Introduction
With the rapid development of cataract surgery,
post-operative refractive errors have been markedly reduced
(1). Compared to myopia or
hyperopia, which may be easily corrected by spherical lenses,
corneal astigmatism of >1.5 D occurs in 19% of cataract
patients, and its correction with glasses remains less efficient.
Traditional surgical methods include making incisions in the
cornea, such as limbal or corneal relaxing incisions, the outcome
of which has been proved somewhat unpredictable and variable as a
result of different healing responses and operating skills. Other
disadvantages include possible risks of overcorrection, perforation
and wound leakage, which have all limited this application in
clinical practice (2–4).
In comparison with the abovementioned methods, Toric
intraocular lenses (Toric IOL) are able to more precisely correct
astigmatism. Numerous studies have demonstrated the efficacy of
Toric IOL vs. spherical IOL plus limbal relaxing incisions in
correcting astigmatism (5,6). According to a retrospective study
performed by Sun et al (7),
the mean post-operative refractive cylinder was smaller in the
Toric IOL group compared with that in the group subjected to
spherical IOL implantation combined with limbal relaxing incisions
(P<0.05), suggesting its advantages in efficiency and
precision.
However, the crucial factor for the obtainment of
such positive results is the long-term maintenance of a concrete
rotational stability of the Toric IOL in the capsular bag (8). A small deviation of 10 degrees is
estimated to reduce the correction power by 35% (9). Capsular bag shrinkage due to fibrosis,
which occurs in the first three months post-operatively, has been
considered as the crucial recovery time (10).
Other risk factors affecting the rotational
stability of Toric IOL include axial length (AL), IOL design and
material, axis of IOL alignment, capsulorhexis size, capsular bag
diameter and overall haptic diameter (6,11). In a
study by Shah et al (12), a
higher rotation was observed in eyes with a greater AL, and it was
indicated that AL is the primary factor affecting rotational
stability, rendering a high AL a disadvantage. The above study
reported a worse outcome for Toric IOL implantation in eyes with
high myopia and a high AL. Further factors associated with a worse
outcome of Toric IOL implantation in myopia eyes include looser
capsular bag, high chamber depth and vitreous liquefaction. All of
these factors increase the difficulty of performing the operation,
and may possibly lead to a worse prognosis (13,14).
The outcome of Toric IOL implantation in eyes with
high myopia has remained to be quantitatively assessed and
associated studies are currently lacking. With this regard, the
present prospective observational study was performed to compare
the efficiency of astigmatism correction by Toric IOL and the
rotational stability achieved between eyes with high myopia and
those with low myopia or emmetropia, and thus to evaluate the
clinical application of Toric IOL in patients with astigmatism and
high myopia.
Patients and methods
Patients
The present prospective clinical observational study
included 27 cataract patients who received phacoemulsification
surgery from January 2011 to June 2012 at the Department of
Ophthalmology of the Tenth People's Hospital (Shanghai, China). The
inclusion criteria were as follows: Pre-existing corneal regular
astigmatism >1.50 D with conformable results from IOL master and
corneal topography; at least one obvious episcleral vessel near the
limbus for use as a reference to align the images acquired from the
follow-ups; clear display and explicit identification of the
axis-marking spots on the lens, without the mask of undilated
pupils or capsular opacity as a result of post-operative
proliferation. The exclusion criteria were as follows:
Pre-operative abnormalities as irregular corneal astigmatism,
zonular weakness, a history of glaucoma, uveitis, macular disease,
intraocular or keratorefractive surgery and intraoperative
complications including posterior capsule rupture. All patients
provided written informed consent prior to the study according to
the Declaration of Helsinki and the protocol of the study was
approved by the Ethics Committee of Shanghai Tenth People's
Hospital.
Pre-operative assessment
The age and gender of each patient were recorded.
