Introduction
The incidence of Klebsiella pneumoniae liver
abscesses (KPLA) has increased in a number of Asian countries over
the past 30 years, particularly in Taiwan and Korea (1,2). Shi
et al (3)analyzed clinical
data from 296 cases of bacterial liver abscess collected between
1994 and 2015. Their results revealed that KPLA accounted for 63.9%
of cases, while 13.2% were attributable to Escherichia coli
liver abscesses (3). K.
pneumoniae is a Gram negative bacteria that is colonized as
normal flora in the human gut but is prone to causing parenteral
infection and even bacteremia in patients with poor immunity
(4).
A number of factors predispose patients with
diabetes mellitus (DM) to infections, including genetic
susceptibility to infection, altered cellular and humoral immune
defense mechanisms, local factors (e.g., poor blood supply and
nerve damage) and DM-associated metabolic changes (5). Increasing evidence suggests that
DM-induced hyperglycemia may cause vascular endothelial damage
(6,7), which contributes to infections. K.
pneumoniae is considered to be a common pathogen in patients
with DM (8). A previous study
reported that patients with DM were 3.6× more likely to develop
bacterial liver abscesses compared with control subjects (9). Lin et al (10) reported that patients with poor blood
glucose control (glycosylated hemoglobin HbA1c <7%) and had a
higher prevalence of recessive liver abscesses, liver abscesses and
gas transfer compared with patients with good blood glucose control
(HbA1c=7%) (11). However, the
molecular mechanism underlying the increased incidence of K.
pneumoniae liver abscess in patients with DM remains unclear.
It has been demonstrated that K. pneumoniae infection
increases the inflammation responses (12).
In the present study, a streptozotocin (STZ)-induced
DM mouse model was constructed and infected with K.
pneumoniae to induce KPLA. Pathological changes in liver
tissues and the expression of NFκB pathway components were
assessed.
Materials and methods
Bacterial strains and animals
K. pneumoniae was isolated from a male 58
year old patient with a liver abscess confirmed by the Department
of Interventional and Vascular Surgery, Tenth People's Hospital of
Tongji University. This procedure included a fine-needle aspiration
of the patients' fluctuant mass from which and 1 ml of purulent
fluid was obtained and sent for culture under 37°C and with a Sheep
Blood AGAR Plate (Shanghai Yihua Medical Technology Co., Ltd.)
which yielded Klebsiella pneumoniae, all process was under
anesthesia with 3% sevoflurane (anesthetized by inhalation). A
total of 48 6–8-week-old male C57BL/6 mice (n=48) were purchased
from Fudan University Animal Care Committee (Shanghai, China) and
were individually housed at 22°C with 60% humidity, a 12 h
light/dark cycle and free access to tap water and food. Animals
were acclimated to the laboratory environment for ≥3 weeks prior to
experiments.
Ethics statement
All experiments were approved by the Ethics
Committee of the Tenth People's Hospital of Tongji University
(Shanghai, China) and performed in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (National Institutes of Health Bethesda, MD, USA). All mice
were anesthetized by intraperitoneal injection of sodium
pentobarbital (30 mg/kg) prior to euthanasia.
Generation of the type 1 DM mouse
model
Mice were intraperitoneally injected with 50, 75,
100 or 120 mg/kg of streptozotocin (STZ; Sigma-Aldrich, Merck KGaA,
Darmstadt, Germany) dissolved in 0.05 M citrate buffer (pH
4.1–4.5). Blood glucose levels and weight were measured at
different times (including 0, 7, 14, 21 and 28 days). Based on the
results (Fig. 1), treatment with 100
mg/kg, STZ resulted in a significant increase in blood glucose
compared with lower dosages, however no significant difference in
blood glucose levels was observed between the 100 and 120 mg/kg
dosage groups. As such, 100 mg/kg STZ was selected as previously
reported (3). Control mice were
treated with the same amount of citrate buffer without STZ. Mice
with blood sugar levels >20 mmol/l were considered to have DM.
Following 7 days of STZ treatment, mice exhibited symptoms of DM,
including polydipsia, polyphagia, polyuria and emaciation. Blood
glucose levels were >20 mmol/l. At 1 month following injection,
the blood glucose levels stabilized at 27–30 mmol/l.
