Introduction
Acute kidney injury comprises of a group of symptoms
that include a sudden drop in renal function during a short time
(<24 h) defined as an increase of ≥0.5 mg/dl in serum creatinine
(SCr) (1). The occurrence of
azotemia, and imbalances of water and/or electrolytes and/or pH are
associated with oliguria (<400 ml/24 h or 17 ml/h) or aurine
(<100 ml/24 h) (2). Acute renal
injury can be divided into pre-renal, renal and post-renal, each
with separate etiologies and pathogeneses (3). Drug-induced acute kidney injuries are
common in clinical practice, and misdiagnosis and late diagnosis of
drug-induced acute kidney injury have occurred (4).
Pantoprazole is used clinically as an irreversible
proton pump inhibitor (PPI) to reduce gastric acid secretion
(5). Pantoprazole is activated in
the acidic environment of gastric parietal cells as cyclic
sulphonamides and specifically binds to mercapto groups on the
proton pump (i.e., HtK + -ATPase) to inhibit H+
secretion (6). A number of side
effects of pantoprazole have been reported, with a small number of
patients reporting headache, dizziness, nausea, diarrhea, bloating,
skin itching and skin rash, as well as reports of elevated
aminotransferase, leukopenia and thrombocytopenia (7–9).
However, there have been few reports of kidney damage associated
with pantoprazole. Early and correct diagnosis of
pantoprazole-induced acute kidney injury may be the key to
treatment. The right diagnosis and early treatment are closely
associated with improved prognosis.
In the present study, a case of acute kidney injury
induced by pantoprazole is presented. The patient presented with
extensive interstitial inflammation and eosinophil infiltration in
the kidney tissue. Following 1 month of glucocorticoid therapy,
serum creatinine and urea nitrogen levels returned to normal.