Pre-operative assessment included uncorrected visual acuity (UCVA),
best-corrected visual acuity (BCVA), AL (IOL master; Carl Zeiss AG,
Oberkochen, Germany); phoropter examination (Nidek 9000; Nidek Co.,
Ltd., Gamagori, Aichi, Japan) to determine the refractive status,
corneal topography (Orbscan; Bausch & Lomb, Bridgewater, NJ,
USA) and optical coherence interferometry measurement (IOL master;
Karl Zeiss AG) to determine the preexisting corneal astigmatism,
and the contrast sensitivity (Vector Vision; Greenville, OH, USA)
under BCVA to assess the visual quality at the photopic and
scotopic status. Other pre-operative examinations for cataract
surgery, including the corneal endothelial scope, were also
routinely performed.
Groups and measurements
According to the pre-operative refractive status,
all patients were divided into 2 groups: Group1, high myopia
(−12.5–6.0 D) group including 11 patients (18 eyes); and Group 2,
emmetropia or low myopia group (−3.0–0.0 D) consisting of 16
patients (21 eyes). The eyes in each group received cataract
phacoemulsification surgeries with the implantation of an
AcrySof® Toric IOL (Alcon Laboratories, Inc., Fort
Worth, TX, USA) under local anesthesia. The horizontal axis was
marked pre-operatively at the limbus with a marker pen under the
slit lamp (BQ900; Haag Streit, Köniz, Bern, Switzerland). While the
alignment axis of the Toric lens was pre-generated with the
Calculation Tool software (version 3.2.4) provided on the Alcon
website (http://www.acrysoftoriccalculator.com) according to
the data from the IOL master examination and marked with a marker
ring at the beginning of the surgery. According to the different
pre-operative refractive status and personal reading habits, the
post-operative refractive status was set as emmetropia in group 2
to generate a good far visual acuity, while it was set at −3.0 D of
myopia for patients in group 1 to achieve a clear near vision for
their reading habits. These settings were fully accepted by the
patients after it was explained to them prior to the surgery.
Different models of Toric IOL from SA60T3 to SA60T8 were selected
according to the diopter of corneal astigmatism calculated
according to the manufacturer's instructions for the
AcrySof® Toric IOL.
Surgical procedure
The standard surgical procedure for the cataract
phacoemulsification, which was performed by the same surgeon (TG)
for all cases, was as follows: A 3.0 mm-wide superior-temporal
clear limbal incision at 120 degrees, an auxiliary spot-penetrating
incision at 30 degrees, continuous curvilinear capsulorhexis, and
phacoemulsification with the cleaver technique and
irrigation/aspiration. Thereafter, the Toric IOL was implanted into
the capsular bag with a Monarch II injector and a B-Cartridge, and
subsequently rotated clockwise to the target alignment axis as
specified prior to surgery. In the standard procedure of the
surgery, the posterior capsule was carefully polished as routine
practice using an irrigation and aspiration handpiece tip, while
the anterior capsule was not polished.
To ensure stable implantation of the IOL in the
capsular bag and prevent its instant shift or rotation, several
specific techniques were applied: First, in certain cases, a second
auxiliary spot-penetrating incision at 180 degrees was adopted to
facilitate the precise rotation of the IOL by the surgeons to the
target alignment axis. This second auxiliary incision, which was
made in the same way as the first one at 30 degrees, neither
increased the complexity of the surgery nor added to the
post-operative astigmatism. Furthermore, the visco-elastic
substances at the inferior capsular membrane-IOL gap and the
posterior capsular membrane-IOL gap were completely removed, as
residual reagent may cause a rotation of the IOL in the capsular
bag. Finally, after the accurate alignment of the IOL, the lens was
pushed down at the optical center to tightly adhere to the
posterior capsular membrane and the incisions were then made
watertight, thus preventing an upward movement of the IOL caused by
the surge of the anterior chamber.
Post-operative treatment and
examination
Tobramicin and dexamethasone eye drops (Alcon
Laboratories, Inc.) were applied 4 times daily for 2–3 weeks. The
follow-up was arranged at the 1st day, 1st week, 1st and 3rd month
post-operatively. The following tests were performed: UCVA, BCVA,
phoropter examination and contrast sensitivity. The BCVA was
converted to the logarithm of the minimum angle of resolution
(logMAR). The abilities to count fingers, see hand motion and
perceive light were assigned the logMAR units of 1.9, 2.3 and 2.7,
respectively.