Infection of mice with K. pneumoniae
bacteria
The K. pneumoniae strain was cultured on
sheep blood agar plates (Shanghai Yihua Medical Technology Co.,
Ltd.) overnight under 37°C and harvested using PBS to make a
bacterial suspension of 3×107 colony-forming units (CFU)
per 50 µl. The 50-µl bacterial suspensions were orally inoculated
into the mice. Mice were divided into four groups: PBS control
normal mouse group (n=6), PBS-control DM group (n=6), K.
pneumoniae-infected DM group (n=18) and K.
pneumoniae-infected normal mouse group (n=18).
Liver tissue pathological
examinations
Liver tissues were dissected 7 days following
inoculation with K. pneumoniae, fixed in 10% formalin for 24
h at room temperature, washed twice with PBS and maintained in PBS
for 24 h prior to dehydration in a graded series of ethanol for 45
min. Finally, the samples were immersed in xylene for 20 min at
room temperature and tissues were embedded in wax. Sections from
the tissue block were cut on a microtome to a thickness of 5 µm and
fixed on a glass slide. Paraffin was removed using xylene and then
the slices were rinsed with a graded series of ethanol followed by
distilled water. The tissue sections were stained with hematoxylin
solution at room temperature for 3 min and rinsed with acidified
water and ammonia with water for 30 sec. Tissue sections were
rinsed in water for 1 h and then stored in distilled water,
following which they were rinsed with 70 and 90% alcohol for 10 min
each. Sections were stained with eosin for 2 min at room
temperature. The stained sections were dehydrated using absolute
alcohol and xylene and subsequently covered with sealing gum and
glass coverslips.
The degree of liver inflammation was determined by a
blinded investigator as previously described (13). Scoring was as follows: 1, <10
micro-abscesses on each liver section and no necrotic regions; 2,
>10 and <20 micro-abscesses on each liver section and no
necrotic regions; 3, >20 and <30 micro-abscesses on each
liver section and that no necrotic regions; 4, >30
micro-abscesses on each liver section and no necrotic regions; 5,
<5 necrotic regions; 6, >5 and <10 necrotic regions; 7,
>10 and <15 necrotic regions; 8, >15 necrotic regions. A
total of three different sections from the largest liver lobule of
each mouse were examined. Histological assessments of intestines
were performed using a scoring system as previously described
(14).
ELISA
The expression of inflammatory factors and
chemokines, including interleukin (IL)-1β (cat. no. EMC021), IL-2
(cat. no. EMC0623), IL-6 (cat. no. EMC0003), IL-10 (cat. no.
EMC106), tumor necrosis factor (TNF)-α (cat. no. EMC323) and
macrophage inflammatory protein (MIP)-1α (cat. no. EMC266) in the
liver lysates (liver tissues were harvested in lysis buffer (BD
Biosciences, Franklin Lakes, NJ, USA) and lysates were cleared
using centrifugation at 12,000 × g for 10 min at 4°C) was measured
by using standard ELISA kits (QuantiCyto, Shenzhen, China)
according to the manufacturer's protocol and normalized to the
levels of total proteins.
Western blot analysis
Livers from all experimental groups were homogenized
with lysis buffer (8 M urea, 50 mM DTT, 2% CHAPS and complete
protease and phosphatase inhibitors). The concentration of
resulting suspensions (liver tissues were harvested in lysis buffer
(BD Biosciences) and lysates were cleared by centrifugation at
12,000 × g for 10 min at 4°C) was quantified with the
two-dimensional Quant kit (GE Healthcare Life Sciences, Little
Chalfont, UK). A total of 30 µg protein/lane was separated by 8%
SDS-PAGE and transferred onto a polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). Membranes were blocked with 2%
skim milk at room temperature for 1 h and probed with the following
antibodies at 4°C overnight: anti-inhibitor of κB (IκB) kinase α
(IKKα; cat. no. ab32041; 1:500; Abcam, Cambridge, UK), anti-IKKβ
(cat. no. 2684; 1:500), anti-IKBα (cat. no. 9242; 1:500), anti-NFκB
(cat. no. 4810; 1:500), anti-phosphorylated (p)-IKKα (cat. no.