Case report
A 50-year-old woman with a >5 year history of
diabetes mellitus presented with pantoprazole-induced acute kidney
disease in July 2017 at Shandong University Qilu Hospital (Jinan,
China). Blood glucose was usually controlled within the normal
range (reference range, 3.6–6.1 mmol/l) and there was no prior
medical history of hypertension, hematuria, proteinuria or other
kidney disease. According to her own narrative, the patient had a
history of chronic gastritis, which was not treated. There was no
history of treatment with and pharmacological agents or Chinese
herbal medicine with the exception of long-acting insulin by
subcutaneous injection once per day. The protocol of the current
study was approved by the ethics committee of Shandong University
Qilu Hospital. Written informed consent was obtained from the
patient after the patient and the patient's family seriously and
carefully read and understood a written summary of the study
plan.
Prior to admission to the emergency department, the
patient had elevated serum glucose (16.3 mmol/l, measured at a
community clinic) for 1 day and complained of mild nausea without
vomiting, abdominal pain or diarrhea. The results of laboratory
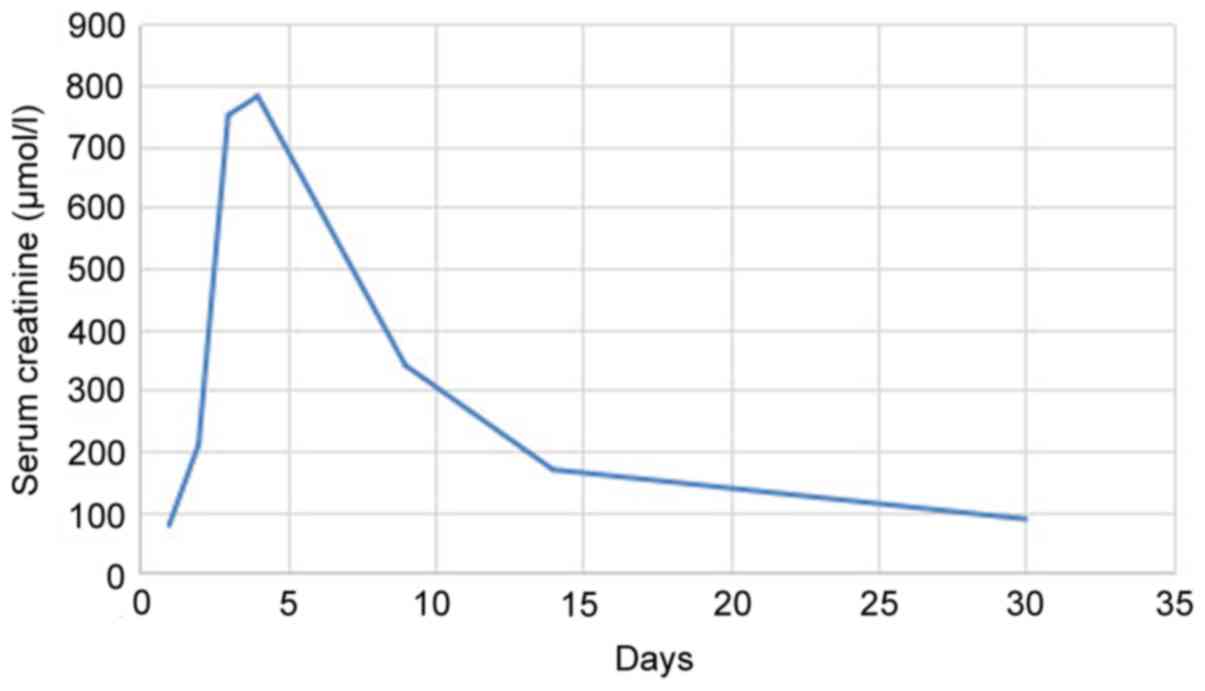
tests revealed serum creatinine (Scr) 78 µmol/l (normal reference
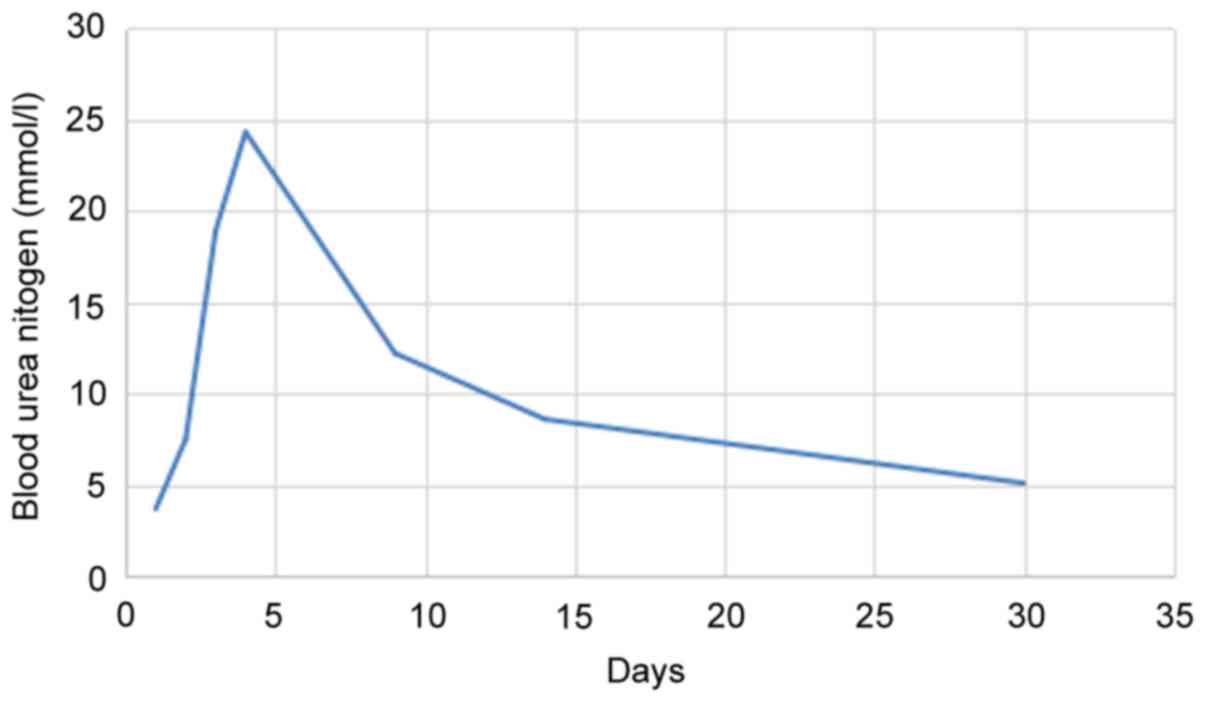
value, 53–97 µmol/l), blood urea nitrogen 3.7 mmol/l (normal
reference value, 2.3–7.8 mmol/l), hemoglobin (Hgb) 136 g/l (normal
reference value, 115–165 g/l), serum glucose (Glu) 15.7 mmol/l
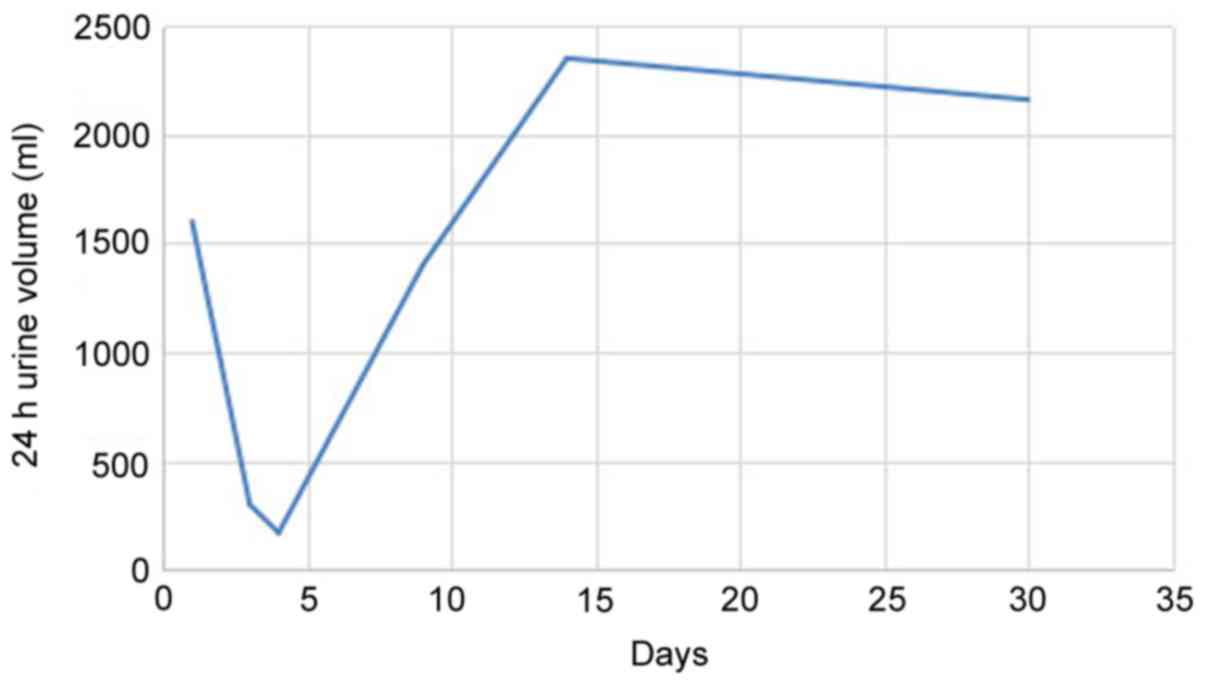
(normal reference value, 3.1–5.6 mmol/l) and 24 h urine volume
1,600 ml (normal reference value, 1,000–2,000 ml/24 h). All
relative indicators were measured from venous blood drawn 8 h after