To examine the rotational stability of the IOL in
the capsular bag, images of the anterior segment were captured and
then analyzed by using purpose-designed comparison of Pre-op And
Post-op Image Software V2.0 (supplied by the Department of
Ophthalmology, Shanghai Tenth People's Hospital and Shanghai ShuJin
Information Technology Co., Ltd., Shanghai, China), which was able
to align the images from different time-points according to the
reference episcleral vessel, as indicated in Fig. 1. The axis shift of the IOL was then
determined using the standard astigmatism chart.
Statistical analysis
Statistical data analysis was performed with SPSS
10.0 software (SPSS, Inc., Chicago, IL, USA). Values are expressed
as the mean ± standard deviation. For comparative statistics, the
Wilcoxon signed-rank test, chi-square test and one way ANOVA with
an SNK post hoc test were used for independent samples. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
The demographics of the cohort and pre-operative
data are presented in Table I. No
significant differences in pre-operative corneal astigmatism were
noted. However, the mean spherical equivalent and AL were
significantly different between the two groups (P<0.05).
 | Table I.Patient demographics and pre-operative
data. |
Table I.
Patient demographics and pre-operative
data.
| Parameter | High myopia (group 1;
n=11) | Emmetropia or low
myopia (group 2; n=16) | P-value |
|---|
| Eyes | 18 | 21 |
|
| Sex (%) |
|
|
|
| Male | 6 (54.55) | 7 (43.75) | 0.704 |
|
Female | 5 (45.45) | 9 (56.25) | 0.704 |
| Age
(years)a | 66.5±7.5; 67
(57–76) | 69.3±8.2; 69
(61–75) | 0.373 |
| Mean spherical
equivalent (D)a | −9.5±2.7;
−9.75 -(12.50–6.00) |
−2.25±1.1; −1.75
(−3.75–0.00) | <0.001 |
| AL (mm)a | 26.43±0.82; 26.88
(25.27–27.63) |
24.16±0.68; 24.06
(23.36–25.02) | <0.001 |
| IOL master diopter
(D) | 6.37±3.68 | 20.42±2.30 | <0.001 |
| Corneal
astigmatism |
|
|
|
| IOL
master cylinder (D) | 2.23±0.63 | 2.07±0.58 | 0.414 |
| Orbscan
topography (D) | 2.17±0.59 | 2.11±0.61 | 0.758 |
| Phoropter
cylinder (D) | 1.89±0.61 | 1.93±0.77 | 0.860 |
Improvement of UCVA, BCVA and mean
spherical equivalent after surgery
The visual acuity was reduced in the UCVA group
compared with the BCVA group at the pre-operative stage (Table II). At the 1st day post-operative
the UCVA and BCVA groups were significantly improved compared with
the pre-operative stage (P<0.001). At all stages, pre- and
post-operative UCVA and BCVA were better in group 2 (low myopia and
emmetropia; P<0.05) (Table II).
The post-operative mean spherical equivalent was similar to the
preset refractive endpoint (Table
II). In group 1 the post-operative mean spherical equivalent
was changed to around −3.0D as designed, while in group 2 it
switched from low myopia or emmetropia pre-operatively to near
emmetropia post-operatively as designed (Table II).
 | Table II.Visual acuity and mean spherical
equivalent at the pre-operative stage and at post-operative
follow-up. |
Table II.
Visual acuity and mean spherical
equivalent at the pre-operative stage and at post-operative
follow-up.
