Introduction
The anterior cruciate ligament (ACL) is an important
stabilizing structure of the knee, and ACL injury is listed as one
of the most common injuries in sports medicine; >120,000 ACL
reconstruction (ACLR) surgeries are reportedly performed annually
in the United States alone (1).
Early rehabilitation and return to sports following an operation
are considered core objectives following an ACLR. To reach these
targets, graft and bone healing is crucial in the postoperative
course; therefore, various studies have been conducted under the
scope of graft and bone healing processes (2–4).
In clinical studies, the graft and bone healing
condition is primarily evaluated indirectly via physical
examination for ligament stability (e.g. Lachman test or
pivot-shift test (5) and return to
sports capability (e.g. single-leg hop test, and isokinetic
evaluation (6), subjective
functional scoring scales (e.g. International Knee Documentation
Committee (IKDC) Knee Ligament Evaluation Form (7) and a personal questionnaire regarding
patients' return to sports, activity frequency and condition.
Radiographic assessments are typically performed in the form of
bilateral weight-bearing X-ray scans based on the IKDC knee
arthritis grading system (8). Recent
studies have utilized a magnetic resonance imaging (MRI) scan to
evaluate graft maturity; this approach may illustrate graft and
bone healing conditions at each time point more clearly (9–11). For
animal studies, histological examination is thought to be the most
suitable method in the evaluation of graft bone healing processes.
Specimens are typically harvested, fixed, embedded, sectioned and
prepared for multiple staining protocols to evaluate different
components, and graft and bone healing conditions are revealed
clearly in slides under microscopy. However, a number of
limitations are also present with histological examination
modalities, as histology studies analyze graft and bone healing in
segments, focusing more on the regional healing condition and
failing to elaborate on the entire graft bone healing result.
Micro-MRI has previously been introduced in
preclinical studies in orthopedics research (12,13). Due
to its superiority in high spatial resolution, high soft tissue
contrast and visualizing tendons, ligaments, cartilage, menisci and
other non-osseous tissues directly, micro-MRI with a high field
coil has been applied in a variety of osteoporosis, osteoarthritis
and cartilage lesion animal studies (14,15).
Disease progression and cartilage derogation have been identified
in animal models, including mouse, rat, rabbit and dog models
(14–16). In the study of ACL, however, few
studies have been performed with micro-MRI scanning as a possible
method for evaluating graft bone healing processes. This may be
associated with difficulties in accessing devices or lack of
previous experience.
In the present study, high-field micro-MRI was
performed on animal models that underwent ACLR surgery at different
time points following surgery to assess progressive changes in
tunnel diameter and MRI signal noise ratio in graft and bone
structures. It was hypothesized that high-field micro-MRI scanning
may provide additional information on graft bone healing processes,
thus serving as a promising supplementary method in graft and bone
healing evaluations following ACLR surgery during preclinical
studies.
Materials and methods
Animal ACLR model
The present animal study was reviewed and approved
by the Animal Experiment Ethics Committee of the College of
Pharmacy, Fudan University (Shanghai, China). A total of 12
skeletally mature male New Zealand white rabbits (age, 1 year;
weight, 3.4±0.4 kg) were provided by the Animal Efficacy Evaluation
Center, College of Pharmacy, Fudan University (Shanghai, China).
Rabbits were housed at 20°C with a 40–60% humidity under a 16/8 h
light/dark cycle. Animals also received ad libitum access to
food and water. Following 2 weeks of quarantine, ACLR surgeries
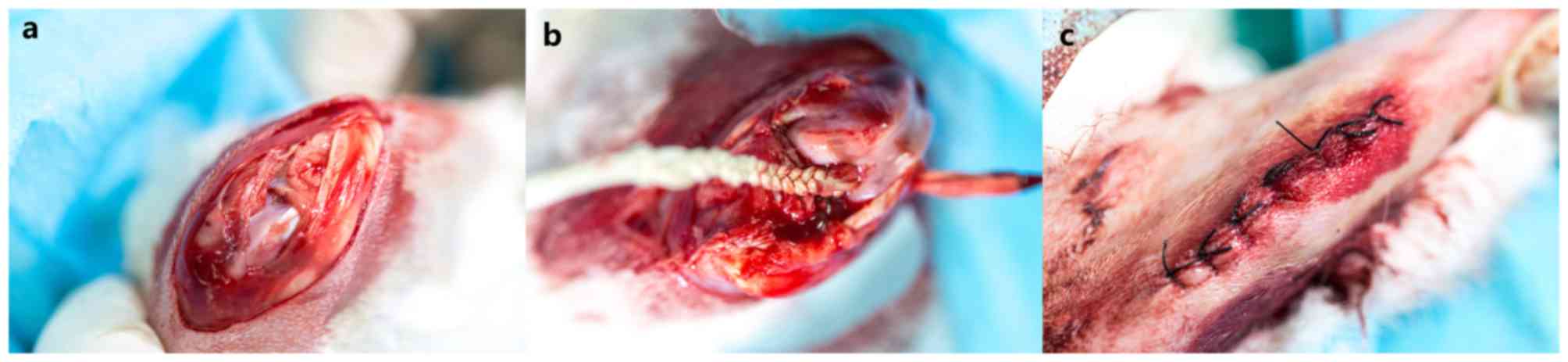
were performed on the rabbits' right legs. Briefly, the rabbits
were fixed on the surgery table in a supine position following
general anesthesia with intravenous administration of 3%
pentobarbital (30 mg/kg body weight; Shanghai Wokai; Sinopharm
Chemical Reagent Co., Ltd. Shanghai, China). A medial parapatellar
incision was made, and the native ACL was exposed and transected.
