Introduction
Esophageal cancer is the most common malignant tumor
of the digestive tract (1). Clinical
data has demonstrated that, worldwide, ~300,000 cases of esophageal
cancer result in mortality each year (2,3). A
previous systematic review and meta-analysis evaluated the
health-related quality of life of patients with esophageal cancer
following potentially curative treatment and results identified
that health-related quality of life of patients with esophageal
cancer is an indicator to evaluate the efficacy of anti-cancer
drugs (4). Although increasing
cancer therapy technologies continue to be investigated in clinical
studies, the five-year survival rate of patients with esophageal
cancer is lower than 12.5% (5).
Previous reports have indicated that C-X-C motif chemokines, their
cognate receptors and tumor metastasis-associated proteins are
involved in the pathogenesis of human esophageal cancer (6,7). In
addition, lymph node, lung and stomach metastases are key
prognostic indicators in late-stage esophageal cancer, with
metastatic tumor cell expansion leading to a shortened survival
rate (8–10). Therefore, the prevention and early
diagnosis of esophageal cancer may enhance the cure rate and
improve survival in patients with early-stage esophageal
cancer.
Currently, the majority of patients clinically
diagnosed with esophageal cancer are in an advanced stage of the
disease (11). Although a number of
reports have introduced various diagnostic techniques for
early-stage esophageal cancer, including Raman spectroscopy,
chemometric techniques, image-enhanced magnifying endoscopy,
endoscopic ultrasound elastography and contrast-enhanced
computerized tomography (CECT), CECT is the most frequently used
method for the diagnosis of esophageal cancer and lymph node
metastasis (12–14). Although CECT is considered to have
many advantages, its relatively low resolution makes it
inconclusive in the final diagnosis of patients with suspected
cancer (15,16). Due to the lack of data on early-stage
cancer, comprehensive treatments and effective therapeutic plans
have not been developed for patients with suspected early-stage
cancer (17,18).
Clinically, contrast agent improves the resolution
of CT, and the most commonly used contrast agents are employed
during ultrasound examination, due to their resonation following
exposure to ultrasound waves (19,20).
Previous results have indicated that contrast agent combined with
CT may be used for the diagnosis of tumor stage and progression
(21). More recently, nanoscale
microbubbles of contrast agents have been developed and clinically
applied in disease diagnosis (22).
Therefore, nanoscale microbubbles specific to the tumor markers of
esophageal cancer may improve the diagnostic sensitivity and
resolution of CT in the diagnosis of patients with early-stage or
suspected esophageal cancer.
Previous reports have suggested that fibroblast
growth factor receptor (FGFR) is overexpressed in tumor cells, and
tumor vasculature may be regulated by FGF/FGFR signaling-mediated
angiogenesis and bone marrow-derived cell recruitment (23,24).
Previous results have also indicated that vascular endothelial
growth factor receptor (VEGFR) is overexpressed and associated with
tumor growth, aggressiveness and tumor angiogenesis in the
progression of human cancers (25,26). In
the present study, the efficacy of CECT combined with nanoscale
microbubble contrast agent targeting of FGFR and VEGFR was
investigated in patients with suspected esophageal cancer. Results
of this clinical analysis demonstrated the potential applications
of CECT combined with Chitosan-Fe3O4
nanoparticles targeting FGFR and VEGFR (CECT-CNFV) for improving
imaging modality and diagnostic sensitivity in the diagnosis of
esophageal cancer. The present outcomes indicated that CECT-CNFV
improved image resolution and diagnostic accuracy for the early
diagnosis and final confirmation of suspected cases, suggesting
that CECT-CNFV may be a potential diagnostic method for early-stage
esophageal cancer.
Materials and methods
Ethics statement
The present clinical design (approval number:
DPHSP20080124M) was carried out in accordance with the
recommendations in the Guide for the Care and Use of Clinical Study
of the Pharmaceutical Administration Law of China (27). The present study was approved by the
Ethics Committee of Dongying People's Hospital (Dongying, China).
All clinical examination and testing procedures were performed
according to standard operating procedures, and all patients
provided written informed consent.
Patients and volunteers
A total of 320 patients with suspected esophageal
cancer aged 24.3–75.4 years old from Dongying People's Hospital
(Dongying, China) were enrolled in the present study between May
2014 and January 2016. Among these patients, 156 were male and 164
were female. Control tissues and cells were obtained from 6
patients (3 male and 3 female) who were diagnosed as tumor free.
The mean age of control patients was 42.5 years old. All patients
were subjected to CECT and CECT-CNFV for the detection of
early-stage esophageal cancer. The characteristics of patients with
suspected esophageal cancer are summarized in Table I. After diagnosis by CECT and
CECT-CNFV, patient survival was investigated in a 60-month
long-term observation period. All patients were investigated and
patients were assessed every two months.
 | Table I.Characteristics of patients with
esophageal cancer. |
Table I.
Characteristics of patients with
esophageal cancer.
| Characteristic | Male | Female |
|---|
| Number, n | 156 | 164 |
| Age, range | 24.3–66.6 | 26.3–75.4 |
| Medical history of
cancer | 2 | 3 |
| Blood pressure (mm
Hg) | 108.4±17.2 | 103.5±16.1 |
| Blood glucose
(mmol/l) | 7.2±3.7 | 8.2±3.2 |
| Diagnostic
method |
|
|
|
CECT | 156 | 164 |
|
CECT-CNFV | 156 | 164 |
Nanoparticles contrast agent
Novel Chitosan-Fe3O4
nanoparticles encapsulated by bispecific antibody against FGFR and
VEGFR (BisFV) were used as a CNFV contrast agent to improve the
imaging resolution ratio and diagnostic sensitivity of early-stage
esophageal cancer diagnosis. BisFV antibody was a gift from
Professor Yuan Hui of the Biological Pharmaceutical Laboratory at
Shandong University of Science and Technology (Qingdao, China)
Chitosan-Fe3O4-encapsulated BisFV was
manufactured according to the covalent bonding method described in
a previous study (28). The CNFV
contrast agent (1–10 mg/kg, n=10; 10–25 mg/kg, n=14; 25–40 mg/kg,
n=18) was orally taken at 20 min prior to CECT-CNFV to allow
adherence to esophageal cancer cells. Following the 20 min
pretreatment, CNFV was visualized using a CECT system. The signal
intensity was analyzed using CECT system.
Pharmacodynamics of BisFV
Plasma concentration of BisFV was analyzed in
patients with suspected esophageal cancer following treatment with
CNFV contrast agent. Blood samples were collected from 32
participators at 0, 6, 12, 18 and 24 h following administration of
CNFV contrast agent. Plasma BisFV levels were determined using
liquid chromatography-tandem mass spectrometry as described
previously (29).
Scan protocol
A 64-multidetector CECT diagnosis system (Philips
Medical Systems, Inc., Bothell, WA, USA) was used to measure the
efficacy of CNFV using a preprogrammed setting. The preprogrammed
setting was optimized to record the best image formation. The
esophagus of all patients was scanned by CECT, according to the
instructions of the CECT system. The parameters and settings of
CECT were as described in a previous study (30). CECT and CECT-CNFV imaging was
performed in all patients with suspected esophageal cancer.
Image data analysis
Data from the CECT-CNFV and CECT image sets were
analyzed using a CT system (Version 2.3, Shimadzu, Kyoto, Japan).
Esophageal cancer masses were detected in the CECT or CECT-CNFV
images. The patients with suspected early-stage gastric cancer were
analyzed CECT-CNFV. The sizes of tumor nodules were evaluated in
regions of stomach tumor lesions using the CT system.
Treatment of esophageal cancer
patients diagnosed by CECT-CNFV
Following diagnosis of early-stage esophageal cancer
using CECT-CNFV, patients immediately received different treatments
following diagnosis, including radiotherapy, chemotherapy, Chinese
medicine, biological therapy and comprehensive therapy (Table II). The median overall survival and
median progression-free survival of patients with esophageal cancer
were analyzed according to a previously described method (31).
 | Table II.Treatment of patients with esophageal
cancer diagnosed by contrast-enhanced computed tomography combined
with Chitosan-Fe3O4 nanoparticles targeting
fibroblast growth factor receptor and vascular endothelial growth
factor receptor. |
Table II.
Treatment of patients with esophageal
cancer diagnosed by contrast-enhanced computed tomography combined
with Chitosan-Fe3O4 nanoparticles targeting
fibroblast growth factor receptor and vascular endothelial growth
factor receptor.
| Variable | Male | Female |
|---|
| Number, n | 56 | 64 |
| Treatment |
|
|
|
Radiotherapy | 12 | 16 |
|
Chemotherapy | 14 | 12 |
| Chinese
medicine | 15 | 18 |
|
Biological therapy | 15 | 18 |
|
Comprehensive therapy | 34 | 45 |
Western blot analysis
Western blot analysis was performed as previously
described (32). Tumor cells were
lysed in lysate buffer containing protease-inhibitor (cat no.