11930; 1:1,000), anti-p-IKKβ (cat. no. 8943; 1:1,000), anti-p-IKBα
(cat. no. 4814; 1:1,000) and anti-p-NFκB (cat. no. 8242; 1:1,000;
all Cell Signaling Technology, Inc., Danvers, MA, USA). Following
hybridization with horseradish peroxidase (HRP)-conjugated
secondary antibodies (cat. no. 5836; 1:1,000; Cell Signaling
Technology, Inc.) for 1 h at room temperature, the blots were
developed with Immobilon™ Western Chemiluminescent HRP Substrate
(EMD Millipore) and visualized with an ImageQuant™ LAS 4000 Mini
Biomolecular Imager (GE Healthcare Life Sciences). The band
intensities were quantified using a UN-SCAN-IT gel, version 6.0
software (Silk Scientific, Orem, UT, USA).
Statistical analysis
Statistical analyses were performed using SPSS
version 2.0 (SPSS, Inc., Chicago, IL, USA). Statistical results are
expressed as the mean ± standard deviation. Statistical analyses
were performed using one-way analysis of variance and a post-hoc
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
DM mouse model construction
In order to construct a model of mice with stable
DM, 0–120 mg/kg STZ was administered via intraperitoneal injection.
Following 7 days, blood glucose levels were detected and the
results revealed that 100 mg/kg STZ resulted in a stable increase
in blood glucose, and so was selected for further experiments.
Pathological changes in mouse liver
tissues
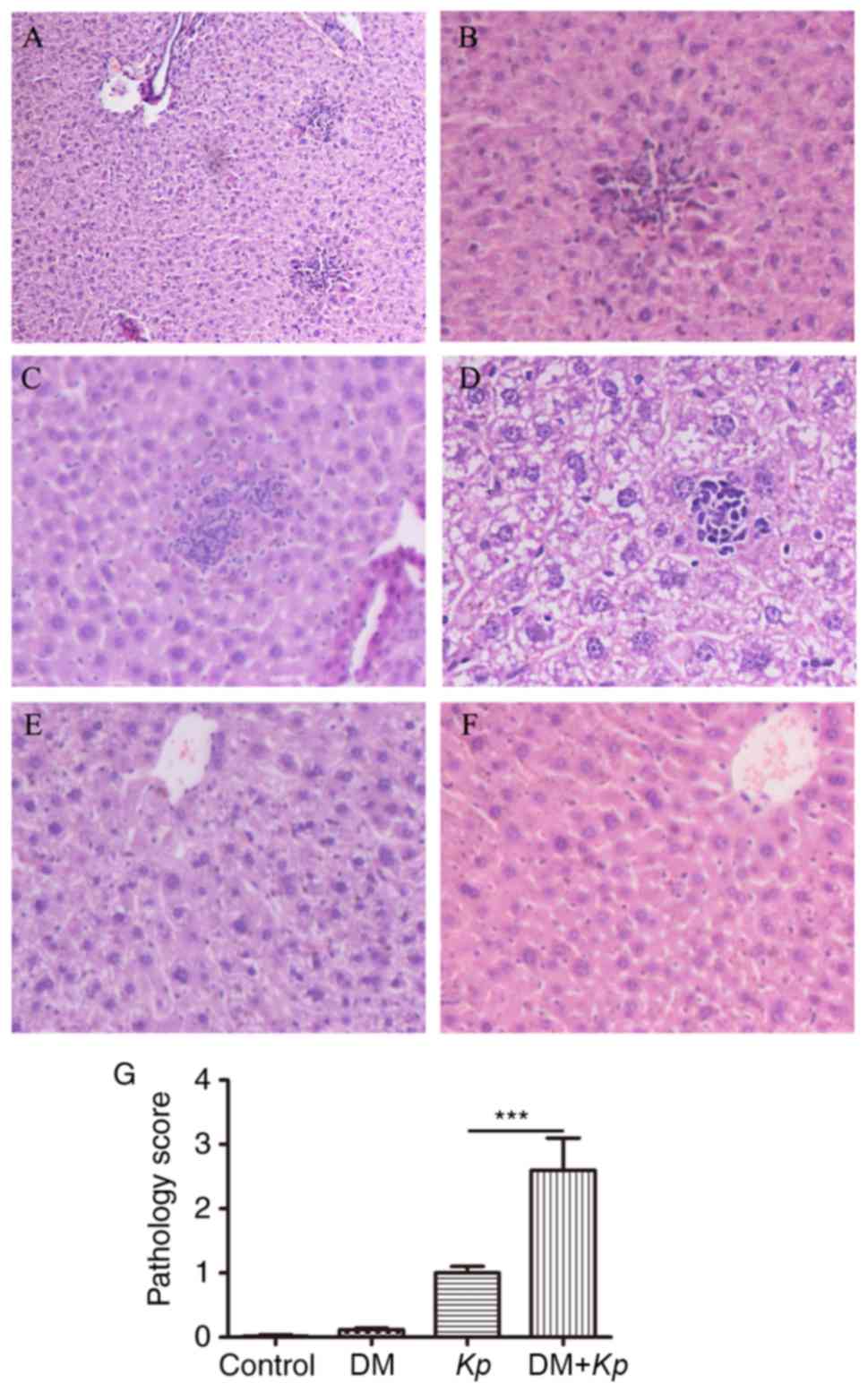
Liver tissue abscesses were observed under an
optical microscope (magnification, ×200). The liver cells from
normal mice and mice with DM were round, borders were clear, cell
nuclei and cytoplasm were stained normally and there were no
inflammatory cell aggregations in the portal areas. However, in
normal mice and mice with DM infected with K. pneumoniae,
foci of hepatic necrosis, diffuse infiltration of neutrophils,
small amounts of lymphocytes in the necrotic areas and balloon-like
lesions of the peripheral liver cells were observed. In 2/18 K.