Joint laxity was confirmed by a Lachman test following removing the
native ACL. A 3.0-mm diameter Kirschner wire was used to drill the
bone tunnels from the ACL footprints and remnants towards the
femoral and tibial insertion sites of the ACL. The polyethylene
terephthalate (PET) ligament fabricated prior to surgery was fed
through the tunnel with the help of a PDS II wire (Ethicon, Inc.,
Cincinnati, OH, USA). The femoral and tibial ends of the ligament
were fixed with the periosteum adjacent soft tissue. Following
graft transplantation, consecutive cycling loads were performed 20
times to ensure the ligaments were functional. Then, the wounds
were sutured in layers (Fig. 1).
Postoperatively, rabbits were returned to their cages and allowed
free cage activity without immobilization. Intramuscular
prophylactic antibiotic injections (800,000 IU penicillin) and
wound cleaning were performed immediately following the operation
and once daily for 3 consecutive days.
Rabbits were euthanized via administration of 3%
pentobarbital (100 mg/kg) in the auricular vein at 4, 8 and 16
weeks following surgery and the femur-graft-tibia complex specimens
(n=4 at each time point) were harvested immediately from the
rabbits following sacrifice. Soft tissues around the complex were
well preserved.
Micro-MRI scan and data analysis
In all rabbit specimens, MRI examinations were
performed with a 7.1T Biospec 70/20R MRI magnet (Bruker AXS GmbH,
Karlsruhe, Germany). An automatic shimming process was performed at
the beginning of each scan to homogenize the magnetic field.
Specimens were placed supine on the animal bed with a 38-mm
transmit-receive cylindrical birdcage radio frequency coil fixed to
it. A two-dimensional T2-TurboRare sequence (repetition time, 3,500
ms; echo time, 26 ms; flip angle, 90°; field of view, 16×16 mm;
slice thickness, 0.35 mm; gap between slices, 0 mm; acquisition
resolution, 256×256 matrix size) was used in the scanning.
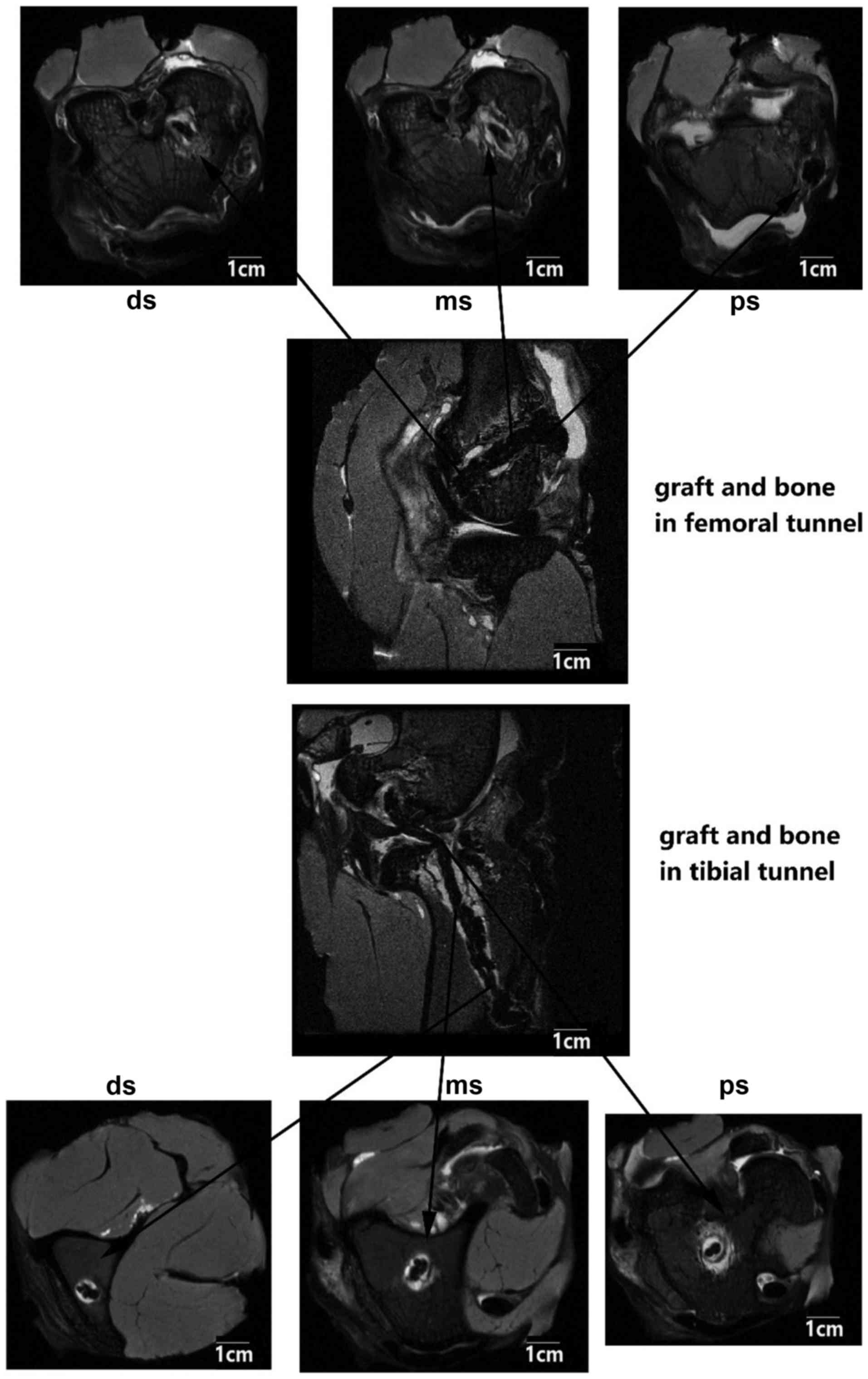
The PET ligament and bone tunnel interface distance
were measured in axial position images, and the distance was
identified as the largest diameter from the center of the ligament
fiber to the edge of the bone tunnel. The tunnel diameters at the
proximal, middle and distal sites in the bone tunnel were measured
simultaneously, both in the femoral and tibial tunnels (Fig. 2).