P3480, Sigma-Aldrich; Merck KGaA) and were centrifuged at 8,000 × g
at 4°C for 10 min. Protein concentration was measured using a BCA
protein assay kit. Protein samples (20 µg) were resolved using 15%
SDS-PAGE and then transferred to polyvinylidene fluoride membranes
(Merck KGaA). After 1 h blocking at 37°C using 10% blocking reagent
(Roche Applied Science, Penzberg, Germany), membranes were
incubated with primary antibodies: mouse anti-human FGFR (1:1,000,
cat no. ab10646), mouse anti-human VEGFR (1:1,000, cat no. ab2349)
and β-actin (1:1,000, cat no. ab124721, all Abcam, Cambridge, UK)
for 12 h at 4°C. Membranes were then washed three times in TBST and
incubated with HRP-conjugated Immunoglobulin G mAb (1:2,000; cat
no. PV-6001; ZSGB-BIO, Beijing, China) for 1 h at 37°C. Following
three washed in TBST, membranes were developed using a
chemiluminescence assay system (Roche) and exposed on Kodak
exposure films. Densitometric quantification of the immunoblot data
was performed using the software of Quantity-One (Bio-Rad
Laboratories, Hercules, CA, USA).
ELISA
Serum levels of FGFR (cat no. DYC766-2, Bio-Rad
Laboratories), VEGFR (cat no. DYC1780-2, Bio-Rad Laboratories) were
detected using ELISA kits according to the manufacturer's protocol.
Serum concentration levels of FGFR and VEGFR were measured using
enzyme micro-plate reader at a wavelength of 450 nm.
Immunofluorescence staining
After esophageal cancer was confirmed in patients by
CECT-CNFV, esophageal tumor cells isolated from patients on day 7
using tumor resection were cultured in minimum essential medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) in vitro. Esophageal tumor
cells were incubated with mouse anti-human FGFR (1:1,000, cat no.
ab10646, Abcam, Cambridge UK) or mouse anti-human VEGFR (1:1,000,
cat no. ab2349, Abcam) for 2 h at 37°C, then washed three times
with phosphate-buffered saline. Cells were incubated with red
fluorescence-labeled goat anti-mouse (1:1,000, cat no. ab150117,
Abcam) or red fluorescence-labeled (1:1,000, cat no. ab150115,
Abcam) goat anti-mouse IgG for 30 min at 37°C. Esophageal tumor
cells were observed under a fluorescence microscope. The
immunofluorescence procedures have previously been reported in
detail (33).
Immunohistochemistry
Esophageal tumors and normal esophageal tissues
(tumor free individuals) from patients were fixed with 10%
formaldehyde for 2 h at 37°C, then embedded in paraffin and cut
into tumor 4 µm thick sections. Antigen retrieval was performed on
the tumor sections using eBioscience™ IHC Antigen Retrieval
Solution (cat no. 00-4955-58, Invitrogen; Thermo Fisher Scientific,
Inc.), sections were washed with PBST (Sigma-Aldrich; Merck KGaA)
and subsequently incubated with mouse anti-human FGFR (1:1,000, cat
no. ab10646, Abcam) or mouse anti-human VEGFR antibodies (1:1,000,
cat no. ab2349, Abcam) for 12 h at 4°C. Following antibody
incubation, proteins were washed with PBST three times and
incubated with Alexa Fluor 488-labeled secondary antibodies (1:500;
Beyotime Institute of Biotechnology, Haimen, China) for 2 h at 37°C
and the specimens were visualized. A Diaminobenzidine staining
system (D7679MSDS, Sigma-Aldrich; Merck KGaA) was used to detect
target protein expression according to manufacturer's protocol. For
histological staining, tumor sections were stained with hematoxylin
and eosin and observed using a light microscope (Olympus BX51,
Olympus Corporation, Tokyo, Japan) as described previously
(34).
Statistical analysis
Data were presented as the mean ± standard deviation
of triplicate experiments. All data were analyzed using SPSS
Statistics 19.0 (IBM Corp., Armonk, NY, USA). Unpaired data were
analyzed using a Student's t test and comparisons between the data
of multiple groups were performed using one-way analysis of
variance followed by Dunnett's t test. P<0.05 was considered to
indicate a statistically significant difference. Kaplan-Meier
analysis was used to estimate the survival rate of patients from a
60-month long-term observation.
Results
FGFR and VEGFR expression in the tumor
cells of esophageal cancer patients
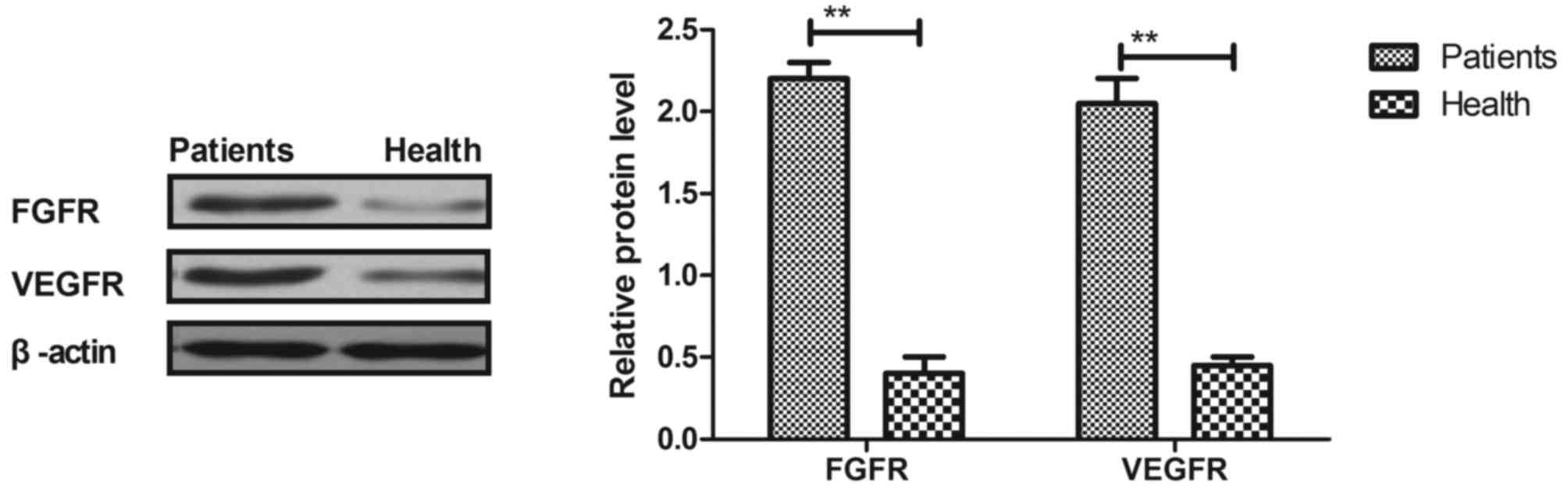
The expressions of FGFR and VEGFR in the tumor cells
of patients with esophageal cancer were assessed. As depicted in
Figs. 1 and 2, the expression and plasma levels of FGFR
and VEGFR were upregulated in the tumor cells of esophageal cancer
patients when compared with normal esophageal cells (P<0.01).