pneumoniae-infected normal mice the focal liver cells were
enlarged, cell boundaries were unclear, cytoplasm was pale and
loose, and a small amount of neutrophils and lymphocytes had
collected in the portal areas. The liver tissues of 5/18 mice in
this group exhibited no inflammatory responses. A total of 3/18
K. pneumoniae-infected normal mice presented with focal
hepatic necrosis, diffuse infiltration of neutrophils, small
numbers of lymphocytes in the necrotic areas and balloon-like
lesions of the peripheral liver cells. Furthermore, 4/18 K.
pneumoniae-infected mice with DM had enlarged focal liver
cells, unclear cell boundaries, pale and loose cytoplasm and small
numbers of neutrophils and lymphocytes were collected in the portal
areas (Fig. 2A-F). To obtain further
insight into the role of the diabetic microenvironment in KPLA,
semi quantitative analyses were performed on liver sections
prepared from K. pneumoniae treated mice. Significantly more
liver damage (assessed by a higher pathology score) was observed in
K. pneumoniae-infected mice with DM compared with K.
pneumoniae-infected normal mice (P<0.001; Fig. 2G). Little to no liver damage was
observed in normal mice and mice with DM.
Expression of tissue inflammatory
factors
The results revealed that the expression of IL-1β,
IL-2, IL-6, MIP-1α and TNFα was significantly increased in liver
tissues from K. pneumoniae-infected mouse with DM compared
with K. pneumoniae-infected normal mice (Fig. 3). The expression of IL-1β, IL-2,
IL-6, MIP-1α and TNFα was increased in liver tissues from K.
pneumoniae-infected mice with DM compared with PBS control mice
with DM (P<0.05; Fig. 3). The
expression of IL-1β, IL-2, IL-6, MIP-1α and TNFα were not
significantly different in liver tissues from K.