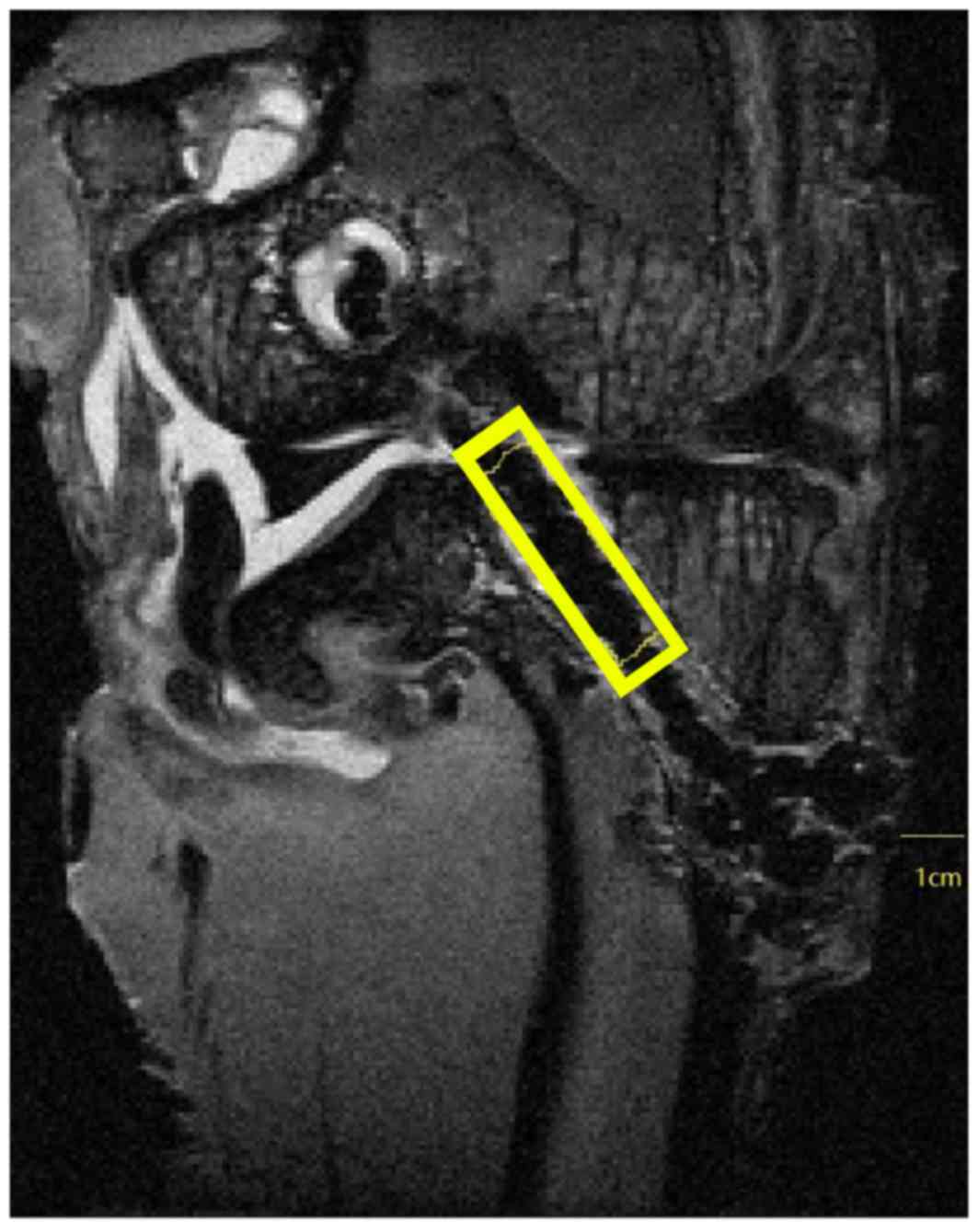
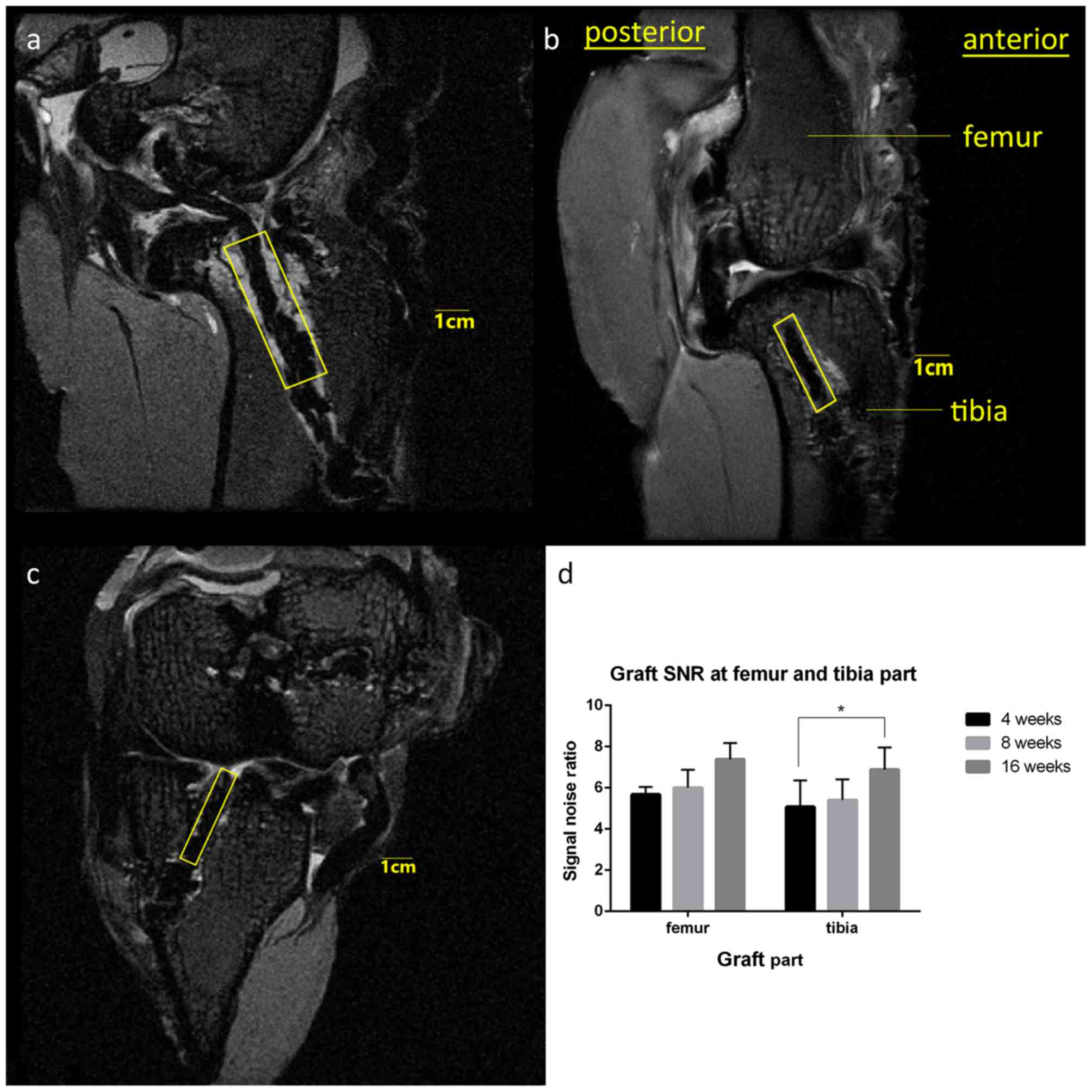
The signal noise ratio (SNR) is a measure of signal
strength in a certain area relative to background noise. SNR
analyses were performed using ImageJ 1.51e (National Institutes of
Health, Bethesda, MD, USA). Briefly, the range of interest (ROI)
was set based on the oblique sagittal plane in which the PET
ligament graft and bone tunnels were mostly linear and parallel to
each other in the longitudinal direction. The PET ligament in the
femoral bone tunnel was highlighted in a linear region with a width
of 10 mm set on the boundary of the joint surface side parallel to
Blumensaat's line. The PET ligament in the tibia bone tunnel was
highlighted in a linear region with a width of 10 mm set on the
boundary of the joint surface side parallel to the joint surface at
the opening site of the bone tunnel. The signal intensity of the
background was measured at 1 cm anterior to the tibia tuberosity.
The SNR was calculated as signal intensity in the ROI divided by
signal intensity in the background area (Fig. 3) (17). Two sports medicine surgeons who were
blinded to the sample information at the measurement moment were
requested to provide an analysis, and the mean values were regarded
as final results.
Histological examination
Bone-graft complexes were harvested from each group
at 4, 8 and 16 weeks (n=4 at each time point) following micro-MRI
and fixed in 10% formalin for 48 h at 10°C immediately prior to
histological examination. Specimens prepared for undecalcified bone
slicing were embedded in resin and sectioned with a sliding
microtome (SM2500; Leica Microsystems GmbH, Wetzlar, Germany)
perpendicular to the longitudinal axis of the femur and tibia
tunnel at a thickness of 5 µm. The sections were stained at 20 kC
for 6 h with hematoxylin-eosin to evaluate the graft, host bone and
the interface between them. Images were visualized via inverted
microscopy (IX71SBF2; Olympus Corporation, Tokyo, Japan) and
captured with DP72 Manager (Olympus Corporation).
Statistical analysis
Statistical analysis was performed using SPSS 22.0
(IBM Corp., Armonk, NY, USA). Data are presented as the mean ±
standard deviation. One-way analysis of variance with Fisher's
Least Significant Difference was used to analyze the mean diameter
between the graft and bone tunnel in the femoral and tibial
tunnels, and the mean SNR at the femur and tibia. P<0.05 was
considered to indicate a statistically significant difference.