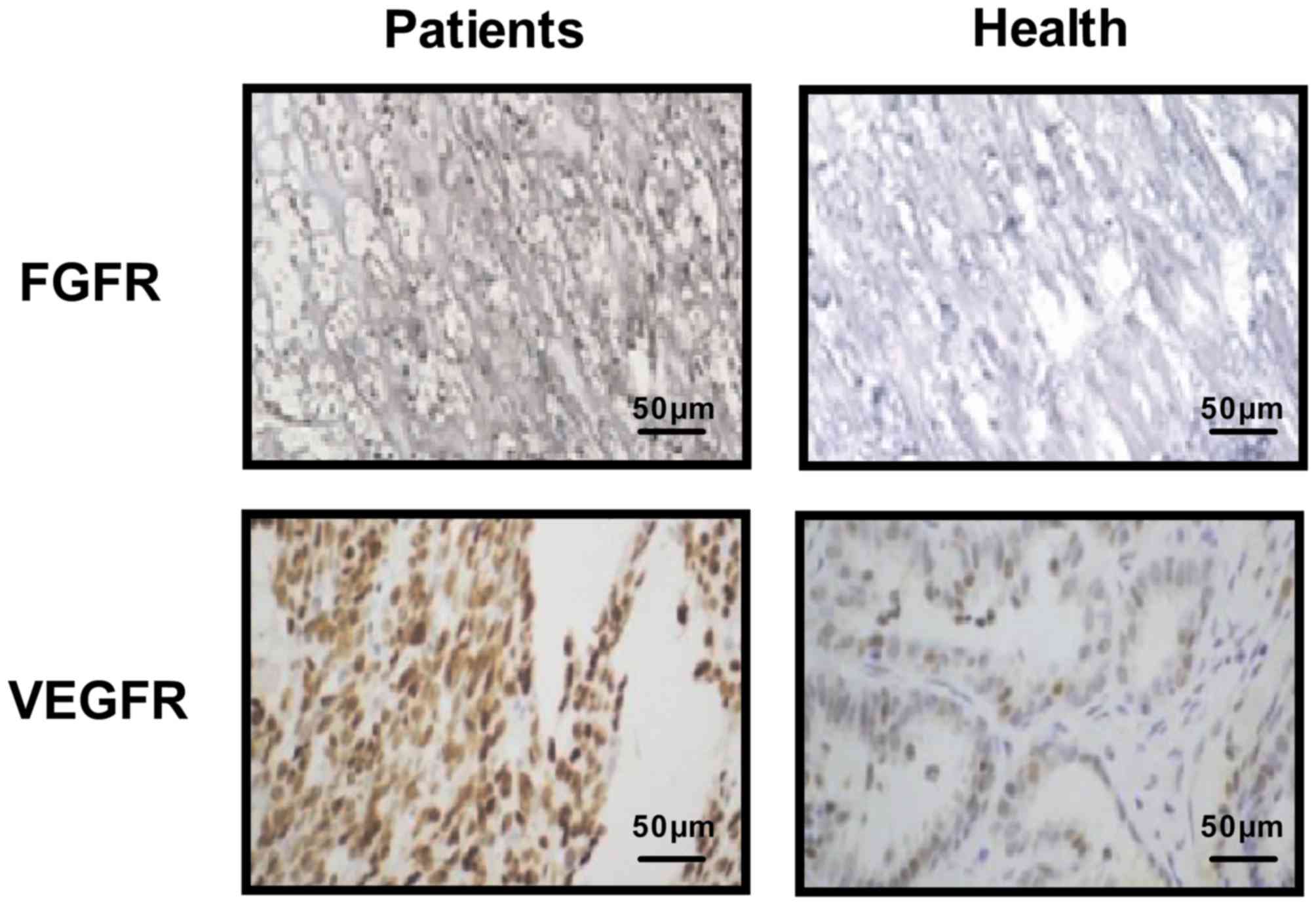
Immunohistochemistry also indicated that FGFR and VEGFR were
upregulated in clinical esophageal tumor tissues when compared with
normal esophageal tissues (Fig. 3).
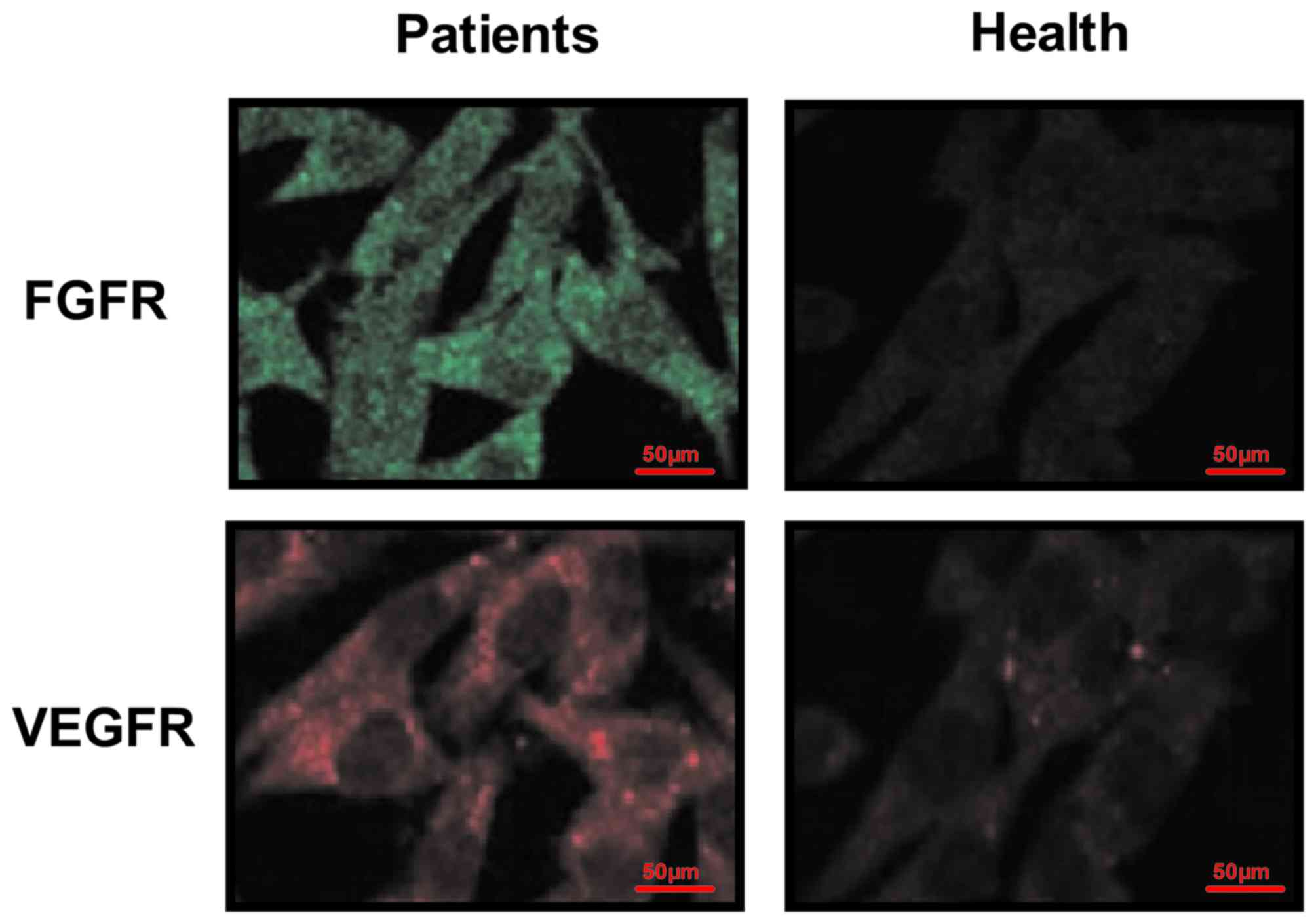
Furthermore, immunofluorescence demonstrated that FGFR and VEGFR
were expressed on the surface of esophageal tumor cells (Fig. 4). These results suggest that FGFR and
VEGFR are upregulated in the tumor cells and tissues of patients
with esophageal cancer.
Efficacy of CECT-CNFV in the early
diagnosis of patients with suspected esophageal cancer
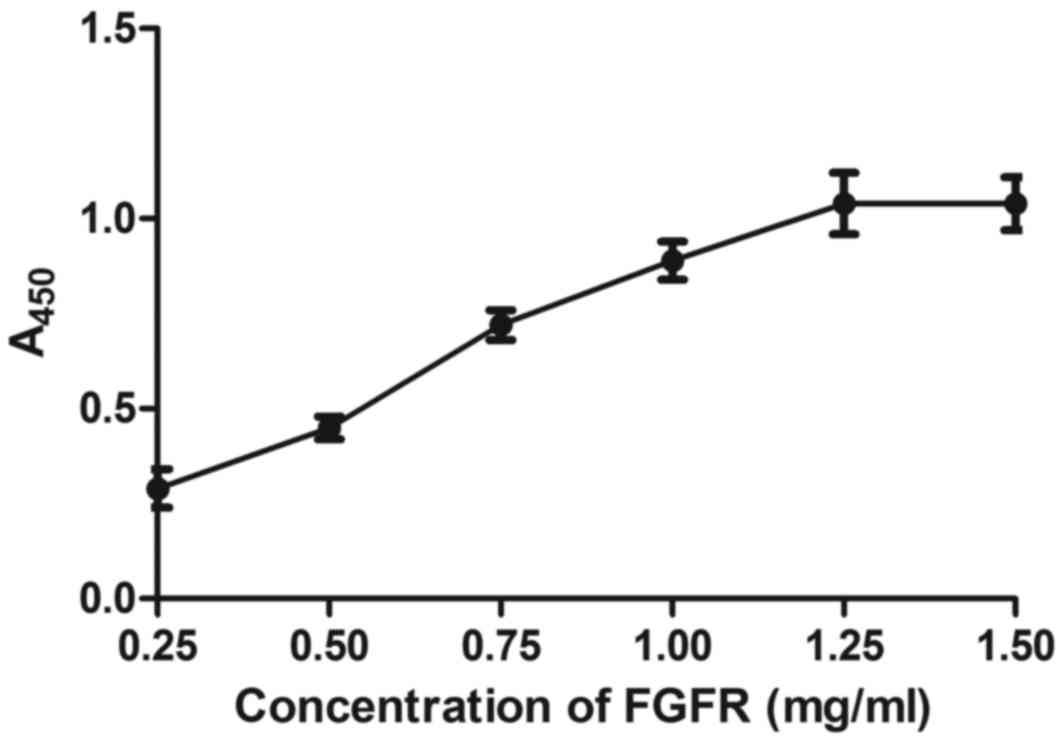
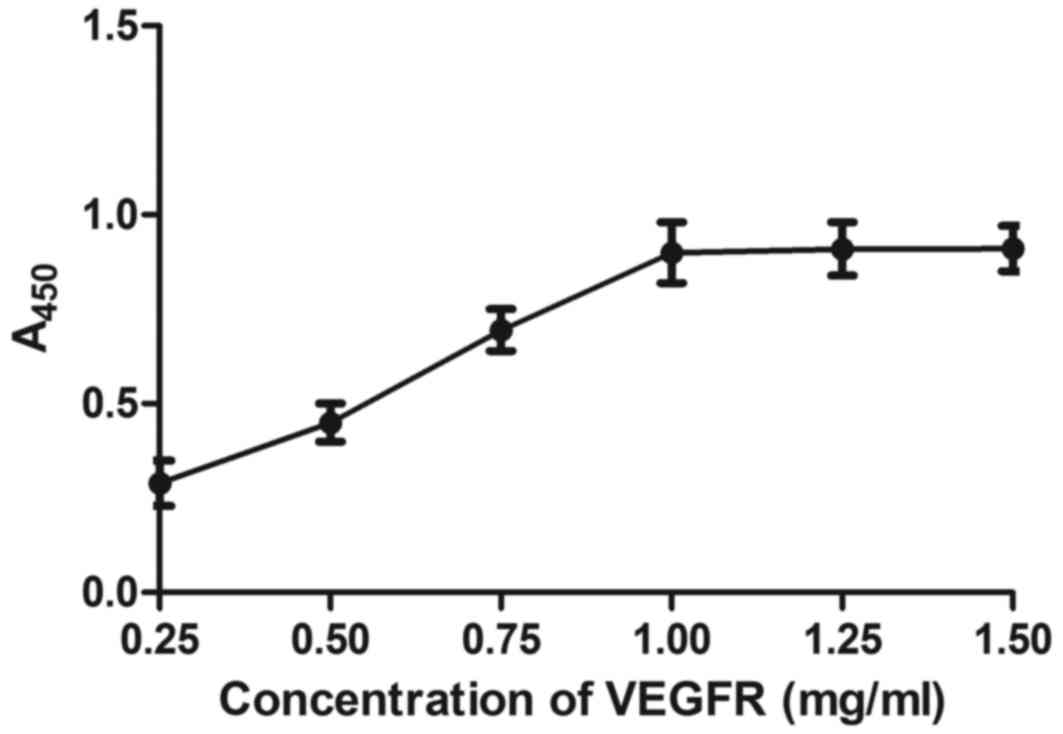
The affinity of BisFV for FGFR and VEGFR was
evaluated. As depicted in Figs. 5
and 6,
Chitosan-Fe3O4-encapsulated BisFV was able to
bind FGFR and VEGFR, as determined by ELISA. The dose of
nanoparticles that achieved optimum targeting efficiency was
identified as 25 mg/kg (Table II).