pneumoniae-infected normal mice compared with control mice
(P<0.05; Fig. 3), suggesting that
K. pneumoniae infection promotes inflammatory responses.
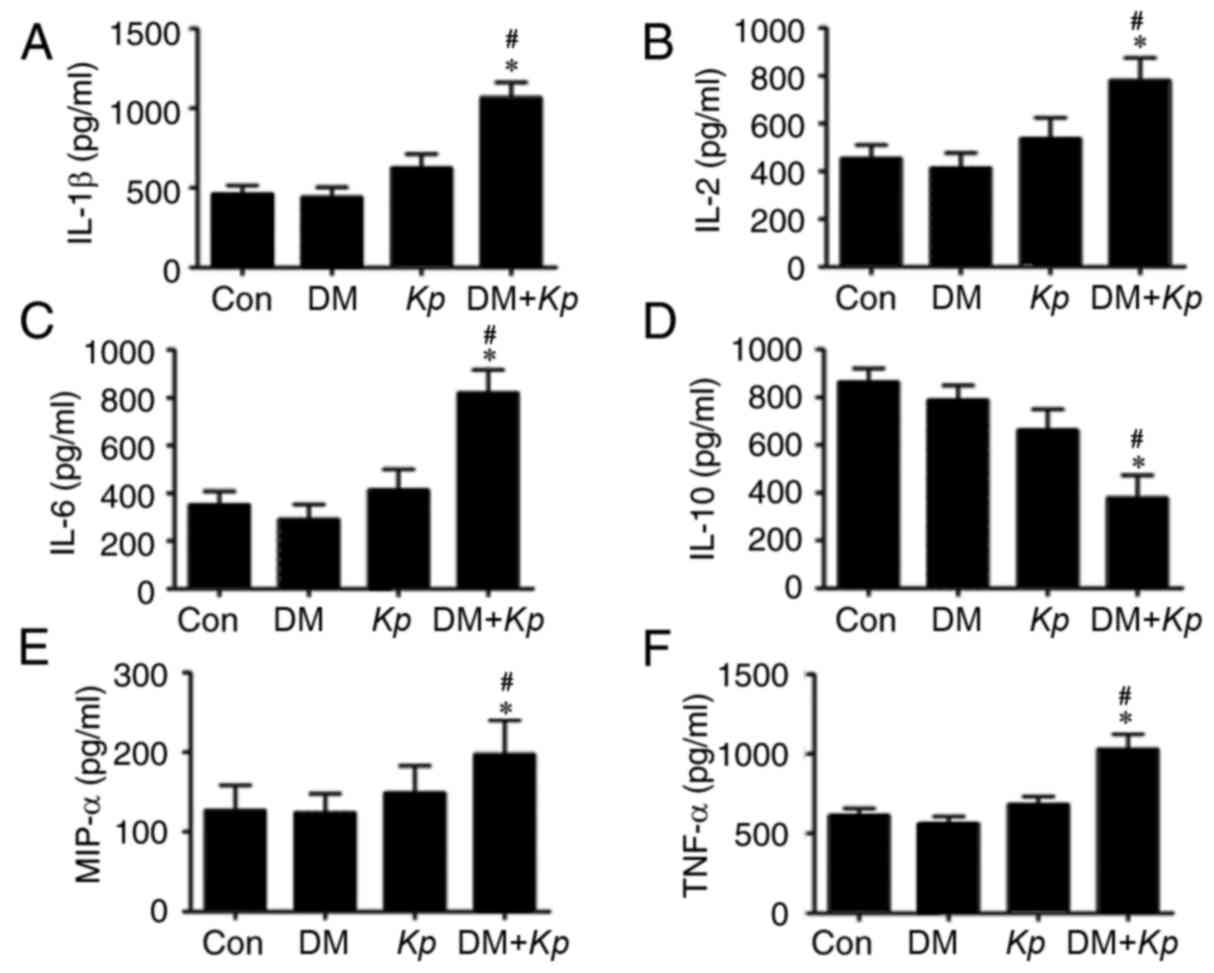 | Figure 3.ELISA assays for (A) IL-1β, (B) IL-2,
(C) IL-6, (D) IL-10, (E) MIP-1α, (F) TNFα in mice with or without
Kp and DM. *P<0.05 vs. Con; #P<0.05 vs.
K. pneumoniae infected normal mice. IL, interleukin; MIP,
macrophage inflammatory protein; TNF, tumor necrosis factor; Con,
control; Kp, Klebsiella pneumoniae; DM, diabetes
mellitus. |
Western blot analysis
The results of western blotting revealed that the
expression of p-IKKβ, p-IKBα and p-NFκB was significantly higher in
liver tissues from K. pneumoniae-infected mice with DM
compared with K. pneumoniae-infected normal mice (P<0.05;
Fig. 4). But the expression of IKKα,
IKKβ, IKBα and p-NFκB did not exhibit any significant differences.
The expression of p-IKKα, p-IKKβ, p-IKBα and p-NFκB in the liver
tissues of K. pneumoniae-infected mice with DM was
significantly higher compared with normal mice with DM (P<0.05;
Fig. 4). The expression of p-IKKα,
p-IKKβ, p-IKBα and p-NFKB in liver tissues from K.
pneumoniae-infected normal mice was significantly higher
compared with control mice (P<0.05; Fig. 4).
 | Figure 4.(A) Expression of NFκB signaling
pathway-associated proteins was measured using western blotting.