fasting and centrifuged at 11,000 × g for 15 min at 25°C. Due to
the patient's mild nausea and history of chronic gastritis, the
patient was treated with pantoprazole (40 mg once a day,
intravenous infusion) and intermediate acting insulin (12 U at 8
a.m. and 10 U at 5 p.m., subcutaneous injection) for 2 days.
Following treatment, Glu recovered to 6.4 mmol/l; however, the
patient reported increased nausea, 24 h urine output was
significantly reduced to 300 ml (Fig.
1) and Scr increased from 78 to 750 µmol/l (Fig. 2). Blood urea nitrogen also increased
from to 18.9 mmol/l (Fig. 3). The
patient was admitted to the Department of Nephrology.
Upon admission a physical examination revealed mild
edema in bilateral eyelids and lower limbs without malar rash, oral
ulcers or diffuse alopecia. Pertinent laboratory findings included
Scr 781 µmol/l, Hgb 101 g/l, blood urea nitrogen 24.3 mmol/l, Glu
5.7 mmol/l and 24 h urine volume 170 ml. White blood cell and
platelet counts were normal. Parathyroid hormone, Ca2+
and P3+ levels were normal. Tests for anti-glomerular
basement membrane antibody and anti-neutrophil antibody were
negative. Glycosylated hemoglobin was 6.1% and the brain
natriuretic peptide (BNP) concentration was 1,863 pg/ml. Urinary
β2 microglobulin concentration was 1.2 mg/l and the
urinary albumin-creatinine ratio was 0.01 g/gCr. Anti-nuclear
antibody spectrum, tumor markers, thyroid function, immunoglobulin,
complement C3 and C4, hepatitis B virus quantification and
coagulation results were all within the normal range. Serum
immunofixation electrophoresis was negative. Renal ultrasonography
revealed that the kidney volume was above normal (right,
12.1×6.3×4.4 cm; left, 11.7×6.9×4.9 cm; reference range
10–12.1×5-6×3–4 cm) (10). Lung
X-rays revealed no evidence of inflammation.