| Parameter | High myopia (group 1;
n=11) | Emmetropia or low
myopia (group 2; n=16) | P-value |
|---|
| Pre-operative visual
acuity |
|
|
|
| UCVA | 0.04±0.08 | 0.23±0.11 | <0.001 |
| BCVA | 0.13±0.11 | 0.28±0.12 | <0.001 |
| Post-operative
visual acuity |
|
|
|
|
UCVA |
|
|
|
|
1st day | 0.21±0.18 | 0.67±0.11 | <0.001 |
|
1st week | 0.26±0.16 | 0.69±0.21 | <0.001 |
|
1st month | 0.27±0.17 | 0.77±0.20 | <0.001 |
|
3rd month | 0.28±0.18 | 0.72±0.19 | <0.001 |
|
BCVA |
|
|
|
|
1st day | 0.49±0.21 | 0.69±0.13 | <0.001 |
|
1st week | 0.57±0.23 | 0.70±0.15 | 0.041 |
|
1st month | 0.58±0.24 | 0.72±0.16 | 0.036 |
|
3rd month | 0.60±0.22 | 0.73±0.17 | 0.045 |
| Mean spherical
equivalent (D) |
|
|
|
|
Pre-operative | −9.5±2.7 | −2.25±1.1 | <0.001 |
| 1st
day | −2.65±0.41 | −0.53±0.32 | <0.001 |
| 1st
week | −2.77±0.38 | −0.29±0.36 | <0.001 |
| 1st
month | −2.83±0.37 | −0.26±0.31 | <0.001 |
| 3rd
month | −2.79±0.46 | −0.28±0.29 | <0.001 |
Improvement of astigmatism after
surgery
In each of the two groups, the astigmatism was
significantly reduced after surgery (P<0.05). No significant
difference in post-operative residual astigmatism between the two
groups was noted (P>0.05; Fig.
2).
Toric IOL rotation in the two
groups
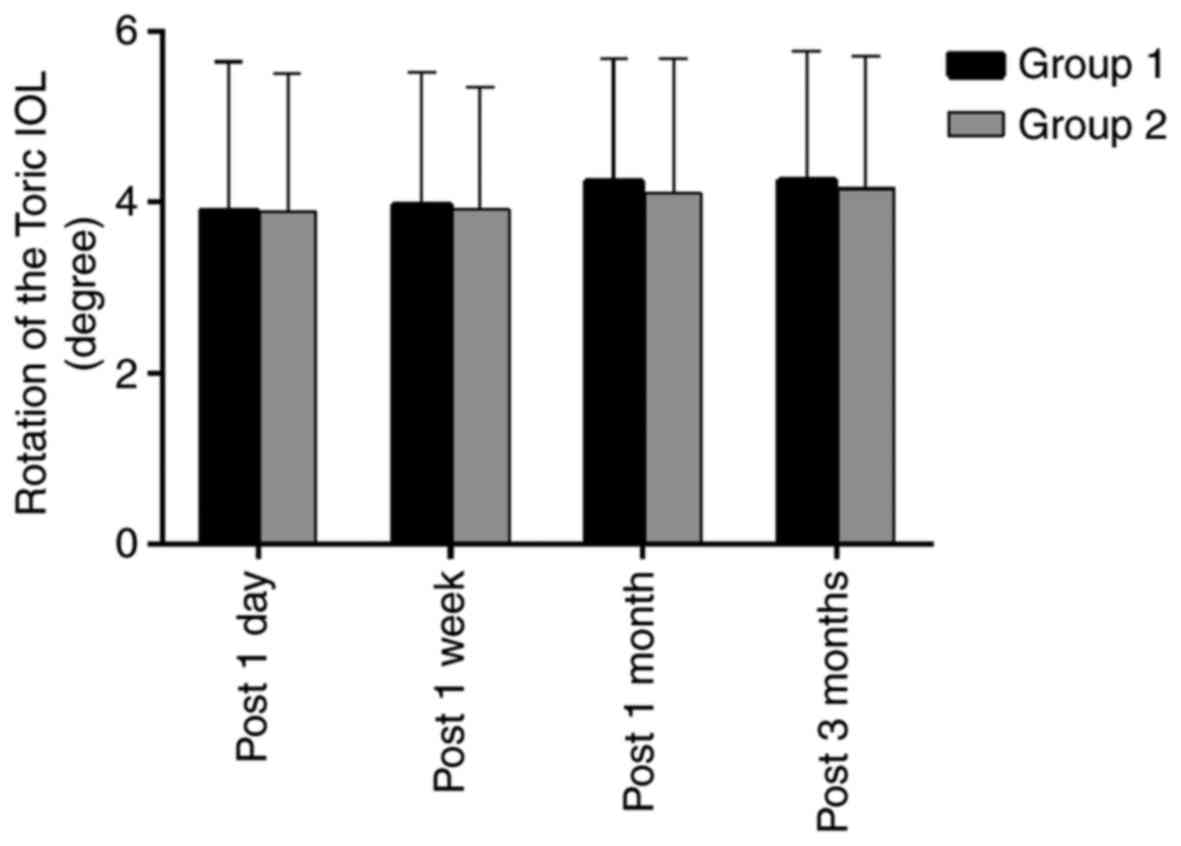
The Toric IOL rotation in groups 1 and 2,
respectively, was quantified as 3.91±1.73 vs. 3.89±1.62 degrees at
1 day, 3.97±1.55 vs. 3.93±1.41 degrees at 1 week, 4.25±1.42 vs.