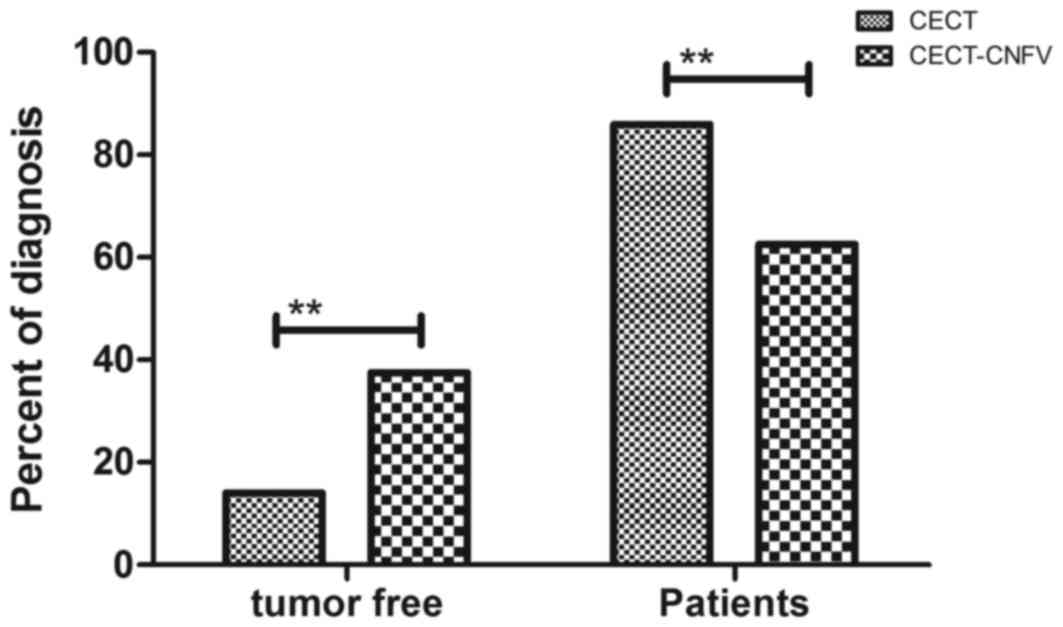
Subsequent analysis of patient clinical outcomes showed that 120
patients (37.50%) were diagnosed as tumor-free and 200 patients
(62.50%) were confirmed to have esophageal cancer after diagnosis
with CECT-CNFV. By contrast, the CECT method only diagnosed 45
patients (14.06%) with esophageal cancer Fig. 7). These outcomes demonstrated that
CECT-CNFV presented significantly higher diagnostic efficacy
compared with CECT (P<0.01).
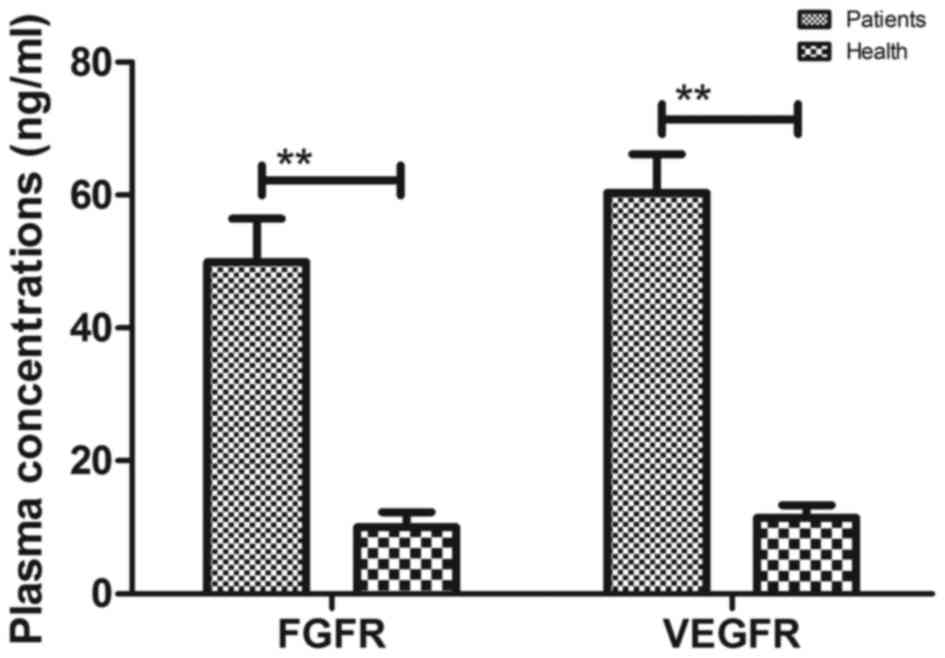
Pharmacodynamics of CNFV in the plasma
of patients with suspected esophageal cancer
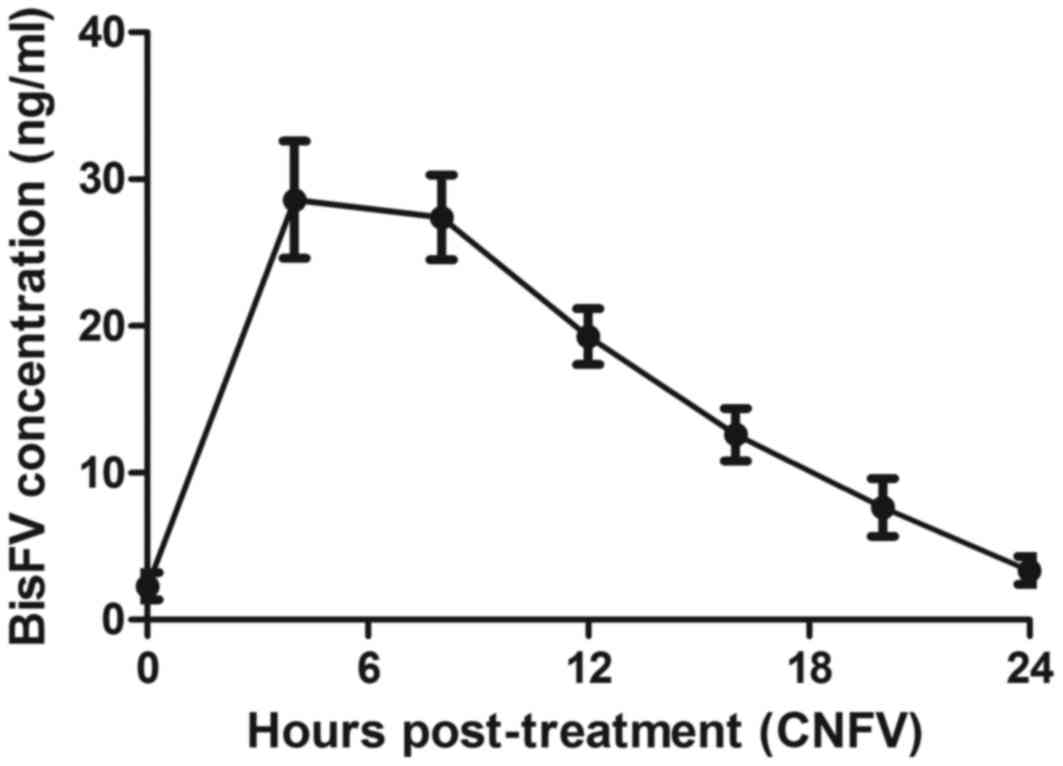
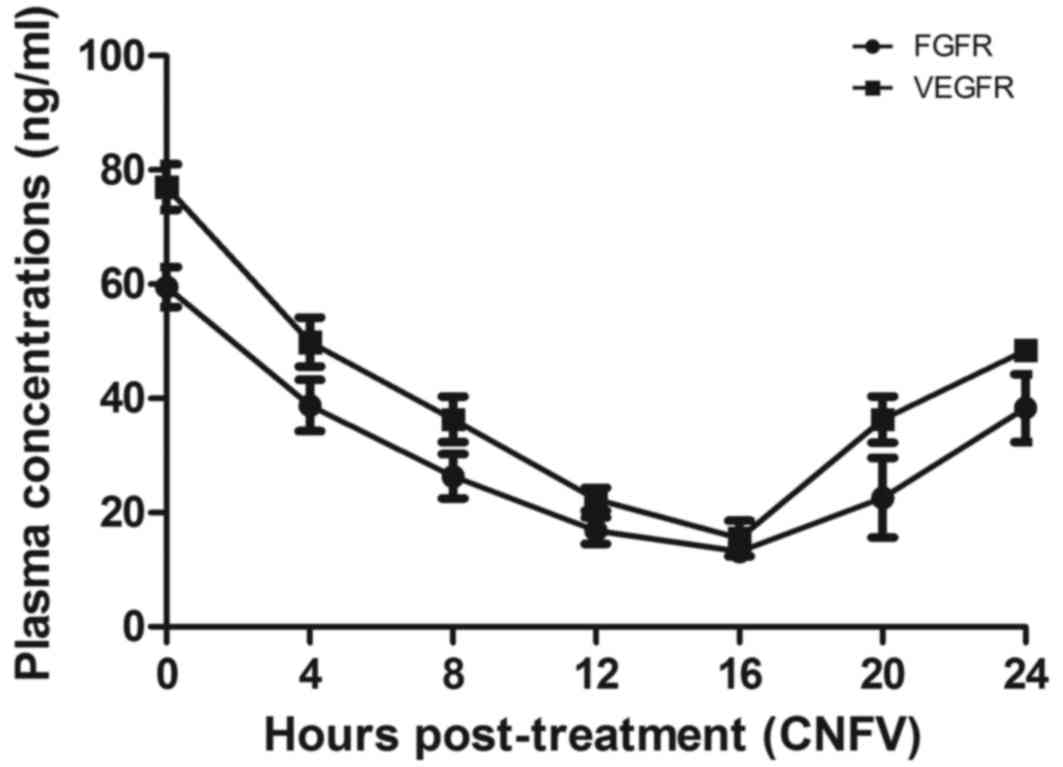
The pharmacodynamics of BisFV was assessed in the
plasma of patients with suspected esophageal cancer. Results
indicated that the plasma concentration of BisFV was increased and
metabolized within 24 h using liquid chromatography-tandem mass
spectrometry (Fig. 8). Patients that
underwent CECT-CNFV exhibited reduced plasma concentrations of FGFR