Results
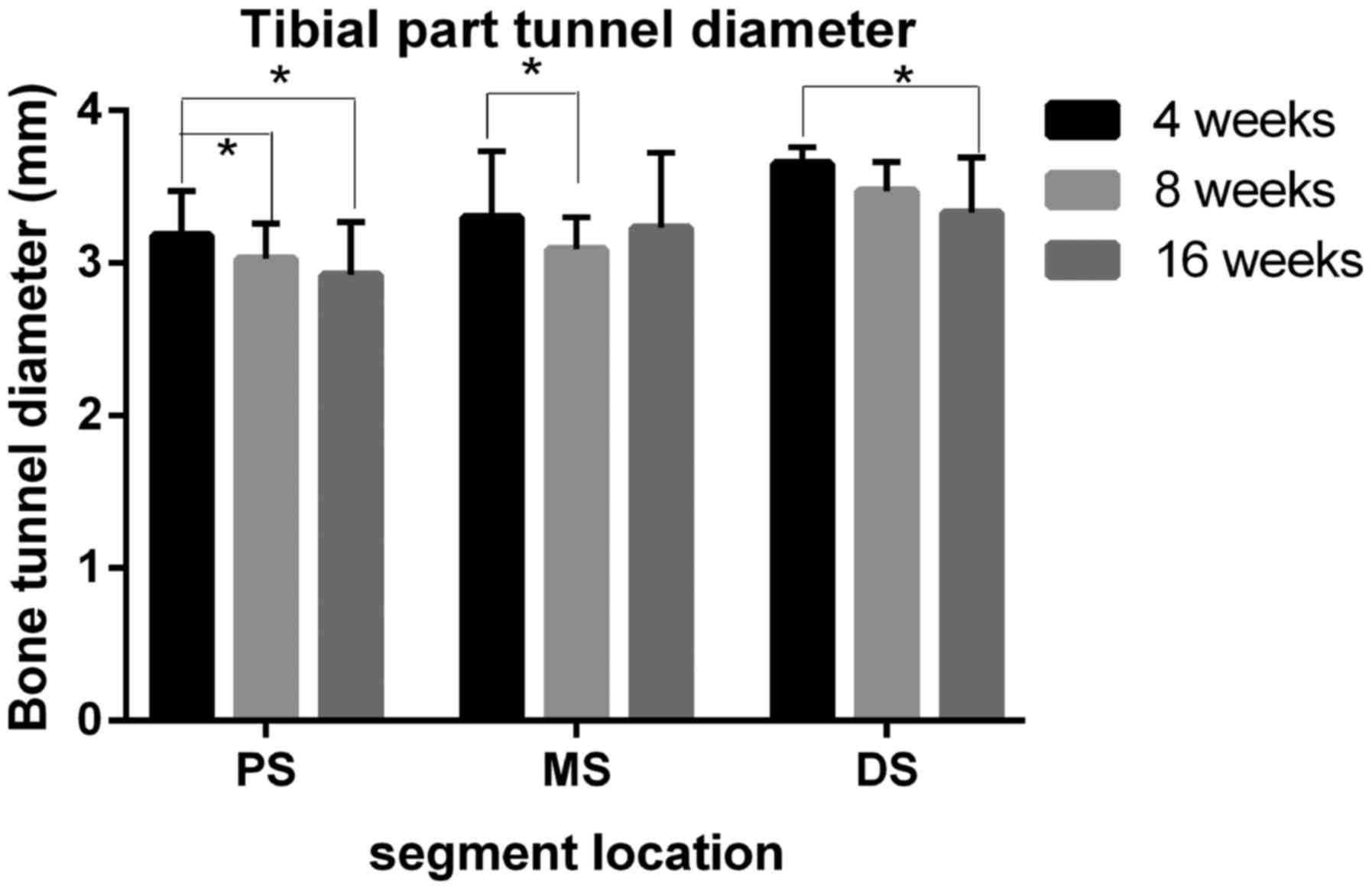
Bone tunnel diameter measurements
The bone tunnel diameter at the graft tunnel
interface site was largest at 4 weeks following surgery (femoral
part, proximal site=3.55±0.18 mm, middle site=3.33±0.41 mm and
distal site=3.20±0.41 mm; Fig. 4;
tibial part, proximal site=3.15±0.41 mm, middle site=3.68±0.43 mm,
and distal site: 3.80±0.19 mm; Fig.
5). Compared with samples at 4 weeks, the size was decreased in
the samples collected at 8 and 16 weeks following surgery. At 8
weeks, the tunnel diameter at the middle site in the femoral part
and at the proximal site in the tibial part showed significant
shrinkage when compared with the results at the same site at 4
weeks (P<0.05). At 16 weeks following surgery, all 6 measuring
sites, except the tibial medial site, in the tibial tunnel reported
significantly decreased tunnel diameters in comparison with the
4-week results (P<0.05). It is worthwhile to note that in
certain measuring sites (proximal, middle and distal site of the
femur part and proximal site of the tibia part;
diameters=2.93±0.69, 2.88±0.40, 2.58±0.33 and 2.92±0.35 mm,
respectively), the tunnel diameter was smaller than the diameter of
the Kirschner wire used in tunnel drilling at 16 weeks following
surgery.
SNR analysis
SNR analysis revealed that both the femoral and
tibial PET ligaments selected in the ROI exhibited a marked
increase in SNR from the initial 4-week results (femoral at 4
weeks, 5.68±0.36; tibial at 4 weeks, 5.08±1.28). However,
statistical significance was only detected in the comparison
between 16 and 4 weeks in the SNR value in the tibia (P=0.0464;
Fig 6).
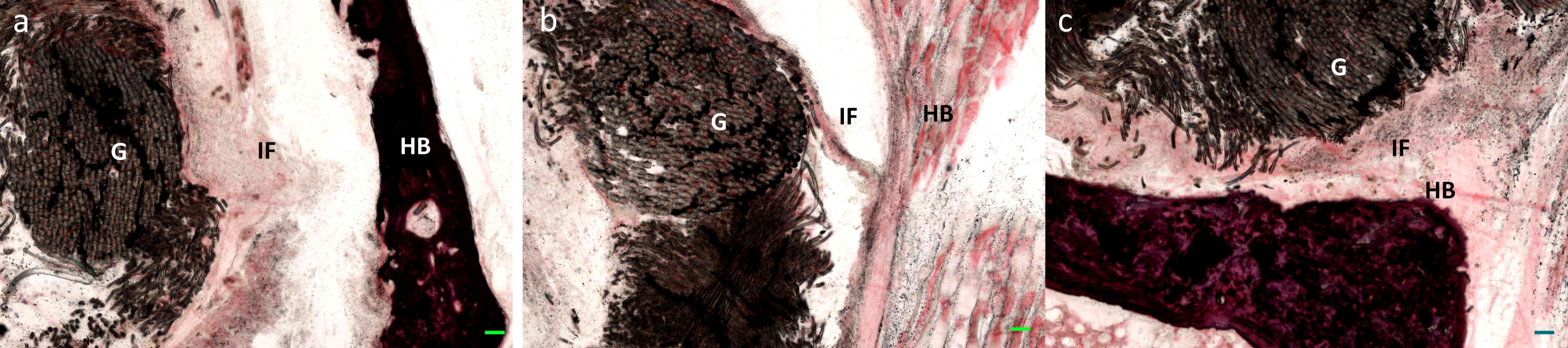
Evaluation of graft bone
interfaces
As presented in Fig.