Following admission to the Department of Nephrology,
the patient immediately underwent renal biopsy. A total of two
renal biopsy specimens ~1.5 cm in length were obtained containing
100% cortex. Specimens were stained with hematoxylin and eosin at
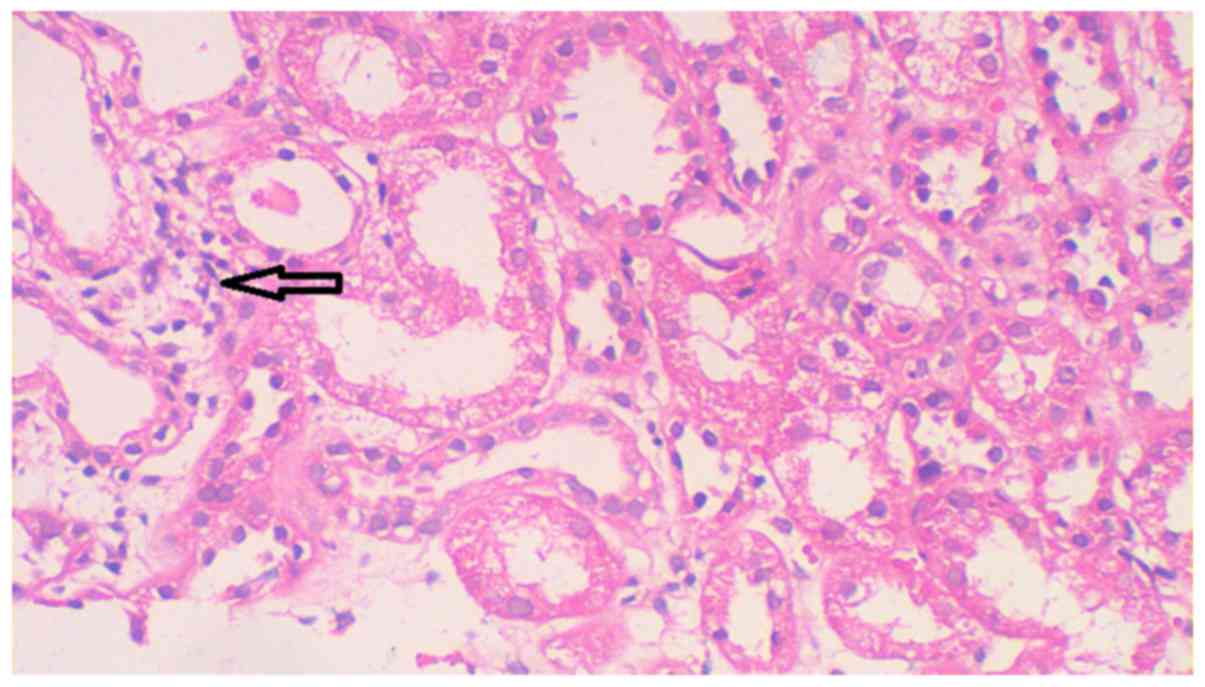
25°C for 40 min. Microscopy revealed 17 glomeruli in each section
without complete or peribulbar fibrosis (Fig. 4). No proliferation was observed in
the mesangial cells and matrix and no glomerular capillary
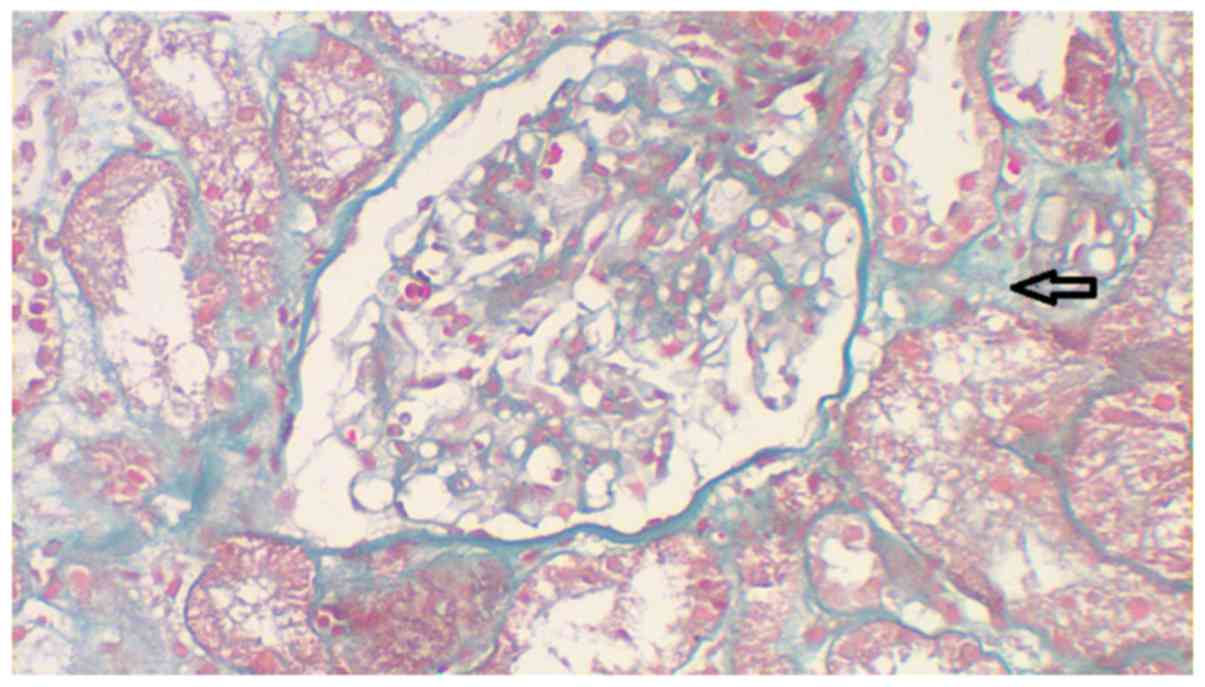
thickening was reported. Masson staining was performed at 25°C for
60 min and no immune complex deposition in the capillary walls was
observed (Fig. 5). Periodic
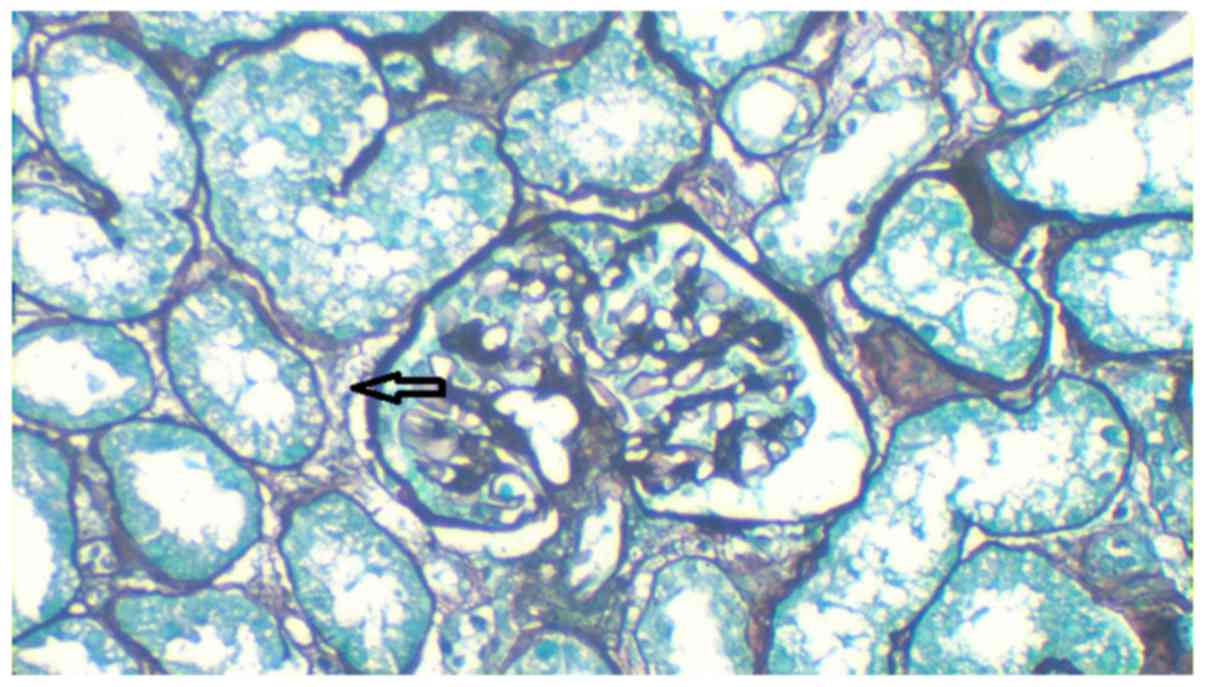
acid-silver methenamine staining was performed at 25°C for 30 min
and revealed no atrophy in the tubules and only a few tubules were
dilated with flat epithelial cells (Fig.
6). Severe edema with multifocal lymphocyte, monocyte and
eosinophil infiltration was observed in the renal interstitium;
however, there was no thickening of arterial walls (Figs. 4–6).
All samples were observed using a light microscope (magnification,
×400).
Sections (70-nm-thick) were fixed by 2.5%
glutaraldehyde solution and 1% osmium tetroxide at 4°C for 4 and 2
h, respectively. The sections were then double stained with 3%
uranyl acetate and lead citrate at 25°C for 2 min, and embedded by
epon812, an epoxy resin, for 30 min at room temperature. Electron