and VRGFR, both of which reached a minimum at 16 h post-treatment
compared with the plasma concentrations prior to diagnosis
(Fig. 9).
Accuracy of CECT-CNFV in the diagnosis
of esophageal cancer
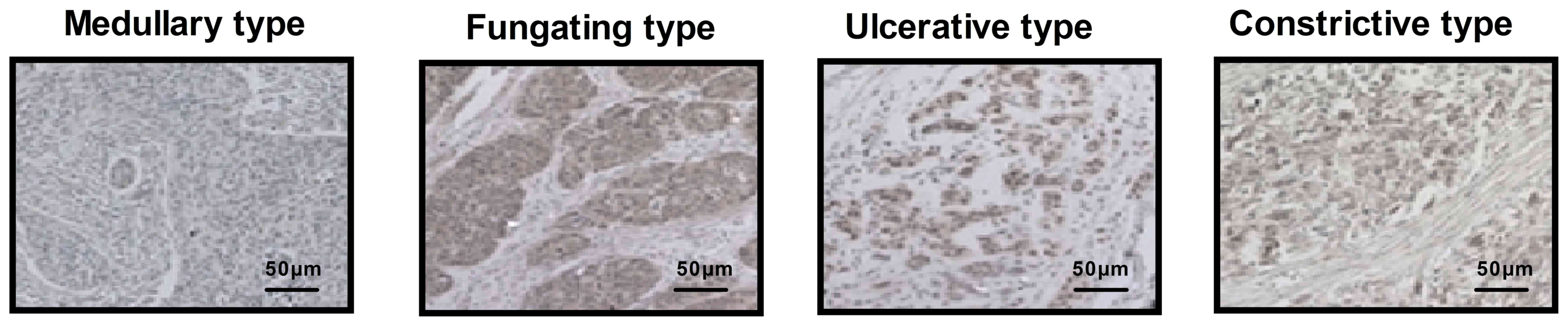
Histopathological analysis was performed to confirm
that CECT-CNFV had successfully diagnosed esophageal cancer. The
different types of early-stage esophageal cancer in patients,
namely medullary, fungating, ulcerative and constrictive, were
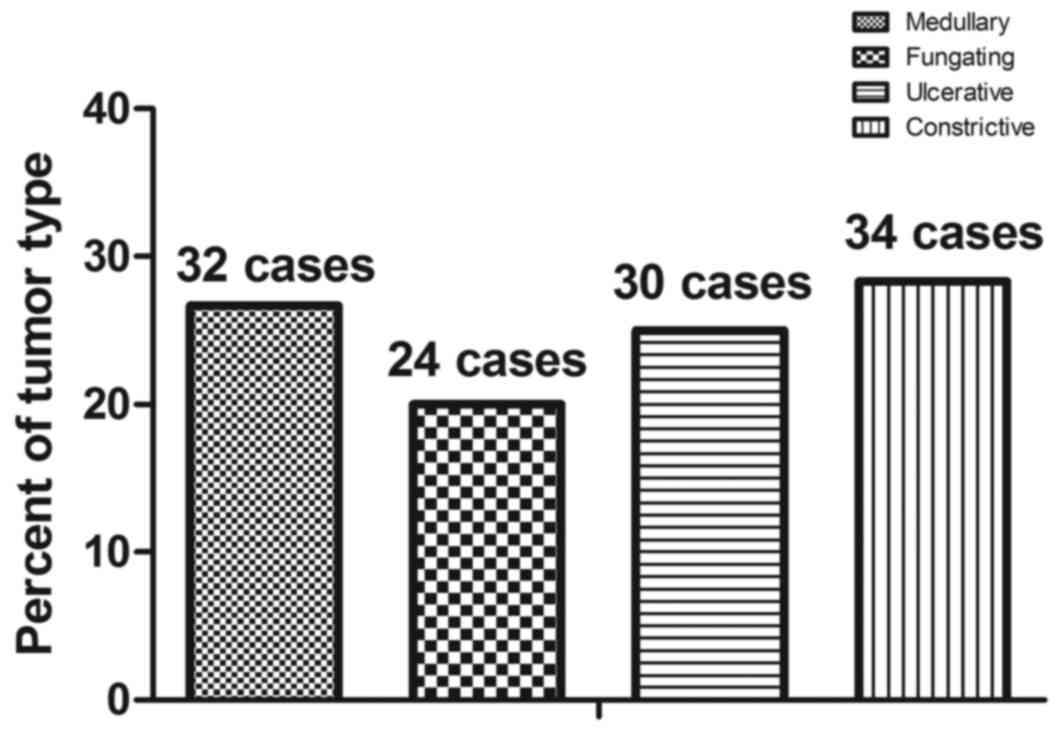
identified by immunohistochemistry (Fig. 10). A total of 120 cases of
esophageal cancer were identified by immunohistochemistry, and the
incidence rates of medullary, fungating, ulcerative and
constrictive type esophageal cancer were 26.67% (32 cases), 20.00%
(24 cases), 25.00% (30 cases) and 28.33% (34 cases), respectively
(Fig. 11). As CECT-CNFV also
identified 120 cases of esophageal cancer, these data suggest that
CECT-CNFV may be a useful clinical method for the diagnosis of
early-stage esophageal cancer.