7, the interface between host bone and the PET ligament graft
was notable at 4 weeks following surgery. At 8 weeks following
animal surgery, the gap interface had shrank but remained visible
via hematoxylin-eosin staining. At 16 weeks, the PET graft was
surrounded with ingrowth bony tissue and the interface was markedly
smaller than that observed at 4 weeks.
Discussion
The graft and bone healing processes following
surgery heavily affect the therapeutic effects as well as patients'
abilities to return to sports, which has always been emphasized in
sports medicine practice (1). In the
hope of accelerating the ligamentation process, various methods
have been applied in preclinical studies (18,19). To
analyse the association between those enhancement solutions and
graft bone healing processes, daily observation, gross observation,
anatomic study, biomechanics test, histology, and medical imaging
were included in the present study. New Zealand Rabbits are
frequently utilized in animal experimental research. The size and
morphological similarity to human knee joint made the animal an
appropriate choice in ACL animal studies. ACLR surgery has also
become a common choice for the treatment of ACL injury.
Daily observation, though not performed in the
present study due to resource limitation, may be carried out prior
to and following the ACLR surgery. Items listed in the daily
observation include, but are not limited to, the animal's weight,
hair color, wound healing condition, knee joint flexion and
extension angle regarding range of motion and active activity level
(19). These indicators partially
reflect the animal's quality of life following surgery and, to a
certain extent, the graft functions in daily movement. However,
difficulties in quantitative analysis with the majority of these
indicators impede further progress of indicator analysis (20). The most frequently used assessment
system of ACLR may be the adjusted Oswestry Arthroscopy Score (OAS)
on a macroscopic assessment. Items listed on the evaluation form
include levels at the insertion site with the surrounding
cartilage, integration at the insertion site with the surrounding
tissue, stiffness of the tendon graft, appearance of articular
surface and color of the articular surface, with a total score of
10 (21). Gross evaluation provides
investigators with an initial semi-quantitative analysis of the
samples and set a rational foundation for further study. Cheng
et al (18) recently used the
OAS in the assessment of fresh specimens harvested from ACLR rabbit
models. As an assessment scale output results in ordinal data, some
evaluation bias is inevitable due to results at boundaries between
the two classes. Anatomic study following the sample harvest
provides an opportunity to further understand general anatomical
structure following graft transplantation and its association with
structures adjacent to it (22,23).
Like the daily observation, those studies failed to offer
quantitative data for assessment. Molecular biology study refers to
the testing of certain biomedical markers associated with ligament
integration processes based on the serum or synovium extracted
during or following caging (24–26). By
conducting molecular biology study, researchers may determine
whether certain enhancement methods induce a concentration rise in
certain growth factors and proteins that have been renowned for
their cartilage or bone incorporation capacity. Functional
biological networks, however, should not be neglected, as it is
believed that signals and factors function through networks instead
of a simple downstream signal or factor. Thus, a confounding bias
should be noted. Biomechanical testing has been emphasized in
recent years in ACLR research such as testing dynamic stress loads
and stiffness with certain fixing devices including a Biopuls
prosthetic limb fixture test and a three-point bending/breaking
device (27,28). The boundedness of biomechanical
testing is a result of the improper design and use of a fixation
device at the beginning of a test. Certain vehicles, including
prosthetic limb fixture testing devices may fail to stretch the
knee joint in a functional way, resulting in misleading data
(27,28). Histology study is crucial in ACLR
preclinical study. Slides stained with hematoxylin and eosin,
toluidine blue, Masson's trichrome stain, and safranin O help
researchers observe grafts, newly born bone, graft-bone interface
and types of collagen specifically and clearly (29–32).
Examination via histology also has some disadvantages. The
mechanical and biological properties of the whole ACL structure are
evaluated using localized sections. Furthermore, tissue wastage
during slicing hinders continuous observation of the graft bone
complex.
Imaging study methods used previously in ACLR
preclinical study primarily involve X-ray and micro computed
tomography (micro-CT) scans. The former is beneficial in knee joint
arthritis detection based on the Kellgren and Lawrence system and
IKDC scale, offering second-hand ACL graft healing information
(29). Micro-CT scan has been used
in the study of bone architecture and mineral density during
preclinical investigations of osteoporosis in several animal models
(31,32). High contrast quality enables a
non-invasive method in evaluating changes in bone stereology and
bone trabecular structure following ACLR (30,31).
However, the ligament grafted in the surgery is not markedly
developed under screening as ligament grafts are typically made
with materials that are low density and are close to the air
background.
Micro-MRI is a novel study solution tool in ACLR
preclinical studies. With the use of small high-efficiency coils
and high field imagery, micro-MRI is superior in soft tissue
imaging. In osteoarthritis animal models, non-invasive quantitative
high field micro-MRI is more specific for quantitatively detecting
menisci and cartilage lesions at different stages when compared
with the results used with common 3T MRI devices (11,16,30).
With the use of micro-MRI, the present study
demonstrated that graft tunnel diameters decreased over time
following ACLR surgery. The preliminary result was rationalized as
a micro-bone fracture due to drilling in the tunnel formation,
which was unavoidable, and the following callus formation and
remodeling are natural courses for fracture healing, resulting in
decreases in the diameters. On further examination, however, at 16
weeks following surgery it was observed that the diameter of the
whole femoral and partial tibial parts were <3 mm, which was the
diameter of the Kirschner wire used in drilling. A difference of
0.4 mm between the observed and estimated diameter at the distal
site in the femoral part may be attributed to bone incorporation
from the surrounding area, as reported recently in the use of
micro-CT scanning on ACLR in animal models (2,30).
