Introduction
Pain has been classified into three different
categories by Woolf et al (1)
and Woolf (2), including
nociceptive, inflammatory and pathological pain. Tissue damage and
infiltration of immune cells have been suggested to be associated
with inflammatory pain, while pathological pain is considered to be
induced by damage or abnormal function of the nervous system
(1,2).
Inflammatory pain, a complicated pathological
process that occurs at the central and peripheral nervous systems,
severely impacts the life quality of patients. Mitogen activated
protein kinase (MAPK) is a type of protein kinase specific to the
amino acids tyrosine, threonine and serine, and is known to
modulate cell proliferation, differentiation, mitosis, survival and
apoptosis (3). In addition, the MAPK
family, which is located in the spinal cord, has been reported to
modulate inflammatory pain (4).
Furthermore, extracellular signal-regulated kinase 1/2 (ERK1/2) was
verified to be activated in inflammatory pain that was induced by
complete Freund's adjuvant (CFA) (5).
The features of inflammation are known to include
accumulation of various pro-inflammatory cytokines and
prostaglandins, as well as increased expression of cyclooxygenase-2
(COX-2) (6,7). Notably, ERK1/2 activation followed by
COX-2 expression was identified to serve a crucial role in
generating inflammatory pain (8).
Dezocine is an effective opioid analgesic and has
been used for the treatment of pain (9). A previous study has demonstrated that
dezocine may be a novel molecular target, with clinical
implications (10). Dezocine was
also revealed to antagonize morphine analgesia upon simultaneous
administration in mice models of acute nociception (11). In the current study, the aims were to
examine the effects of dezocine on a CFA-induced inflammatory pain
model in rats and to investigate the possible underlying molecular
mechanisms.
Materials and methods
Animals
Animal care and handling procedures were approved by
the Northwest University for Nationalities (Yinchuan, China), and
conducted according to the National Institutes of Health Guide for
the Care and Use of Laboratory Animals. A total of 30 adult male
(6–8 weeks) Sprague-Dawley rats (weight, 220–250 g) were obtained
from Department of Anesthesiology, the Northwest University for
Nationalities (Yinchuan, China). Rats were housed under controlled
temperature (23±1°C), humidity (70±10%) and 12-h light/dark
lighting, and were provided with distilled water and food ad
libitum.
Rats were randomly divided into three groups as
follows: i) Control group, subcutaneously injected with 100 µl
saline (n=10); ii) CFA group, subcutaneously injected with 100 µl
CFA (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; n=10); and iii)
dezocine+CFA group, pretreated with 1 ml dezocine injection (0.4
µg/kg) at 30 min before 100 µl CFA injection in the plantar surface
of right hind paw (n=10). Subsequently, the rats were anesthetized
with 10% choral hydrate (0.35 ml/100 g; intraperitoneal injection)
and the ipsilateral spinal cord (L4-L6) was removed on the 10th day
following experimentation, immediately applied for the extraction
of mRNA and protein for the determination of mRNA level and protein
level, respectively.
Behavioral testing
At 1 day before the experiment (baseline) and 6 h
after CFA injection, the paw withdrawal threshold (PWT) and paw
withdrawal latency (PWL) of the rats were measured with an
automated von Frey testing device (Dynamic Plantar Aesthesiometer
37450; Ugo Basile, Gemonio, Italy). Rats were allowed to adapt to
the new environment for ~30 min. A steel rod (0.5 mm diameter) was
then pushed against the hind paw of the rat with an ascending force
between 0 and 50 × g over a period of 20 sec. The mechanical
stimulus was stopped when the rat withdrew its hind paw and the
corresponding force and latency were recorded.
Western blot analysis
The ipsilateral lumbar 4–6 spinal cord samples
obtained from the rats were homogenized in lysis buffer containing
a mixture of phosphatase and proteinase inhibitors (Roche
Diagnostics Ltd., Burgess Hill, UK). Next, the protein
concentration was determined using a BCA assay kit (Beyotime
Institute of Biotechnology, Haimen, China), protein sample (10 µg)
was separated by 8–12% SDS-polyacrylamide gel electrophoresis and
transferred onto nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA). Subsequent to blocking the membranes with 5%
skim milk at 37°C for 1 h, the samples were incubated overnight at
4°C with primary antibodies: COX-2 (cat. no. 12282, 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA); IL-1β (cat. no.
I2393, 1:1,000; Sigma, St. Louis, MO, USA); TNF-α (cat. no.
OAAB21998, 1:1,000; Aviva Systems BIOLOGY, San Diego, CA, USA);
p-ERK1/2 (cat. no. 5726, 1:1,000; Cell Signaling Technology, Inc.);
ERK1/2 (cat. no. 4696, 1:1,000; Cell Signaling Technology, Inc.);
P65 (cat. no. 8242, 1:1,000; Cell Signaling Technology, inc.) and
p-P65 (cat. no. 3033, 1:1,000; Cell Signaling Technology, Inc.).