Relative expression of (B) p-IKKα, (C) IKKα, (D) p-IKKβ, (E) IKKβ,
(F) p-IKBα, (G) IKBα, (H) p-NFκB and (I) NFκB. *P<0.05 vs.
control. #P<0.05 vs. K. pneumoniae-infected
normal mice. &P<0.05 vs. uninfected mice with DM.
NF, nuclear factor; p, phosphorylated; IKK, inhibitor of κB kinase;
Kp, Klebsiella pneumoniae; DM, diabetes mellitus. |
Discussion
In the present study, DM was induced in mice via
intraperitoneal injections of 100 mg/kg STZ. Each mouse was orally
inoculated with K. pneumoniae to establish the liver abscess
model and liver tissues were separated 7 days later.
Histopathological examination revealed focal necrosis of liver
cells, diffuse infiltration of neutrophils and lymphocytes,
hepatocyte swelling, unclear cell boundaries, slightly stained
cytoplasm, cytoplasmic osteoporosis and ballooning, all of which
are indicative of liver abscess formation. In this study, the
incidence of liver abscess formation in mice with DM was 50%, which
is lower than previously reported (15). This may be because liver extraction
was performed prior to liver cell edema and the infiltration of
small amounts of inflammatory cells did not result in abscess
formation. Alternatively, the K. pneumoniae strain may have
been to the previous study and have different virulence. Lastly,
when bacterial suspensions were orally inoculated, the operation
procedure may have resulted in the bacterial suspension failing to
enter the stomach of the mouse.
In the present study, the number of liver abscesses
observed in the K. pneumoniae-infected mice with DM was
higher compared with the K. pneumoniae-infected normal mice.
This indicated that that high glucose induced the NFκB signaling
pathways exerted a promotional effect on the pathogenesis of K.
pneumoniae bacteria induced liver abscess. After microbial
infection, the host immune system recognizes pathogen-associated
molecular patterns and stimulates the production of inflammatory
mediators, including ILs, complement, TNFα and neutrophils, as well
as macrophage aggregation, bacterial phagocytosis and degradation,
resulting in a protective immune response (16–18). In
the present study, the expression of IL-1β, IL-2, IL-6, MIP-1α and
TNFα in the K. pneumoniae-infected mice with DM was
increased compared with the control mice with DM. Furthermore, the
expression of these factors was also increased in K.
pneumoniae-infected normal mice compared with control mice.
Kupffer cells, capillary endothelial cells, monocytes and
macrophages produce IL-1β, IL-2, IL-6, MIP-1α and TNFα to activate
the inflammatory signaling pathways of NFκB, neutrophil
aggregation, phagocytosis and degradation (19,20). In
acute inflammation, IL-1β and TNFα are the main inflammatory
mediators and may be quickly released to activate the inflammatory
reaction cascade, or even induce endotoxin shock, with the
synergistic effects of NFκB (21).
MIP-1α is a chemotactic factor secreted by
macrophages that attracts neutrophils and macrophages to the site
of infection (16). High IL-1β, TNFα
and MIP-1α expression in mice with DM may increase the expression
of NFκB signaling pathway proteins and the acute inflammatory
reaction in the liver. Excessive inflammatory reactions in the body
can cause uncontrollable tissue damage and liver inflammation,
especially in hyperglycemic tissue microenvironments, resulting in
necrosis of the liver cells and abscess formation (22).
NFκB is a nuclear transcription factor that exists
as a dimer and is widely distributed in multicellular organisms.
IKK, IKB, NFκB and other family members coordinate inflammatory
responses, innate and adaptive immune responses and cell
differentiation and proliferation (21,23–25). In
inflammatory conditions, extracellular stimulation is able to
activate the NFκB signaling pathway proteins, whose expression is
crucial for the progression of the inflammatory response. IKK is a
IKB protein kinas, comprising three subunits; IKKα, IKKβ and IKK
(26). IKK is inactive in resting
cells; however, extracellular K. pneumoniae, IL-1β and TNFα
stimulate IKKα, IKKβ and NFκB activation via phosphorylation
(24,25,27). In
the present study, the expression of p-IKKα, p-IKKβ, p-IKBα and
p-NFκB in the K. pneumoniae-infected mice with DM was
increased compared with normal mice with DM. Furthermore, levels of
these proteins were also increased in K. pneumoniae-infected
normal mice compared with the control group. Following K.