4.11±1.57 degrees at 1 month and 4.27±1.51 vs. 4.16±1.55 degrees at
3 months post-surgery. Although the curves of the rotational degree
exhibit a slight shift from 1 week to 1 month post-surgery in each
group, there was no significant difference in the degree of
rotation between the two groups during the entire post-operative
period (P>0.05; Figs. 1 and
3).
Toric IOL achieves better photopic and
scotopic contrast sensitivity in patients with astigmatism and high
myopia
Photopic and scotopic contrast sensitivity was
significantly improved at the third post-operative month when
compared with the baseline status (P<0.05). Group 1 achieved
better photopic and scotopic contrast sensitivity than group 2
(P<0.05; Fig. 4).
Discussion
The development and implementation of the Toric IOL
provided surgeons with an alternative to correct astigmatism during
phacoemulsification cataract surgery. Emerging studies have
indicated high accuracy and efficacy of Toric IOL in correcting
astigmatism compared to spherical IOLs plus limbal relaxing
incisions (5,6). In the present study, comparable results
regarding the efficiency of astigmatism correction and rotational
stability were obtained. The two groups exhibited improvements in
the UCVA and BCVA, and reduced astigmatism. No significant
difference in residual astigmatism was observed between the two
groups. During the follow-up, no significant difference in the
rotation degree was noted between the two groups (P>0.05). The
present results corroborate with those of previous studies
(7,11,12).
Therefore, it may be concluded that implantation of Toric IOL in
high myopia eyes is effective.
A high AL, a significantly looser capsular bag,
higher chamber depth and vitreous liquefaction in eyes with high
myopia may increase the difficulty of the operation as follows
(13–15): The higher chamber depth in the
surgery increases the vertical distance for operation and calls for
a higher depth of the visual field, along with leakage from the
corneal incision, and thus, surgeons are required to apply their
instruments in a more vertical angle under compromised
visualization. A looser capsular bag allows for more room for the
IOL to move unpredictably and induces instability. A relaxed
zonular ligament causes higher difficulty in IOL restoration. These
issues may possibly lead to a worse outcome of Toric IOL
implantation in high-myopia eyes, thus limiting its
application.
Specific surgical techniques are essential in
performing the surgery. With the help of the second auxiliary
spot-penetrating incision at 180 degrees, IOLs are easier to
rotate. Complete removal of visco-elastic substances ensured stable
IOL implantation in the capsular bag. Irrigation and aspiration
provided a tight adherence of the IOL to the posterior capsular
membrane. All of these procedures contribute to maintain the
stability of Toric IOLs.
As for the pre- and post-operative UCVA and BCVA,
group 2 (emmetropia or low-myopia eyes) had better vision group 1
(high-myopia eyes). A similar trend was observed for pre- and
post-operative contrast sensitivity under the photopic and scotopic
status. This result may be explained by the larger optical
aberrations in the high-myopia eyes at low-order (e.g., myopia and
astigmatism) and high-order (e.g., coma aberration) levels
(13). However, each of the two
groups gained a significant improvement in UCVA, BCVA and contrast
sensitivity. For far vision, patients only required
myopia-correcting glasses post-operatively rather than
myopia-astigmatism-correcting glasses used at the pre-operative
stage, and for near vision, no glasses were required rather than
cylinder glasses used at the pre-operative stage. However,
according to several previous studies, controversy remains
regarding this issue (16–19). Further investigation and discussion
is therefore required to validate the present findings.