Survival rate of patients with
esophageal cancer
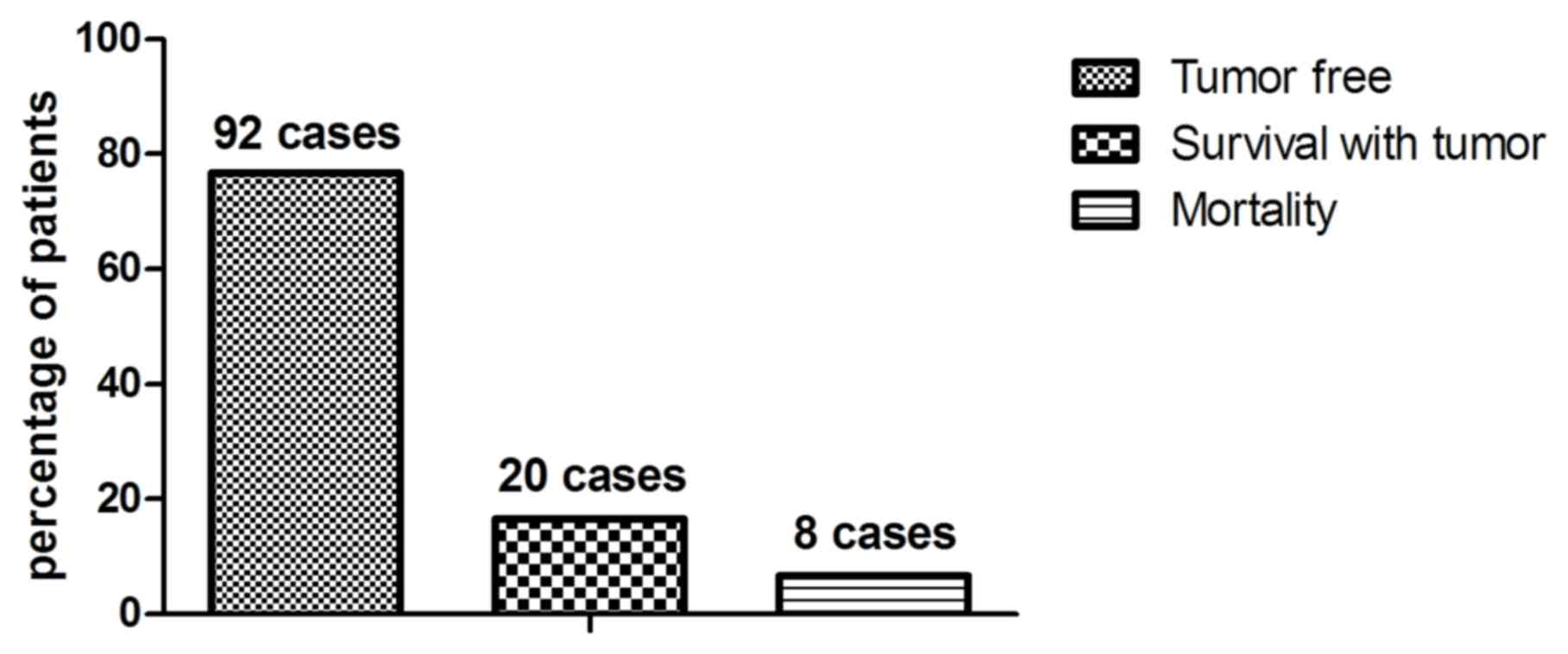
Patients diagnosed with early-stage esophageal
cancer received different treatments to inhibit or eradicate tumor
growth (Table III). The overall
survival rate of patients with esophageal cancer following
diagnosis with CECT-CNFV was subsequently evaluated. At a 60-month
follow-up, 92 patients (76.67%) had survived and were tumor-free,
and 20 patients (16.66%) had survived with tumors. A total of 8
patients (6.67%) did not survive (Fig.
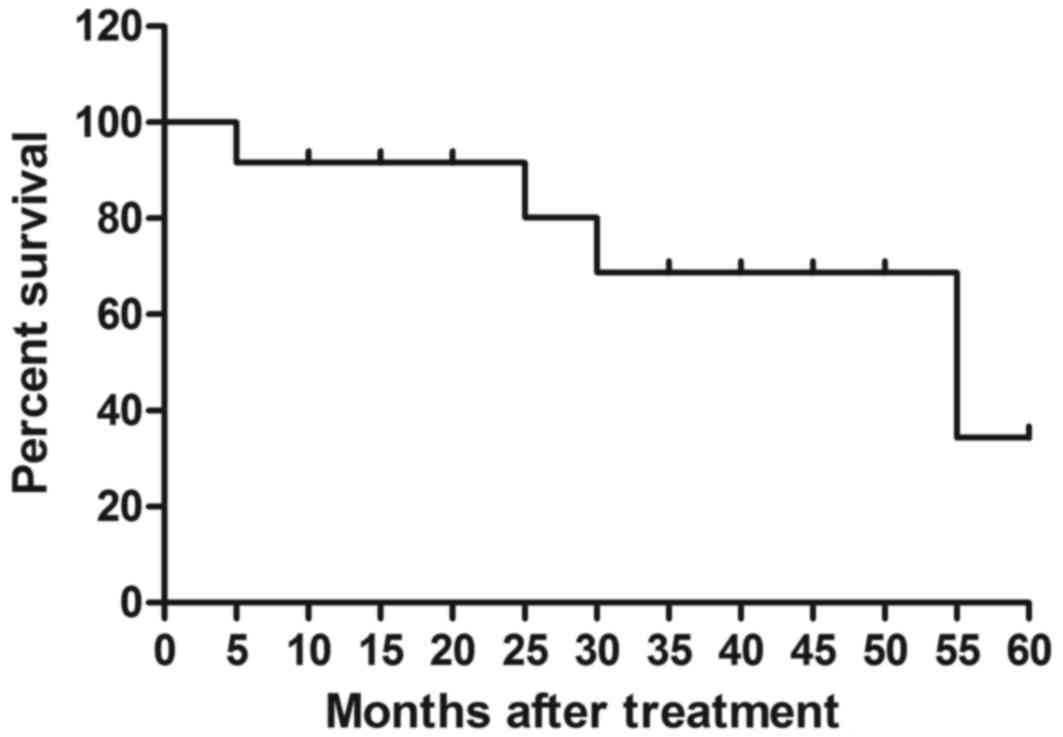
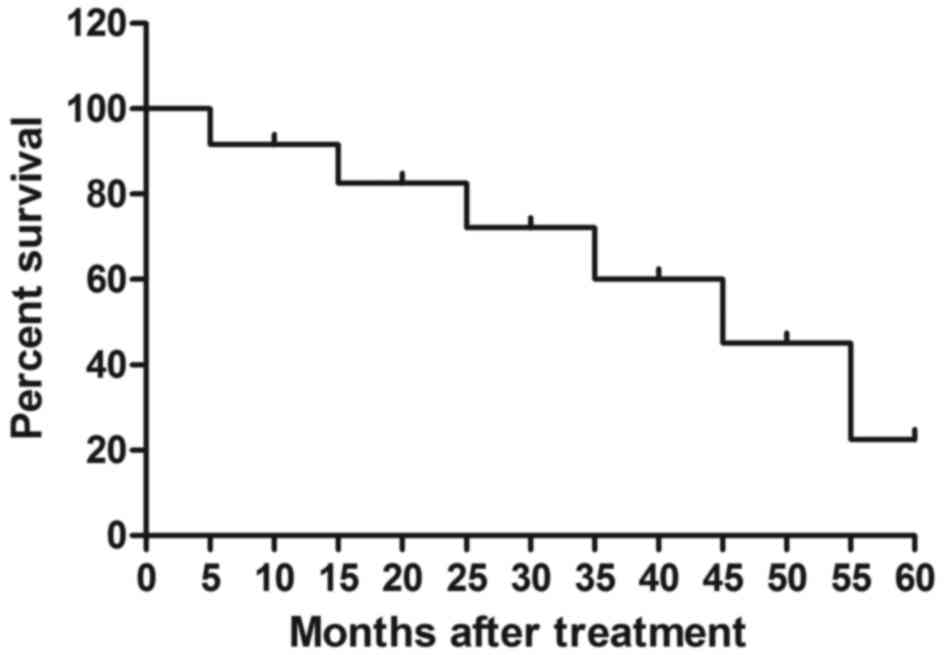
12). The median overall survival rate was 55.2 months (Fig. 13) and median progression-free
survival rate was 44.6 months (Fig.
14). These data suggest that patients with early-stage
esophageal cancer diagnosed by CECT-CNFV and administered with
anti-cancer treatments present with longer survival rates.
 | Table III.Confirmation of contrast agent
nanoparticle dosage for patients with esophageal cancer. |
Table III.