Inward growth decreased the graft bone interference distance in
micro-scale assessment. An alternative explanation is required for
the variance in diameters among proximal, middle and distal sites
in both femoral and tibial parts. This is primarily due to the
technique used in drilling the tunnel. The tunnel was initially
drilled from the native ACL remnants within the knee joint towards
femoral and tibial insertion sites. To feed the PET ligament
through the tunnel, extra drilling was performed to expand the
tunnel size at the femoral and tibial sites of the tunnel. SNR
analysis provided another perspective in evaluating graft
conditions following ACLR. The graft used in ACLR was set to
present a relatively fixed signal intensity. The rise in SNR
between 4 and 16 weeks following surgery in both femoral and tibial
parts of the PET ligament graft indicated potential tissue growth
on the surface or inside the ligament graft, which is consistent
with the aforementioned decreases in diameters. Based on the
hematoxylin-eosin staining results, it was demonstrated that the
results were consistent with micro-MRI findings at 4, 8 and 16
weeks. The interface decreased over time, which was in accordance
with micro-MRI findings. These findings suggest that micro-MRI is
an effective tool and possible supplement to the histology,
biomechanical study, gross evaluation, and micro-CT scans in the
ACLR preclinical research field.
However, there are several limitations to the
present study. First, a small sample size of 12 New Zealand white
rabbits was investigated. Further studies with larger sample
amounts are required for better analysis of micro-MRI. Second, due
to the limitation of the coil size, in vivo micro-MRI
scanning, which is accessible for rats and mice in preclinical
study, was not available in the current study, as reported above.
With in vivo observation, a single knee joint may be
continuously observed at different time points. Despite the short
interval between euthanasia, harvesting and micro-MRI scanning, the
in vitro micro-MRI scan may not fully reflect the true
structural circumstance inside the knee joint. A more suitable MRI
coil may solve this problem. Third, a further limitation is the
imaging of the graft bone complex samples with micro-MRI. The
T2-Turborare Sequence used in the present study may detect the
ligament and cartilage lesion more easily than T1 sequences, but
specific scan protocols, such as T2 mapping, fat saturated gradient
echo, and 3D gradient echo, may be more feasible in subsequent
research on ACLR preclinical studies (31,32).
Furthermore, CT scanning or Micro-CT scanning would be helpful in
tunnel diameter evaluation in our future study.
The present study applied micro-MRI scans in an
animal model that underwent ACLR surgery with a PET ligament, and
determined that micro-MRI is promising in quantitatively observing
graft bone healing processes directly, with a focus on graft tunnel
distances and the SNR.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National 863 Hi-Tech Project (grant no. 2015AA033703), the National
Natural Science Foundation of China (grant nos. 81271958, 81572108
and 81370052) and the Specialized Research Fund for the Doctoral
Programme of Higher Education (grant no. 20120071110067).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FC and FW performed the experiment and wrote the
manuscript. JJ conceived the idea and designed the study with SC.
In addition, SC reviewed and rewrote the manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Animal Experiment Ethics Committee of the College of Pharmacy,
Fudan University (Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mall NA, Chalmers PN, Moric M, Tanaka MJ,
Cole BJ, Bach BR Jr and Paletta GA Jr: Incidence and trends of
anterior cruciate ligament reconstruction in the United States. Am
J Sports Med. 42:2363–2370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong S, Huangfu X, Xie G, Zhang Y, Shen P,
Li X, Qi J and Zhao J: Decellularized versus Fresh-frozen
allografts in anterior cruciate ligament reconstruction: An in
vitro study in a rabbit model. Am J Sports Med. 43:1924–1934. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuang GM, Yau WP, Lu WW and Chiu KY: Use
of a strontium-enriched calcium phosphate cement in accelerating
the healing of soft-tissue tendon graft within the bone tunnel in a
rabbit model of anterior cruciate ligament reconstruction. Bone
Joint J. 95-B:923–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsumoto T, Kubo S, Sasaki K, Kawakami Y,
Oka S, Sasaki H, Takayama K, Tei K, Matsushita T, Mifune Y, et al:
Acceleration of tendon-bone healing of anterior cruciate ligament
graft using autologous ruptured tissue. Am J Sports Med.
40:1296–1302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katz JW and Fingeroth RJ: The diagnostic
accuracy of ruptures of the anterior cruciate ligament comparing
the Lachman test, the anterior drawer sign, and the pivot shift
test in acute and chronic knee injuries. Am J Sports Med. 14:88–91.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Undheim MB, Cosgrave C, King E, Strike S,
Marshall B, Falvey É and Franklyn-Miller A: Isokinetic muscle
strength and readiness to return to sport following anterior
cruciate ligament reconstruction: Is there an association? A
systematic review and a protocol recommendation. Br J Sports Med.
49:1305–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hefti F, Müller W, Jakob RP and Stäubli
HU: Evaluation of knee ligament injuries with the IKDC form. Knee
Surg Sports Traumatol Arthrosc. 1:226–234. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Meer BL, Meuffels DE, van Eijsden WA,
Verhaar JA, Bierma-Zeinstra SM and Reijman M: Which determinants
predict tibiofemoral and patellofemoral osteoarthritis after
anterior cruciate ligament injury? A systematic review. Br J Sports
Med. 49:975–983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Tao H, Cho S and Chen S, Yao Z and
Chen S: Difference in graft maturity of the reconstructed anterior
cruciate ligament 2 years postoperatively: A comparison between
autografts and allografts in young men using clinical and 3.0-T
magnetic resonance imaging evaluation. Am J Sports Med.
40:1519–1526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Packer JD, Bedi A, Fox AJ, Gasinu S,
Imhauser CW, Stasiak M, Deng XH and Rodeo SA: Effect of immediate
and delayed high-strain loading on tendon-to-bone healing after
anterior cruciate ligament reconstruction. J Bone Joint Surg Am.