pneumoniae infection of liver tissues, macrophages and Kupffer
cells secrete IL-1β, TNFα, MIP-1α and other cytokines, causing
hepatocyte IKKα and IKKβ phosphorylation, as well as the activation
and further phosphorylation of IKBα and NFκB (26). The activation of these proteins, in
turn, promotes the transcription of inflammatory cytokines and the
cascade of inflammatory processes, leading to liver cell damage,
necrosis and liver abscess formation (27). In the present study, the expression
of p-IKKβ, p-IKBα and p-NFκB in K. pneumoniae-infected mice
with DM was significantly higher compared with K.
pneumoniae-infected normal mice. Increased p-IKKβ, p-IKBα and
p-NFκB expression in DM mouse liver cells stimulates monocytes,
macrophages and liver Kupffer cells to secrete larger amounts of
IL-1β, IL-2, IL-6, MIP-1α, TNFα and other cytokines, attracting
inflammatory cells to the infected foci and causing hepatic edema,
lysis, death and even abscess formation (28).
In summary, the results of the present study suggest
that mice with DM have a higher incidence of KPLA due to the
excessive activation of NFκB cell signaling pathway proteins,
increased inflammation and increases liver cell necrosis. However,
further investigation is required to validate these results and
investigate additional factors that may contribute to KPLA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XL, LP, MZ and ML generated and analyzed the data.
DX, CC, RS, JL and SH designed the experiments and drafted the
manuscript. All authors approved the final version of the
manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee of the Tenth People's Hospital of Tongji University
(Shanghai, China) and performed in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (National Institutes of Health Bethesda, MD, USA). All mice
were anesthetized by intraperitoneal injection of sodium
pentobarbital (30 mg/kg) prior to euthanasia.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chung DR, Lee SS, Lee HR, Kim HB, Choi HJ,
Eom JS, Kim JS, Choi YH, Lee JS, Chung MH, et al: Emerging invasive
liver abscess caused by K1 serotype Klebsiella pneumoniae in
Korea. J Infect. 54:578–583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeh KM, Kurup A, Siu LK, Koh YL, Fung CP,
Lin JC, Chen TL, Chang FY and Koh TH: Capsular serotype K1 or K2,
rather than magA and rmpA, is a major virulence determinant for
Klebsiella pneumoniae liver abscess in Singapore and Taiwan.
J Clin Microbiol. 45:466–471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi R, Jin Y, Cao C, Han S, Shao X, Meng
L, Cheng J, Zhang M, Zheng J, Xu J and Li M: Localization of human
adipose-derived stem cells and their effect in repair of diabetic
foot ulcers in rats. Stem Cell Res Ther. 7:1552016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu JH and Tsai CG: Infectivity of hepatic
strain Klebsiella pneumoniae in diabetic mice. Exp Biol Med
(Maywood). 230:757–761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pozzilli P and Leslie RD: Infections and
diabetes: Mechanisms and prospects for prevention. Diabet Med.
11:935–941. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chao CL, Chuang CP, Cheng YF, Lee KR,
Chang Y, Cheng SP, Chan WK and Ho FM: The protective role of
autophagy in matrix metalloproteinase-mediated cell transmigration
and cell death in high-glucose-treated endothelial cells.
Inflammation. 39:830–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weikel KA, Cacicedo JM, Ruderman NB and
Ido Y: Knockdown of GSK3β increases basal autophagy and AMPK
signalling in nutrient-laden human aortic endothelial cells. Biosci
Rep. 36:e003822016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang YT, Jiang JY, Hsu MS, Hsu HS, Liao
CH and Hsueh PR: The prevalence of rectal carriage of Klebsiella
pneumoniae amongst diabetic patients and their clinical
relevance in Taiwan: A five-year prospective study. J Microbiol
Immunol Infect: S1684-1182(17)30119-6. 2017. View Article : Google Scholar
|
|
9
|
Keynan Y and Rubinstein E: Diabetes
mellitus and pyogenic liver abscess: Risk and prognosis. Clin
Infect Dis. 45:8012007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin YT, Wang FD, Wu PF and Fung CP:
Klebsiella pneumoniae liver abscess in diabetic patients:
Association of glycemic control with the clinical characteristics.