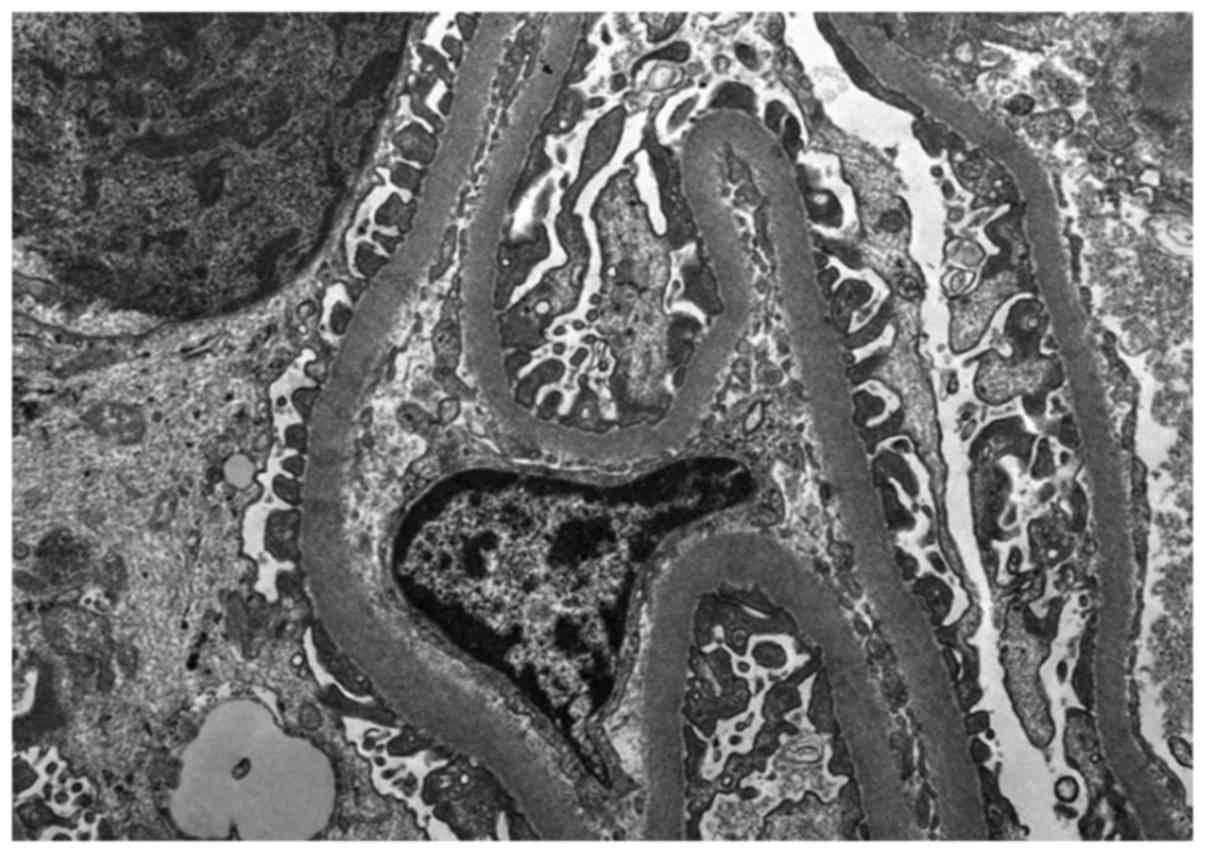
microscopy revealed no significant proliferation or expansion of
mesangial cells or matrix (Fig. 7).
The capillary wall basement membrane exhibited no thickening,
shrinkage or significant electron-dense deposits. The morphology of
podocytes was normal and small segmental foot processes appeared
fused. Tubular epithelial cells and organelles were swollen. The
tubular basement membrane appeared almost normal. Interstitial
focal edema and inflammatory cell infiltration were observed. The
interstitium exhibited severe edema with inflammatory cell
infiltration (Fig. 7).
Based on her history, clinical manifestations and
laboratory results, the patient was diagnosed with
pantoprazole-induced acute kidney injury. Pantoprazole treatment
was ceased and the patient was given renal replacement therapy
(RRT; hemodialysis). The patient was managed with standard
prednisolone (1 mg/kg/day) (11)
and, following 5 days of treatment, 24 h urine volume increased to
1,400 ml, Scr decreased to 340 µmol/l and blood urea nitrogen was
12.2 mmol/l. RRT treatment was ceased and within 2 weeks the
patient's 24 h urine volume increased to 2,350 ml, Scr was 169
µmol/l, Hgb was 131 g/l, BNP was 377 pg/ml, blood urea nitrogen was
8.6 mmol/l and Glu was 5.3 mmol/l. The patient was discharged and
follow up was performed 1 month later. At follow up, the patient's
24 h urine volume had increased to 2,160 ml, Scr was 88 µmol/l, ESR
was 11 mm/l, Hgb was 137 g/l, BNP was 169 pg/ml, blood urea