Fibrosis has been considered the underlying cause
for capsular shrinkage and IOL movement at the post-operative stage
(10). In the present study, the
curve of the rotational degree displays a slight shift from 1 week
to 1 month post-operatively for both each of the two groups
(P<0.05). This result conformed to those of previous studies,
which revealed a climax of capsular bag shrinkage and in
situ fibrosis at that time (6,12).
Previous studies also indicated that a high AL is a risk factor for
Toric IOL rotation in high-myopia eyes; in addition, a thick lens,
large capsular sac and relatively loose capsule are associated with
the likelihood and amplitude of rotation (12,20). Due
to the above reasons, certain experts do not recommend the
implantation of Toric IOL to correct astigmatism in cataract
patients with high myopia (12),
which conflicts with the present results. The discrepancy in
results may arise from differences in the study sample and the
period of observation. Differences in surgical procedures between
various studies may also contribute to this. Further study with a
larger sample size and long follow-up may clarify this.
Furthermore, a previous study indicated that a higher anterior
capsular opacification grade may decrease Toric IOL rotation,
indicating that reducing the polishing of the anterior capsule may
improve the rotational stability of a Toric IOL (20), a point that has not been addressed in
the present study. In the present study the anterior capsule was no
polished, which may have contributed to the rotational
stability.
Various methods have been used to calculate the
rotations of toric IOL. Becker et al (21) introduced digital photography
recording through the slit lamp and operating microscope, and this
method has become a standard for evaluating the centration and
axial positioning of a Toric IOL (22). Viestenz and Langenbucher (23) then used the method of simultaneous
slide projection to evaluate the rotation of the Toric lens. In the
present study, images of the anterior segment were captured with
digital cameras affiliated to the slit-lamp under dilated pupils,
and the image was then analyzed with specific software, which was
able to align the images from different time-points according to
the reference of the episcleral vessel and then calculate the
rotation of the IOL axis. This method was similar to that of Shah
et al (12) and offered
precise data for comparison. However, the fibrosis at the
peripheral capsular bag possibly masked the marking spots in
certain cases at the 1-month follow-up, which forced these cases to
be excluded from the present study, leading to a loss of the
sample.
To the best of our knowledge, the present study was
the first to compare the visual outcomes of patients with varying
degrees of myopia after Toric IOL implantation. An equal efficiency
in astigmatism correction and rotational stability were achieved
for groups 1 and 2, indicating a prospective future for the
application of Toric IOLs in high-myopia eyes. However, the small
size of the sample remains a limitation of the present study.
Therefore, studies with a larger sample size as well as a longer
follow-up are required to further investigate the rotational
stability and visual quality of Toric IOL in high-myopia eyes with
astigmatism.
Acknowledgements
Not applicable.
Funding
This work was supported by the Fund of Shanghai
Ninth People's Hospital (grant no. syzrc2014-003) and The Science
and Technology Commission of Shanghai (grant nos. 15411970000 and
17DZ2260100).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
TG and PG together designed the protocol, analyzed
the data and drafted the manuscript. TG was also the surgeon who
performed all the surgeries in the study. PG and CL designed the
software for the calculation of the IOL rotation degree. LF, LG and
YF contributed to data acquisition and examination of the patients.
All authors agree with the submission and have full responsibility
for all primary data. No part of this paper has been published or
submitted elsewhere.
Ethical approval and consent to
participate
All patients provided written informed consent prior
to the study according to the Declaration of Helsinki and the study
was approved by the Ethics Committee of Shanghai Tenth People's
Hospital.
Consent for publication
Written informed consent was obtained from all
patients for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Toric IOL
|
Toric intraocular lens
|
|
UCVA
|
uncorrected visual acuity
|
|
BCVA
|
best corrected visual acuity
|
|
AL
|
axial length
|
|
logMAR
|
logarithm of the minimum angle of
resolution
|
References
|
1
|
Talley-Rostov A: Patient-centered care and
refractive cataract surgery. Curr Opin Ophthalmol. 19:5–9. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang LH and Tang X: The research progress
in treating astigmatism at the time of cataract surgery. Zhonghua
Yan Ke Za Zhi. 47:573–576. 2011.(In Chinese). PubMed/NCBI
|
|
3
|
Amesbury EC and Miller KM: Correction of
astigmatism at the time of cataract surgery. Curr Opin Ophthalmol.