BMC Infect Dis. 13:562013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fung CP, Chang FY, Lin JC, Ho DM, Chen CT,
Chen JH, Yeh KM, Chen TL, Lin YT and Siu LK: Immune response and
pathophysiological features of Klebsiella pneumoniae liver
abscesses in an animal model. Lab Invest. 91:1029–1039. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen J: The immunopathogenesis of sepsis.
Nature. 420:885–891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin YT, Liu CJ, Yeh YC, Chen TJ and Fung
CP: Ampicillin and amoxicillin use and the risk of Klebsiella
pneumoniae liver abscess in Taiwan. J Infect Dis. 208:211–217.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hasegawa M, Yamazaki T, Kamada N,
Tawaratsumida K, Kim YG, Núñez G and Inohara N: Nucleotide-binding
oligomerization domain 1 mediates recognition of Clostridium
difficile and induces neutrophil recruitment and protection against
the pathogen. J Immunol. 186:4872–4880. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu MF, Yang CY, Lin TL, Wang JT, Yang FL,
Wu SH, Hu BS, Chou TY, Tsai MD, Lin CH and Hsieh SL: Humoral
immunity against capsule polysaccharide protects the host from
magA+ Klebsiella pneumoniae-induced lethal disease by
evading Toll-like receptor 4 signaling. Infect Immun. 77:615–621.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo N, Xu Y and Cao Z: Absinthin
attenuates LPS-induced ALI through MIP-1α-mediated inflammatory
cell infiltration. Exp Lung Res. 41:514–524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gerondakis S, Grumont R, Gugasyan R, Wong
L, Isomura I, Ho W and Banerjee A: Unravelling the complexities of
the NF-kappaB signalling pathway using mouse knockout and
transgenic models. Oncogene. 25:6781–6799. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vallabhapurapu S and Karin M: Regulation
and function of NF-kappaB transcription factors in the immune
system. Annu Rev Immunol. 27:693–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho YC, Kim BR, Le HTT and Cho S:
Antiinflammatory effects on murine macrophages of ethanol extracts
of Lygodium japonicum spores via inhibition of NFκB and p38.
Mol Med Rep. 16:4362–4370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yadav N and Chandra H: Suppression of
inflammatory and infection responses in lung macrophages by
eucalyptus oil and its constituent 1,8-cineole: Role of pattern
recognition receptors TREM-1 and NLRP3, the MAP kinase regulator
MKP-1, and NFκB. PLoS One. 12:e01882322017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hayden MS and Ghosh S: NF-κB, the first
quarter-century: Remarkable progress and outstanding questions.
Genes Dev. 26:203–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giménez-Scherer JA, Cárdenas G,
López-Osuna M, Velázquez JR, Rico G, Isibasi A, Mdel Maldonado C,
Morales ME, Fernández-Diez J and Kretschmer RR: Immunization with a
tetramer derivative of an anti-inflammatory pentapeptide produced
by Entamoeba histolytica protects gerbils (Meriones
unguiculatus) against experimental amoebic abscess of the
liver. Parasite Immunol. 26:343–349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoffmann A and Baltimore D: Circuitry of
nuclear factor kappaB signaling. Immunol Rev. 210:171–186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu F, Xia Y, Parker AS and Verma IM: IKK
biology. Immunol Rev. 246:239–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoffmann A, Levchenko A, Scott ML and
Baltimore D: The IkappaB-NF-kappaB signaling module: Temporal
control and selective gene activation. Science. 298:1241–1245.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin CH, Cheng HW, Ma HP, Wu CH, Hong CY
and Chen BC: Thrombin induces NF-kappaB activation and IL-8/CXCL8
expression in lung epithelial cells by a Rac1-dependent PI3K/Akt
pathway. J Biol Chem. 286:10483–10494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Regueiro V, Moranta D, Campos MA,
Margareto J, Garmendia J and Bengoechea JA: Klebsiella
pneumoniae increases the levels of Toll-like receptors 2 and 4
in human airway epithelial cells. Infect Immun. 77:714–724. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duseja A, Singh SP, Saraswat VA, Acharya
SK, Chawla YK, Chowdhury S, Dhiman RK, Jayakumar RV, Madan K, Misra
SP, et al: Non-alcoholic fatty liver disease and metabolic
syndrome-position paper of the indian national association for the
study of the liver, endocrine society of India, Indian college of
cardiology and indian society of gastroenterology. J Clin Exp
Hepatol. 5:51–68. 2015. View Article : Google Scholar : PubMed/NCBI
|