic factors in
aminoglycoside toxicity. Pharmacogenomics. 6:27–36. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prezant TR, Agapian JV, Bohlman MC, Bu X,
Oztas S, Qiu WQ, Arnos KS, Cortopassi GA, Jaber L, Rotter JI, et
al: Mitochondrial ribosomal RNA mutation associated with both
antibiotic induced and non-syndromic deafness. Nat Genet.
4:289–294. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li R, Greinwald JH Jr, Yang L, Choo DI,
Wenstrup RJ and Guan MX: Molecular analysis of mitochondrial 12S
rRNA and tRNASer(UCN) genes in paediatric subjects with
non-syndromic hearing loss. J Med Genet. 41:615–620. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neefs JM, Van de Peer Y, De Rijk P, Goris
A and De Wachter R: Compilation of small ribosomal subunit RNA
sequences. Nucleic Acids Res. 19 Suppl:S1987–S2015. 1991.
View Article : Google Scholar
|
|
7
|
O'Connor M, Thomas CL, Zimmermann RA and
Dahlberg AE: Decoding fidelity at the ribosomal A and P sites:
Influence of mutations in three different regions of the decoding
domain in 16S rRNA. Nucleic Acids Res. 25:1185–1193. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zwieb CD, Jemiolo DK, Jacob WF, Wagner R
and Dahlberg AE: Characterization of a collection of deletion
mutants at the 3′ end of 16S ribosomal RNA of Escherichia
coli. Mol Gen Genet. 203:256–264. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qian Y and Guan MX: Interaction of
aminoglycosides with human mitochondrial 12S rRNA carrying the
deafness-associated mutation. Antimicrob Agents Chemother.
53:4612–4618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guan MX, Fischel-Ghodsian N and Attardi G:
Biochemical evidence for nuclear gene involvement in phenotype of
non-syndromic deafness associated with mitochondrial 12S rRNA
mutation. Hum Mol Genet. 5:963–971. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rieder MJ, Taylor SL, Tobe VO and
Nickerson DA: Automating the identification of DNA variations using
quality-based fluorescence re-sequencing: Analysis of the human
mitochondrial genome. Nucleic Acids Res. 26:967–973. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Andrews RM, Kubacka I, Chinnery PF,
Lightowlers RN, Turnbull DM and Howell N: Reanalysis and revision
of the Cambridge reference sequence for human mitochondrial DNA.
Nat Genet. 23:1471999. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruiz-Pesini E and Wallace DC: Evidence for
adaptive selection acting on the tRNA and rRNA genes of human
mitochondrial DNA. Hum Mutat. 27:1072–1081. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai ZY, Sun BC, Huang SS, Yuan YY, Zhu YH,
Su Y and Dai P: Correlation analysis of phenotype and genotype of
GJB2 in patients with non-syndromic hearing loss in China. Gene.
570:272–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guan MX, Yan Q, Li X, Bykhovskaya Y,
Gallo-Teran J, Hajek P, Umeda N, Zhao H, Garrido G, Mengesha E, et
al: Mutation in TRMU related to transfer RNA modification modulates
the phenotypic expression of the deafness-associated mitochondrial
12S ribosomal RNA mutations. Am J Hum Genet. 79:291–302. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding Y, Li Y, You J, Yang L, Chen B, Lu J
and Guan MX: Mitochondrial tRNA(Glu) A14693G variant may modulate
the phenotypic manifestation of deafness-associated 12S rRNA A1555G
mutation in a Han Chinese family. J Genet Genomics. 36:241–250.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gruber AR, Lorenz R, Bernhart SH, Neuböck
R and Hofacker IL: The vienna RNA website. Nucleic Acids Res.
36:(Web Server Issue). W70–W74. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bibb MJ, Van Etten RA, Wright CT, Walberg
MW and Clayton DA: Sequence and gene organization of mouse
mitochondrial DNA. Cell. 26:167–180. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gadaleta G, Pepe G, De Candia G,
Quagliariello C, Sbisà E and Saccone C: The complete nucleotide
sequence of the Rattus norvegicus mitochondrial genome:
Cryptic signals revealed by comparative analysis between
vertebrates. J Mol Evol. 28:497–516. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roe A, Ma DP, Wilson RK and Wong JF: The
complete nucleotide sequence of the Xenopus laevis
mitochondrial genome. J Biol Chem. 260:9759–9774. 1985.PubMed/NCBI
|
|
21
|
Purohit P and Stern S: Interactions of a
small RNA with antibiotic and RNA ligands of the 30S subunit.
Nature. 370:659–662. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamasaki K and Rando RR: Specific binding
of aminoglycosides to a human rRNA construct based on a DNA
polymorphism, which causes aminoglycoside-induced deafness.
Biochemistry. 36:12323–12328. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Usami S, Abe S, Akita J, Namba A, Shinkawa
H, Ishii M, Iwasaki S, Hoshino T, Ito J, Doi K, et al: Prevalence
of mitochondrial gene mutations among hearing impaired patients. J
Med Genet. 37:38–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hutchin T, Haworth I, Higashi K,
Fischel-Ghodsian N, Stoneking M, Saha N, Arnos C and Cortopassi G:
A molecular basis for human hypersensitivity to aminoglycoside
antibiotics. Nuclear Acids Res. 21:4174–4179. 1993. View Article : Google Scholar
|
|
25
|
Li Z, Li R, Chen J, Liao Z, Zhu Y, Qian Y,
Xiong S, Heman-Ackah S, Wu J, Choo DI and Guan MX: Mutational
analysis of the mitochondrial 12S rRNA gene in Chinese pediatric
subjects with aminoglycoside-induced and non-syndromic hearing
loss. Hum Genet. 117:9–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu J, Li Z, Zhu Y, Yang A, Li R, Zheng J,
Cai Q, Peng G, Zheng W, Tang X, et al: Mitochondrial 12S rRNA
variants in 1642 Han Chinese pediatric subjects with
aminoglycoside-induced and nonsyndromic hearing loss.
Mitochondrion. 10:380–390. 2010. View Article : Google Scholar : PubMed/NCBI
|