Confirmation of contrast agent
nanoparticle dosage for patients with esophageal cancer.
|
| Dosage, mg/kg |
|---|
|
|
|
|---|
| Variable | 1–10 (n=10) | 10–25 (n=14) | 25–40 (n=18) |
|---|
| Signal intensity
(HU) | 56.2±8.8 | 86.7±10.3 | 83.5±12.5 |
| Sensitivity
(%) | 65.6±12.2 | 81.2±12.6 | 82.6±11.6 |
Discussion
Early diagnosis of cancer is a significant challenge
in the clinical treatment of human cancer (35,36). In
recent years, contrast-enhanced ultrasound, CT and
fluorodeoxyglucose-positron emission tomography have been widely
used in the diagnosis of human cancers (37). In particular, CECT is the most widely
used method in the diagnosis of human tumors (38,39).
However, the accuracy and sensitivity of CECT is insufficient in
the clinical detection of early-stage tumors (40,41). In
the present study, a target nano-scale contrast agent combined with
CECT was used to improve the accuracy and sensitivity of CECT in
the diagnosis of patients with suspected early-stage esophageal
cancer. The target nano-scale contrast agent was
Chitosan-Fe3O4 nanoparticles encapsulated by
BisFV, which may bind to esophageal cancer cells. The results
indicated that CECT-CNFV not only improved the resolution ratio of
images captured by CECT, but also increased the accuracy and
sensitivity of CECT in the diagnosis of patients with suspected
early-stage esophageal cancer.
Theoretically, a nano-scale contrast agent may
improve in vivo tumor imaging made by ultrasound, CT and
magnetic resonance imaging (42–44). Kim
et al (42) demonstrated that
ultrasound enhanced-contrast agents, which may go preferentially to
the target tumor tissue and amplify the ultrasound imaging signal
in vivo, improved the detection limit of ultrasound imaging.
Furthermore, Ding et al (43)
demonstrated that targeted Fe-filled carbon nanotube as a
multifunctional contrast agent improved the resolution ratio of
magnetic resonance imaging of tumors in living mice. These reports
indicate that nano-scale contrast agents may be useful for
detecting tumor masses in early-stage cancer.
In the present study, a novel nano-scale contrast
agent composed of Chitosan-Fe3O4
nanoparticles encapsulated by BisFV was introduced to evaluate the
efficacy of CECT-CNFV in the diagnosis of patients with suspected
esophageal cancer. Barium sulfate and iodinated contrast media are
frequently used for angiography studies and in the detection of
tumors in the digestive system (45,46).
Various kinds of electropositive iron and iron oxide nanoparticles
have been used as contrast media in combination with ultrasound, CT
and magnetic resonance imaging for the clinical diagnosis of human
cancers (47,48). In addition, targeted contrast agents
are considered to enhance optical coherence tomography and improve
the accuracy of CT in the diagnosis of tumor tissue masses
(49). However, although contrast
media improve the accuracy of CT to a certain degree, their
sensitivity has not been improved in previous studies (21,50). The
present results indicated that CECT-CNFV was efficient in the
targeting of FGFR and VEGFR, and improved the accuracy and
sensitivity of CT in the diagnosis of early-stage esophageal
cancer. Notably, long-term follow-up investigations suggested that
patients diagnosed by CECT-CNFV at early-stage presented with
higher median overall survival and median progression-free survival
rates compared with the mean survival rate of patients with
esophageal cancer in previous reports (51).
In conclusion, the present study investigated the
efficacy of CECT-CNFV in the diagnosis of suspected early-stage
esophageal cancer. Chitosan-Fe3O4
nanoparticles encapsulated by BisFV not only improved the image
resolution generated by CT, but also enhanced the accuracy and
sensitivity of CT in the diagnosis of early-stage esophageal
cancer. These outcomes indicate that CECT-CNFV may be an efficient
clinical method for diagnosing patients with suspected esophageal
cancer at an early-stage. Thus, CECT-CNFV may be a reliable and
sensitive assessment method in the clinical diagnosis of cancer
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Findlay JM, Middleton MR and Tomlinson I:
A systematic review and meta-analysis of somatic and germline DNA
sequence biomarkers of esophageal cancer survival, therapy response
and stage. Ann Oncol. 26:624–644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malik MA, Zargar SA and Mittal B: Role of
NQO1 609C>T and NQO2-3423G>A gene polymorphisms in esophageal
cancer risk in Kashmir valley and meta analysis. Mol Biol Rep.
39:9095–9104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen M, Cai E, Huang J, Yu P and Li K:
Prognostic value of vascular endothelial growth factor expression
in patients with esophageal cancer: A systematic review and
meta-analysis. Cancer Epidemiol Biomarkers Prev. 21:1126–1134.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacobs M, Macefield RC, Blazeby JM,
Korfage IJ, van Berge Henegouwen MI, de Haes HC, Smets EM and
Sprangers MA: Systematic review reveals limitations of studies
evaluating health-related quality of life after potentially
curative treatment for esophageal cancer. Qual Life Res.
22:1787–1803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samson P, Robinson C, Bradley J, Lockhart
AC, Puri V, Broderick S, Kreisel D, Krupnick AS, Patterson GA,
Meyers B and Crabtree T: Neoadjuvant chemotherapy versus
chemoradiation prior to esophagectomy: Impact on rate of complete
pathologic response and survival in esophageal cancer patients. J
Thorac Oncol. 11:2227–2237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao J, Liu R, Shi YJ, Yin LH and Pu YP:
Exosome-shuttling microRNA-21 promotes cell migration and
invasion-targeting PDCD4 in esophageal cancer. Int J Oncol.
48:2567–2579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo J, Yu X, Gu J, Lin Z, Zhao G, Xu F, Lu
C and Ge D: Regulation of CXCR4/AKT-signaling-induced cell invasion
and tumor metastasis by RhoA, Rac-1, and Cdc42 in human esophageal
cancer. Tumour Biol. 37:6371–6378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan Z, Mao W, Bao Y, Zhang M, Su X and Xu
X: The long noncoding RNA CASC9 regulates migration and invasion in
esophageal cancer. Cancer Med. 5:2442–2447. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi H, Shi D, Wu Y, Shen Q and Li J:
Qigesan inhibits migration and invasion of esophageal cancer cells
via inducing connexin expression and enhancing gap junction
function. Cancer Lett. 380:184–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schiefer AI, Schoppmann SF and Birner P:
Lymphovascular invasion of tumor cells in lymph node metastases has
a negative impact on survival in esophageal cancer. Surgery.
160:331–340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yokota T, Igaki H, Kato K, Tsubosa Y,
Mizusawa J, Katayama H, Nakamura K, Fukuda H and Kitagawa Y:
Accuracy of preoperative diagnosis of lymph node metastasis for
thoracic esophageal cancer patients from JCOG9907 trial. Int J Clin
Oncol. 21:283–288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Visser E, Leeftink AG, van Rossum PS,
Siesling S, van Hillegersberg R and Ruurda JP: Waiting time from
diagnosis to treatment has no impact on survival in patients with
esophageal cancer. Ann Surg Oncol. 23:2679–2689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishigaki M, Maeda Y, Taketani A, Andriana
BB, Ishihara R, Wongravee K, Ozaki Y and Sato H: Diagnosis of
early-stage esophageal cancer by Raman spectroscopy and chemometric
techniques. Analyst. 141:1027–1033. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawada S and Imai Y: Diagnosis of
esophageal cancer and metastatic lymph node using CT and MRI. Nihon
Rinsho. 69 Suppl 6:S174–S181. 2011.(In Japanese).
|
|
15
|
Freling N, Roele E, Schaefer-Prokop C and
Fokkens W: Prediction of deep neck abscesses by contrast-enhanced
computerized tomography in 76 clinically suspect consecutive
patients. Laryngoscope. 119:1745–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang DH, Min JJ, Jeong YY, Ahn JS, Kim YK,
Cho SH, Chung IJ, Bom HS, Kim HJ and Lee JJ: The combined
evaluation of interim contrast-enhanced computerized tomography
(CT) and FDG-PET/CT predicts the clinical outcomes and may impact
on the therapeutic plans in patients with aggressive non-Hodgkin's
lymphoma. Ann Hematol. 88:425–432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanat O, O'Neil B and Shahda S: Targeted
therapy for advanced gastric cancer: A review of current status and
future prospects. World J Gastrointestinal Oncol. 7:401–410. 2015.
View Article : Google Scholar
|
|
18
|
Kakimoto N, Chindasombatjaroen J, Tomita
S, Shimamoto H, Uchiyama Y, Hasegawa Y, Kishino M, Murakami S and
Furukawa S: Contrast-enhanced multidetector computerized tomography
for odontogenic cysts and cystic-appearing tumors of the jaws: Is
it useful? Oral Surg Oral Med Oral Pathol Oral Radiol. 115:104–113.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mazzei MA, Guerrini S, Mazzei FG,
Squitieri Cioffi N, Notaro D, de Donato G, Galzerano G, Sacco P,
Setacci F, Volterrani L and Setacci C: Follow-up of endovascular
aortic aneurysm repair: Preliminary validation of digital
tomosynthesis and contrast enhanced ultrasound in detection of
medium- to long-term complications. World J Radiol. 8:530–536.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schalk S, Demi L, Bouhouch N, Kuenen MPJ,
Postema AW, de la Rosette JJMCH, Wijkstra H, Tjalkens TJ and Mischi
M: Contrast-enhanced Ultrasound Angiogenesis Imaging by mutual
information analysis for prostate cancer localization. IEEE Trans
Biomed Eng. 64:661–670. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi H, Wang Z, Huang C, Gu X, Jia T, Zhang
A, Wu Z, Zhu L, Luo X, Zhao X, et al: A functional ct contrast
agent for in vivo imaging of tumor hypoxia. Small. 12:3995–4006.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wheatley MA, Forsberg F, Dube N, Patel M
and Oeffinger BE: Surfactant-stabilized contrast agent on the
nanoscale for diagnostic ultrasound imaging. Ultrasound Med Biol.