nitrogen was 5.1 mmol/l and Glu was 5.4 mmol/l (Figs. 1–3).
Standard prednisolone was reduced according to her clinical
manifestations and laboratory tests. At first 1 mg/kg/day
prednisolone was administered, 2 weeks later it was reduced to 0.5
mg/kg/day, then 0.2 mg/kg/day 1 month later; after 2 months,
prednisolone administration was stopped.
Discussion
Pantoprazole is used clinically to reduce gastric
acid secretion (11). It is an
anti-ulcer PPI derived from benzimidazole (12). Pantoprazole is able to relieve pain
in patients with duodenal ulcers and improve nausea, bloating, acid
reflux, belching and other ulcer-associated symptoms (13). Pantoprazole begins to act within
15–30 min of intravenous administration and 86% of gastric acid
secretion is inhibited within 60 min (14).
The bioavailability of pantoprazole is >75% and
it is metabolized primarily in the liver without interaction with
cytochrome P450 (15). As such,
pantoprazole metabolism does not affect the metabolism of other
drugs in the liver (16).
Approximately 80% of pantoprazole metabolites are excreted by the
kidneys and retained in the stool (17). The plasma clearance rate is 11 l/h,
as so pantoprazole has relatively fewer adverse side effects
compared with other pharmacological agents (18). Side effects include occasional
dizziness, insomnia, drowsiness, nausea, diarrhea, constipation,
rash, muscle pain, arrhythmia, increased aminotransferases and
decreased granulocytes (19).