20:19–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buckhurst PJ, Wolffsohn JS, Davies LN and
Naroo SA: Surgical correction of astigmatism during cataract
surgery. Clin Exp Optomet. 93:409–418. 2010. View Article : Google Scholar
|
|
5
|
Dick HB and Buchner SE: Toric phakic
intraocular lenses. Ophthalmologe. 104:1032–1040. 2007.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Edrington TB: A literature review: The
impact of rotational stabilization methods on toric soft contact
lens performance. Cont Lens Anterior Eye. 34:104–110. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun XY, Vicary D, Montgomery P and
Griffiths M: Toric intraocular lenses for correcting astigmatism in
130 eyes. Ophthalmology. 107:1776–1782. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horn JD: Status of toric intraocular
lenses. Curr Opin Ophthalmol. 18:58–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Viestenz A, Walter S, Viestenz A,
Behrens-Baumann W and Langenbucher A: Toric intraocular lenses and
correction of astigmatism. Ophthalmologe. 104:620–627. 2007.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurz S, Krummenauer F, Hacker P, Pfeiffer
N and Dick HB: Capsular bag shrinkage after implantation of a
capsular bending or capsular tension ring. J Cataract Refract Surg.
31:1915–1920. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang DF: Early rotational stability of
the longer Staar toric intraocular lens: Fifty consecutive cases. J
Cataract Refract Surg. 29:935–940. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shah GD, Praveen MR, Vasavada AR, Vasavada
VA, Rampal G and Shastry LR: Rotational stability of a toric
intraocular lens: Influence of axial length and alignment in the
capsular bag. J Cataract Refract Surg. 38:54–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saw SM, Gazzard G, Shih-Yen EC and Chua
WH: Myopia and associated pathological complications. Ophthalmic
Physiol Opt. 25:381–391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu YC, Xia WT, Zhou XT, Liu RJ, Bian SZ,
Ying CL and Zhu GY: Eyeball structure changes in high myopic
patients and their significance for forensic assessment. Fa Yi Xue
Za Zhi. 24:356–360. 2008.(In Chinese). PubMed/NCBI
|
|
15
|
Seward H, Packard R and Allen D:
Management of cataract surgery in a high myope. Br J Ophthalmol.
85:1372–1378. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayashi K, Kondo H, Yoshida M, Manabe S
and Hirata A: Higher-order aberrations and visual function in
pseudophakic eyes with a toric intraocular lens. J Cataract Refract
Surg. 38:1156–1165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamiya K, Shimizu K, Aizawa D, Igarashi A,
Komatsu M and Nakamura A: One-year follow-up of posterior chamber
toric phakic intraocular lens implantation for moderate to high
myopic astigmatism. Ophthalmology. 117:2287–2294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alió JL, Piñero DP, Tomás J and Plaza AB:
Vector analysis of astigmatic changes after cataract surgery with
implantation of a new toric multifocal intraocular lens. J Cataract
Refract Surg. 37:1217–1229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Visser N, Nuijts RM, de Vries NE and Bauer
NJ: Visual outcomes and patient satisfaction after cataract surgery
with toric multifocal intraocular lens implantation. J Cataract
Refract Surg. 37:2034–2042. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, He W, Zhang K and Lu Y: Factors
influencing 1-year rotational stability of AcrySof Toric
intraocular lenses. Br J Ophthalmol. 100:263–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Becker KA, Auffarth GU and Völcker HE:
Measurement method for the determination of rotation and
decentration of intraocular lenses. Ophthalmologe. 101:600–603.
2004.(In German). PubMed/NCBI
|
|
22
|
Warlo I, Krummenauer F and Dick HB:
Rotational stability in intraocular lenses with C-loop haptics
versus Z haptics in cataract surgery. A prospective randomised
comparison. Ophthalmologe. 102:987–992. 2005.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Viestenz A and Langenbucher A: Rotational
stability of the eye in standard photography. Klin Monbl
Augenheilkd. 221:262–265. 2004.(In German). View Article : Google Scholar : PubMed/NCBI
|