32:83–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fons P, Gueguen-Dorbes G, Herault JP,
Geronimi F, Tuyaret J, Frédérique D, Schaeffer P, Volle-Challier C,
Herbert JM and Bono F: Tumor vasculature is regulated by FGF/FGFR
signaling-mediated angiogenesis and bone marrow-derived cell
recruitment: This mechanism is inhibited by SSR128129E, the first
allosteric antagonist of FGFRs. J Cell Physiol. 230:43–51. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valesky M, Spang AJ, Fisher GW, Farkas DL
and Becker D: Noninvasive dynamic fluorescence imaging of human
melanomas reveals that targeted inhibition of bFGF or FGFR-1 in
melanoma cells blocks tumor growth by apoptosis. Mol Med.
8:103–112. 2002.PubMed/NCBI
|
|
25
|
Takahashi O, Komaki R, Smith PD,
Jürgensmeier JM, Ryan A, Bekele BN, Wistuba II, Jacoby JJ,
Korshunova MV, Biernacka A, et al: Combined MEK and VEGFR
inhibition in orthotopic human lung cancer models results in
enhanced inhibition of tumor angiogenesis, growth, and metastasis.
Clin Cancer Res. 18:1641–1654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bozec A, Formento P, Lassalle S, Lippens
C, Hofman P and Milano G: Dual inhibition of EGFR and VEGFR
pathways in combination with irradiation: Antitumour supra-additive
effects on human head and neck cancer xenografts. Br J Cancer.
97:65–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Z, Lu A, Wong BC, Chen X, Bian Z,
Zhao Z, Huang W, Zhang G, Chen H and Xu M: Effect of liposomes on
the absorption of water-soluble active pharmaceutical ingredients
via oral administration. Curr Pharm Des. 19:6647–6654. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen CL, Hu GY, Mei Q, Qiu H, Long GX and
Hu GQ: Epidermal growth factor receptor-targeted ultra-small
superparamagnetic iron oxide particles for magnetic resonance
molecular imaging of lung cancer cells in vitro. Chin Med J (Engl).
125:2322–2328. 2012.PubMed/NCBI
|
|
29
|
Granja RH, Salerno AG, de Lima AC,
Montalvo C, Reche KV, Giannotti FM and Wanschel AC: Liquid
chromatography/tandem mass spectrometry method to determine
boldenone in bovine liver tissues. J AOAC Int. 97:1476–1480. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghantous Y, Naddaf R, Barak M, Abd-Elraziq
M and Eln-Naaj Abu I: The role of fine needle aspiration in the
diagnosis of parotid gland tumors: Correlation with preoperative
computerized tomography tumor size. J Craniofac Surg. 27:e192–e196.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Angelov KG, Vasileva MB, Grozdev KS,
Sokolov MB and Todorov G: Clinical and pathological
characteristics, and prognostic factors for gastric cancer survival
in 155 patients in Bulgaria. Hepatogastroenterology. 61:2421–2424.
2014.PubMed/NCBI
|
|
32
|
Sawasdikosol S: Detecting
tyrosine-phosphorylated proteins by Western blot analysis. Curr
Protoc Immunol Chapter. 11:Unit 11 13. 11. 2010.
|
|
33
|
Dirani M, Nasreddine W, Abdulla F and
Beydoun A: Seizure control and improvement of neurological
dysfunction in Lafora disease with perampanel. Epilepsy Behav Case
Rep. 2:164–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kargahi N, Razavi SM, Deyhimi P and
Homayouni S: Comparative evaluation of eosinophils in normal
mucosa, dysplastic mucosa and oral squamous cell carcinoma with
hematoxylin-eosin, Congo red, and EMR1 immunohistochemical staining
techniques. Electron Physician. 7:1019–1026. 2015.PubMed/NCBI
|
|
35
|
Wei R, Guo B and Tan ZM: Application of
tumor markers detection in early diagnosis of oral
maxillofacial-head and neck carcinomas. Zhonghua Kou Qiang Yi Xue
Za Zhi. 43:143–145. 2008.(In Chinese). PubMed/NCBI
|
|
36
|
Neven P, Brouckaert O, Van Belle V, Vanden
Bempt I, Hendrickx W, Cho H, Deraedt K, Van Calster B, Van Huffel
S, Moerman P, et al: In early-stage breast cancer, the estrogen
receptor interacts with correlation between human epidermal growth
factor receptor 2 status and age at diagnosis, tumor grade, and
lymph node involvement. J Clin Oncol. 26:1768–1771. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ebell MH, Culp MB and Radke TJ: A
systematic review of symptoms for the diagnosis of ovarian cancer.
Am J Prev Med. 50:384–394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Moura PM, Hallac R, Kane A and Seaward
J: Improving the evaluation of alveolar bone grafts with cone beam
computerized tomography. Cleft Palate Craniofac J. 53:57–63. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fang L, Jingjing L, Ying S, Lan M, Tao W
and Nan J: Computerized tomography-guided sphenopalatine ganglion
pulsed radiofrequency treatment in 16 patients with refractory
cluster headaches: Twelve- to 30-month follow-up evaluations.
Cephalalgia. 36:106–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Benharroch D, Shvarts S, Jotkowitz A and
Shelef I: Computerized tomography scanning and magnetic resonance
imaging will terminate the era of the autopsy-a hypothesis. J
Cancer. 7:115–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lambert S, Al-Hadithy N, Sewell MD, Hertel
R, Südkamp N, Noser H and Kamer L: Computerized tomography based 3D
modeling of the clavicle. J Orthop Res. 34:1216–1223. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim M, Lee JH, Kim SE, Kang SS and Tae G:
Nanosized ultrasound enhanced-contrast agent for in vivo tumor
imaging via intravenous injection. ACS Appl Mater Interfaces.
8:8409–8418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ding W, Lou C, Qiu J, Zhao Z, Zhou Q,
Liang M, Ji Z, Yang S and Xing D: Targeted Fe-filled carbon
nanotube as a multifunctional contrast agent for thermoacoustic and
magnetic resonance imaging of tumor in living mice. Nanomedicine.
12:235–244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang W, Li J, Liu R, Zhang A and Yuan Z:
Size effect of Au/PAMAM contrast agent on CT imaging of
reticuloendothelial system and tumor tissue. Nanoscale Res Lett.
11:4292016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Konduru N, Keller J, Ma-Hock L, Gröters S,
Landsiedel R, Donaghey TC, Brain JD, Wohlleben W and Molina RM:
Biokinetics and effects of barium sulfate nanoparticles. Part Fibre
Toxicol. 11:552014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liss P, Hansell P, Fasching A and Palm F:
Iodinated contrast media inhibit oxygen consumption in freshly
isolated proximal tubular cells from elderly humans and diabetic
rats: Influence of nitric oxide. Ups J Med Sci. 121:12–16. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mishra SK, Kumar BS, Khushu S, Tripathi RP
and Gangenahalli G: Increased transverse relaxivity in ultrasmall
superparamagnetic iron oxide nanoparticles used as MRI contrast
agent for biomedical imaging. Contrast Media Mol Imaging.
11:350–361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kitoh Y: 5. Inspection of hepatocellular
carcinoma 3-contrast for the diagnosis of hepatocellular carcinoma:
Techniques of image contrast and the choice of MR contrast agent.
Nihon Hoshasen Gijutsu Gakkai Zasshi. 72:441–451. 2016.(In
Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang J, Liu J, Wang LM, Li ZY and Yuan Z:
Retroreflective-type Janus microspheres as a novel contrast agent
for enhanced optical coherence tomography. J Biophotonics.
10:878–886. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
D'Hollander A, Mathieu E, Jans H, Vande
Velde G, Stakenborg T, Van Dorpe P, Himmelreich U and Lagae L:
Development of nanostars as a biocompatible tumor contrast agent:
Toward in vivo SERS imaging. Int J Nanomedicine. 11:3703–3714.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang YM, Wang CH, Huang JS, Tsai CS, Yeh
KY, Lan YJ, Wu TH, Chang PH, Chang YS and Lai CH:
Treatment-associated severe thrombocytopenia affects survival rate
in esophageal cancer patients undergoing concurrent
chemoradiotherapy. Indian J Cancer. 52:454–460. 2015. View Article : Google Scholar : PubMed/NCBI
|