There have been few reports of pantoprazole-induced
acute kidney disease (20,21). Pantoprazole-induced acute kidney
injury was first reported in 2004 and there have been no more than
100 publicly reported cases (22).
The patient presented here was the first case of
pantoprazole-induced acute kidney disease at the Department of
Nephrology, Shandong University Qilu Hospital. The mean duration of
exposure to pantoprazole prior to the onset of acute kidney injury
is 3 months, although it has also been reported to occur within
hours of pantoprazole administration (23). The symptoms of acute kidney injury
are generally nonspecific, for instance fatigue and malaise
(24). Acute renal failure is the
only consistent clinical presentation, although oliguria is unusual
(24). Nausea and vomiting are
present in 1/3 of cases (25). In
the present study, the patient was managed with pantoprazole for 2
days and reported increased nausea without vomiting, fatigue or
malaise. The classic triad of fever, rash and eosinophilia was not
present. Reports about proteinuria in pantoprazole-induced renal
injury are rare (26) and no protein
was identified in urine of the patient in the present study.
In the present case report, Scr and blood urea
nitrogen were markedly increased and 24 h urine output was
decreased compared with normal ranges following pantoprazole
treatment. Following 5 days of treatment with RRT and prednisolone,
these parameters were improved and continued to improve with
prednisolone treatment alone until the patient was discharged 2
weeks later. Upon admission to the Department of Nephrology, a
biopsy revealed that the renal pathology was consistent with acute
kidney injury. Therefore, the diagnosis of pantoprazole-induced
acute kidney injury was established.
Renal biopsy remains the gold standard for
diagnosis. Typical histopathological findings include interstitial
edema with mononuclear cells, T lymphocytes, eosinophils and plasma
cell infiltration around the renal tubules, sparing the glomeruli
and blood vessels (14,26). Initially, interstitial fibrosis is
mild and diffuse, however this may progress to tubular atrophy or
extensive interstitial fibrosis and glomerulosclerosis (13). In the present study, a renal biopsy
was performed two days after admission to the hospital, revealing
that the patient exhibited severe edema with multifocal lymphocytes
and monocytes and eosinophil infiltration. No thickening of the
arterial wall in the renal interstitium was observed in light or
electron micrographs.
The underlying mechanism of pantoprazole-induced
acute kidney injury is complex. Sensitivity to pantoprazole is the
primary reason for the onset of acute kidney injury and may only be
confirmed by renal pathology (27).
The standard diagnostic method for pantoprazole-induced acute
kidney injury is monitoring the response to prednisone treatment
(26). The use of steroids for the
treatment of acute kidney injury is controversial. Intravenous
Methylprednisolone pulses (250–500 mg/day for 3–4 days) followed by
a tapering course of prednisone (0.5–1 mg/kg/day) over 4–6 weeks
have been suggested as a treatment regimen for pantoprazole-induced
acute kidney injury (26). In the
present study, the patient was treated by discontinuing
pantoprazole, administering short term RRT and long-term
prednisolone management.
In the present study, the onset of acute kidney
injury was clearly associated with pantoprazole administration and.
Prednisolone therapy is considered to be an effective treatment for
drug-induced acute kidney injury. Although the incidence of
pantoprazole-induced acute kidney injury is low, the prognosis is
good as long as the condition is correctly diagnosed at the
earliest opportunity.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Outstanding
Young Scientist Research Award Fund Project of Shandong Province
(grant no. BS2013YY042) and the Science and Technology Development
Plan of Shandong Province (grant no. 2014GSF121005).
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
TP, XY and ZH designed the study and drafted the
manuscript. HZ and CM collected the clinical and imaging data. JZ
collected and analyzed the pathological data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Shandong University Qilu Hospital (Jinan, China).
Written informed consent was obtained from the patient after the
patient and the patient's family seriously and carefully read and
understood a written summary of the study plan.
Consent to participate and for
publication
The patient gave her consent for the publication of
this case report and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bellomo R, Kellum JA and Ronco C: Acute
kidney injury. Lancet. 380:756–766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petejova N and Martinek A: Acute kidney
injury due to rhabdomyolysis and renal replacement therapy: A
critical review. Crit Care. 18:2242014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaffney AM and Sladen RN: Acute kidney
injury in cardiac surgery. Curr Opin Anaesthesiol. 28:50–59. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng T, Hu Z, Yang X, Gao Y and Ma C:
Nitrite-induced acute kidney injury with secondary
hyperparathyroidism: Case report and literature review. Medicine
(Baltimore). 97:e98892018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Welsh C, Kasirer MY, Pan J, Shifrin Y and
Belik J: Pantoprazole decreases gastroesophageal muscle tone in
newborn rats via rho-kinase inhibition. Am J Physiol Gastrointest
Liver Physiol. 307:G390–G396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knoth H: Electrochemical behaviour of
pantoprazole. Pharmazie. 59:2312004.(In German). PubMed/NCBI
|
|
7
|
Schiller D, Maieron A, Schöfl R and
Donnerer J: Drug fever due to a single dose of pantoprazole.