96:770–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wachsmuth L, Lindhorst E, Wrubel S,
Hadzhiyski H, Hudelmaier M, Eckstein F and Chrubasik S:
Micro-morphometrical assessment of the effect of Harpagophytum
procumbens extract on articular cartilage in rabbits with
experimental osteoarthritis using magnetic resonance imaging.
Phytother Res. 25:1133–1140. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lan SM, Wu YN, Wu PC, Sun CK, Shieh DB and
Lin RM: Advances in noninvasive functional imaging of bone. Acad
Radiol. 21:281–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wehrli FW: Structural and functional
assessment of trabecular and cortical bone by micro magnetic
resonance imaging. J Magn Reson Imaging. 25:390–409. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boulocher C, Chereul E, Langlois JB,
Armenean M, Duclos ME, Viguier E, Roger T and Vignon E:
Non-invasive in vivo quantification of the medial tibial cartilage
thickness progression in an osteoarthritis rabbit model with
quantitative 3D high resolution micro-MRI. Osteoarthritis
Cartilage. 15:1378–1387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Libicher M, Ivancic M, Hoffmann M and Wenz
W: Early changes in experimental osteoarthritis using the Pond-Nuki
dog model: Technical procedure and initial results of in vivo MR
imaging. Eur Radiol. 15:390–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bolbos R, Benoit-Cattin H, Langlois JB,
Chomel A, Chereul E, Odet C, Pastoureau P, Janier M and Beuf O:
Knee cartilage thickness measurements using MRI: A 4(1/2)-month
longitudinal study in the meniscectomized guinea pig model of OA.
Osteoarthritis Cartilage. 15:656–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanamura H, Arai Y, Hara K, Takahashi T,
Ikoma K, Fujiwara H, Minami G, Terauchi R, Nakagawa S, Honjo K and
Kubo T: Quantitative evaluation of revascularization at bone
tunnels and grafts with contrast-enhanced magnetic resonance
angiography after anterior cruciate ligament reconstruction. Int
Orthop. 40:1531–1536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng P, Han P, Zhao C, Zhang S, Wu H, Ni
J, Hou P, Zhang Y, Liu J, Xu H, et al: High-purity magnesium
interference screws promote fibrocartilaginous entheses
regeneration in the anterior cruciate ligament reconstruction
rabbit model via accumulation of BMP-2 and VEGF. Biomaterials.
81:14–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma R, Ju X, Deng XH and Rodeo SA: A novel
small animal model of differential anterior cruciate ligament
reconstruction graft strain. J Knee Surg. 28:489–495.
2015.PubMed/NCBI
|
|
20
|
Kondo E, Yasuda K, Katsura T, Hayashi R,
Kotani Y and Tohyama H: Biomechanical and histological evaluations
of the doubled semitendinosus tendon autograft after anterior
cruciate ligament reconstruction in sheep. Am J Sports Med.
40:315–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goebel L, Orth P, Müller A, Zurakowski D,
Bücker A, Cucchiarini M, Pape D and Madry H: Experimental scoring
systems for macroscopic articular cartilage repair correlate with
the MOCART score assessed by a high-field MRI at 9.4 T-comparative
evaluation of five macroscopic scoring systems in a large animal
cartilage defect model. Osteoarthritis Cartilage. 20:1046–1055.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JK, Lee S, Seong SC and Lee MC:
Anatomy of the anterior cruciate ligament insertion sites:
Comparison of plain radiography and three-dimensional computed
tomographic imaging to anatomic dissection. Knee Surg Sports
Traumatol Arthrosc. 23:2297–2305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Thambyah A and Broom ND: A
multi-scale structural study of the porcine anterior cruciate
ligament tibial enthesis. J Anat. 224:624–633. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu SC, Cheuk YC, Yung SH, Rolf CG and Chan
KM: Systematic review of biological modulation of healing in
anterior cruciate ligament reconstruction. Orthop J Sports Med.
2:23259671145266872014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuang GM, Yau WP, Lu WW and Chiu KY:
Osteointegration of soft tissue grafts within the bone tunnels in
anterior cruciate ligament reconstruction can be enhanced. Knee
Surg Sports Traumatol Arthrosc. 18:1038–1051. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Proffen BL, Sieker JT and Murray MM:
Bio-enhanced repair of the anterior cruciate ligament. Arthroscopy.
31:990–997. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vavken P, Fleming BC, Mastrangelo AN,
Machan JT and Murray MM: Biomechanical outcomes after bioenhanced
anterior cruciate ligament repair and anterior cruciate ligament
reconstruction are equal in a porcine model. Arthroscopy.
28:672–680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gille J, Gerlach U, Oheim R, Hintze T,
Himpe B and Schultz AP: Functional outcome of septic arthritis
after anterior cruciate ligament surgery. Int Orthop. 39:1195–1201.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bi F, Shi Z, Liu A, Guo P and Yan S:
Anterior cruciate ligament reconstruction in a rabbit model using
silk-collagen scaffold and comparison with autograft. PLoS One.
10:e01259002015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wachsmuth L, Keiffer R, Juretschke HP,
Raiss RX, Kimmig N and Lindhorst E: In vivo contrast-enhanced micro
MR-imaging of experimental osteoarthritis in the rabbit knee joint
at 7.1T1. Osteoarthritis Cartilage. 11:891–902. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Batiste DL, Kirkley A, Laverty S, Thain
LM, Spouge AR and Holdsworth DW: Ex vivo characterization of
articular cartilage and bone lesions in a rabbit ACL transection
model of osteoarthritis using MRI and micro-CT. Osteoarthritis
Cartilage. 12:986–996. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chu CR, Williams AA, West RV, Qian Y, Fu
FH, Do BH and Bruno S: Quantitative magnetic resonance imaging
UTE-T2* mapping of cartilage and meniscus healing after anatomic
anterior cruciate ligament reconstruction. Am J Sports Med.
42:1847–1856. 2014. View Article : Google Scholar : PubMed/NCBI
|