Pharmacology. 94:78–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arbel Y, Birati EY, Finkelstein A, Halkin
A, Kletzel H, Abramowitz Y, Berliner S, Deutsch V, Herz I, Keren G
and Banai S: Platelet inhibitory effect of clopidogrel in patients
treated with omeprazole, pantoprazole, and famotidine: A
prospective, randomized, crossover study. Clin Cardiol. 36:342–346.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Das S, Ganguly A, Ghosh A, Mondal S, Dey
JK and Saha I: Oral pantoprazole-induced acute pancreatitis in an
11-year-old child. Ther Drug Monit. 34:242–244. 2012.PubMed/NCBI
|
|
10
|
Engelhorn Valiente AL, Engelhorn CA,
Salles-Cunha SX, Ehlert R, Akiyoshi FK and Assad KW: Ultrasound
tissue characterization of the normal kidney. Ultrasound Q.
28:275–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Colmenares EW and Pappas AL: Proton pump
inhibitors: Risk for myopathy? Ann Pharmacother. 51:66–71. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lo EA, Wilby KJ and Ensom MH: Use of
proton pump inhibitors in the management of gastroesophageal
varices: A systematic review. Ann Pharmacother. 49:207–219. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klassen S, Krepinsky JC and Prebtani AP:
Pantoprazole-induced acute interstitial nephritis. CMAJ. 185:56–59.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torlot FJ and Whitehead DJ: Acute
interstitial nephritis caused by two different proton pump
inhibitors. Br J Hosp Med (Lond). 77:50–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shakhnovich V, Smith PB, Guptill JT, James
LP, Collier DN, Wu H, Livingston CE, Zhao J and Kearns GL: Best
Pharmaceuticals for Children Act-Pediatric Trials Network: Obese
children require lower doses of pantoprazole than nonobese peers to
achieve equal systemic drug exposures. J Pediatr. 193:102–108.e1.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sherwood MW, Melloni C, Jones WS, Washam
JB, Hasselblad V and Dolor RJ: Individual proton pump inhibitors
and outcomes in patients with coronary artery disease on dual
antiplatelet therapy: A systematic review. J Am Heart Assoc.
4:e0022452015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dias Moreira L: Pantoprazole: A proton
pump inhibitor. Clin Drug Investig. 29 Suppl 2:S3–S12. 2009.
View Article : Google Scholar
|
|
18
|
Jiao HW, Sun LN, Li YQ, Yu L, Zhang HW,
Wang MF, Yu LY, Yuan ZQ, Xie LJ, Chen J, et al: Safety,
pharmacokinetics, and pharmacodynamics of S-(−)-pantoprazole sodium
injections after single and multiple intravenous doses in healthy
Chinese subjects. Eur J Clin Pharmacol. 74:257–265. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aslan M, Celik Y, Karadas S, Olmez S and
Cifci A: Liver hepatotoxicity associated with pantoprazole: A rare
case report. Wien Klin Wochenschr. 126:390–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MJ, Heim M and Mayr M: Effect of
corticosteroids during ongoing drug exposure in
pantoprazole-induced interstitial nephritis. Nephrol Dial
Transplant. 25:1716–1719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao Y, Fan Y, Xie Y, Yin L, Zhang Y, Deng
L, Sun X, Shao X, Tan X, He J and Zhao S: Effect of continuous
renal replacement therapy on kidney injury molecule-1 and
neutrophil gelatinase-associated lipocalin in patients with septic
acute kidney injury. Exp Ther Med. 13:3594–3602. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moore I, Sayer JA, Nayar A, Ahmed S and
Tapson JS: Pantoprazole-induced acute interstitial nephritis. J
Nephrol. 17:580–581. 2004.PubMed/NCBI
|
|
23
|
Simpson IJ, Marshall MR, Pilmore H, Manley
P, Williams L, Thein H and Voss D: Proton pump inhibitors and acute
interstitial nephritis: Report and analysis of 15 cases. Nephrology
(Carlton). 11:381–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Avinash A, Patil N, Kunder SK, Balaji O,
Tilak A, Sori RK and Rao R: A retrospective study to assess the
effect of proton pump inhibitors on renal profile in a south indian
hospital. J Clin Diagn Res. 11:FC09–FC12. 2017.PubMed/NCBI
|
|
25
|
Ra A and Tobe SW: Acute interstitial
nephritis due to pantoprazole. Ann Pharmacother. 38:41–45. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sampathkumar K, Ramalingam R, Prabakar A
and Abraham A: Acute interstitial nephritis due to proton pump
inhibitors. Indian J Nephrol. 23:304–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Geevasinga N, Coleman PL, Webster AC and
Roger SD: Proton pump inhibitors and acute interstitial nephritis.
Clin Gastroenterol Hepatol. 4:597–604. 2006. View Article : Google Scholar : PubMed/NCBI
|