The membranes were incubated for 1 h at 37°C with the horseradish
peroxidase-conjugated secondary antibody (1:10,000; Cell Signaling
Technology). GAPDH antibody (1:1,000; Cell Signaling Technology)
was used as internal control. The bands were visualized by an
enhanced chemiluminescence kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
The prostaglandin E2 (PGE2) level in the protein
samples was measured by a Parameter™ PGE2 Immunoassay ELISA kit
(R&D Systems, USA) according to the manufacturer's
protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from the ipsilateral lumbar 4–6 spinal
cord specimens was extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The RNA concentration was then quantified by
spectrophotometry. Next, cDNA was synthesized from 1 µg RNA by
PrimerScript RT reagent kit with gDNA Eraser (Takara Bio, Inc.,
Otsu, Japan). qPCR was subsequently conducted with a CFX96™
real-time PCR detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The PCR mixture contained 5 µl RT-qPCR mix
(Takara Bio, Inc.), 0.5 µl forward primer (GenScript Co., Ltd.,
Nanjing, China) and 0.5 µl reverse primer (GenScript Co., Ltd.), 1
µl cDNA and 3 µl ddH2O (Invitrogen; Thermo Fisher
Scientific, Inc.). Reactions were incubated at 95°C for 3 min,
followed by 40 cycles of 10 sec at 95°C and 30 sec at 55.9°C. The
primers utilized were as follows: IL-1β forward primer
5′-AGAGTGTGGATCCCAAACAA-3′, reverse primer
5′-AGTCAACTATGTCCCGACCA-3′. TNF-α forward primer
5′-TTCTCATTCCTGCTTGTG-3′, reverse primer 5′-TTGGTGGTTTGCTACG-3′.
COX-2 forward primer 5′-CACGGACTTGCTCACTTTGTT-3′, reverse primer
5′-AAGCGTTTGCGGTACTCATT-3′. GAPDH forward primer
5′-TGCTGAGTATGTCGTGGAG-3′, reverse primer
5′-GTCTTCTGAGTGGCAGTGAT-3′. The quantification cycle (Cq) value was
used for the detection of gene expression levels according to the
formula 2−ΔΔCq, with GAPDH serving as the internal
control (12).
Statistical analysis
Data were analyzed using GraphPad Prism5 software
(GraphPad Software, Inc., La Jolla, CA, USA). Data are expressed as
the mean ± standard error of the mean. Analysis of variance
followed by a post-hoc Newman Keuls was used for independent
samples to compare differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of dezocine on rat behavior as
a result of inflammatory pain
At 6 h after CFA injection, the rats began to limp
and to guard their injected limb. The PWL and PWT values were
measured in rats of the three groups at days 1, 3, 7 and 10 after
the corresponding treatments. A statistically significant
difference was identified among the control, CFA and dezocine+CFA
groups. The PWL and PWT values of the rat hind paw in the CFA group
were significantly decreased as compared with those in the control
group (P<0.01). However, upon pre-administration of dezocine,
the PWL and PWT values in the dezocine+CFA group were significant
higher compared with those in the CFA group (P<0.01; Figs. 1 and 2). These results were consistent with the
findings of a previous study, which also detected the effects of
dezocine in pain behavior (13). In
conclusion, the results of the behavioral tests suggested that
dezocine successfully reduced CFA-induced inflammatory pain in
rats.
Effect of dezocine on COX-2
expression
To detect whether a COX-2 response was induced by
the analgesic effect of dezocine, the mRNA and protein expression
levels of COX-2 in the rat spinal cord were assessed. The results
demonstrated that, compared with the control group, CFA evidently
induced the protein expression of COX-2, whereas dezocine
pretreatment markedly inhibited the upregulation of COX-2 protein
level that was caused by CFA administration (Fig. 3A). In addition, the mRNA expression
profile of COX-2 in the different groups was detected by RT-qPCR.
It was observed that the mRNA expression of COX-2 in the
CFA-treated group was significantly upregulated as compared with
that of the control group (P<0.01). By contrast, COX-2 mRNA
expression was significantly downregulated by pretreatment with
dezocine (P<0.01; Fig. 3B).
Effect of dezocine on the expression
levels of interleukin (IL)-1β and tumor necrosis factor
(TNF)-α
To detect whether IL-1β and TNF-α responses were
caused by the analgesic effect of dezocine, the mRNA and protein
expression levels of IL-1β and TNF-α in the spinal cord samples
were also assessed. The results revealed that, compared with the
control group, CFA significantly induced the mRNA expression levels
of IL-1β and TNF-α (P<0.01), whereas dezocine pretreatment
inhibited the upregulation of these levels resulting from CFA
injection (P<0.05; Fig. 4A and
B). Furthermore, the protein expression levels of IL-1β and
TNF-α were detected by western blot analysis. The results revealed
that the protein expression levels of IL-1β and TNF-α in the CFA
group were clearly upregulated compared with those in the control
group, while these levels were evidently downregulated by
pretreatment with dezocine (Fig.
4C).
Effect of dezocine on PGE2 expression
level
PGE2 levels in the different groups were measured by
ELISA. The results revealed a significant increase of the PGE2
protein level in the CFA group compared with that in the control
group (P<0.001). By contrast, pre-exposure to dezocine in rats
injected with CFA led to an evident decrease in the PGE2 protein
level as compared with that in rats injected with CFA alone
(P<0.01; Fig. 5).
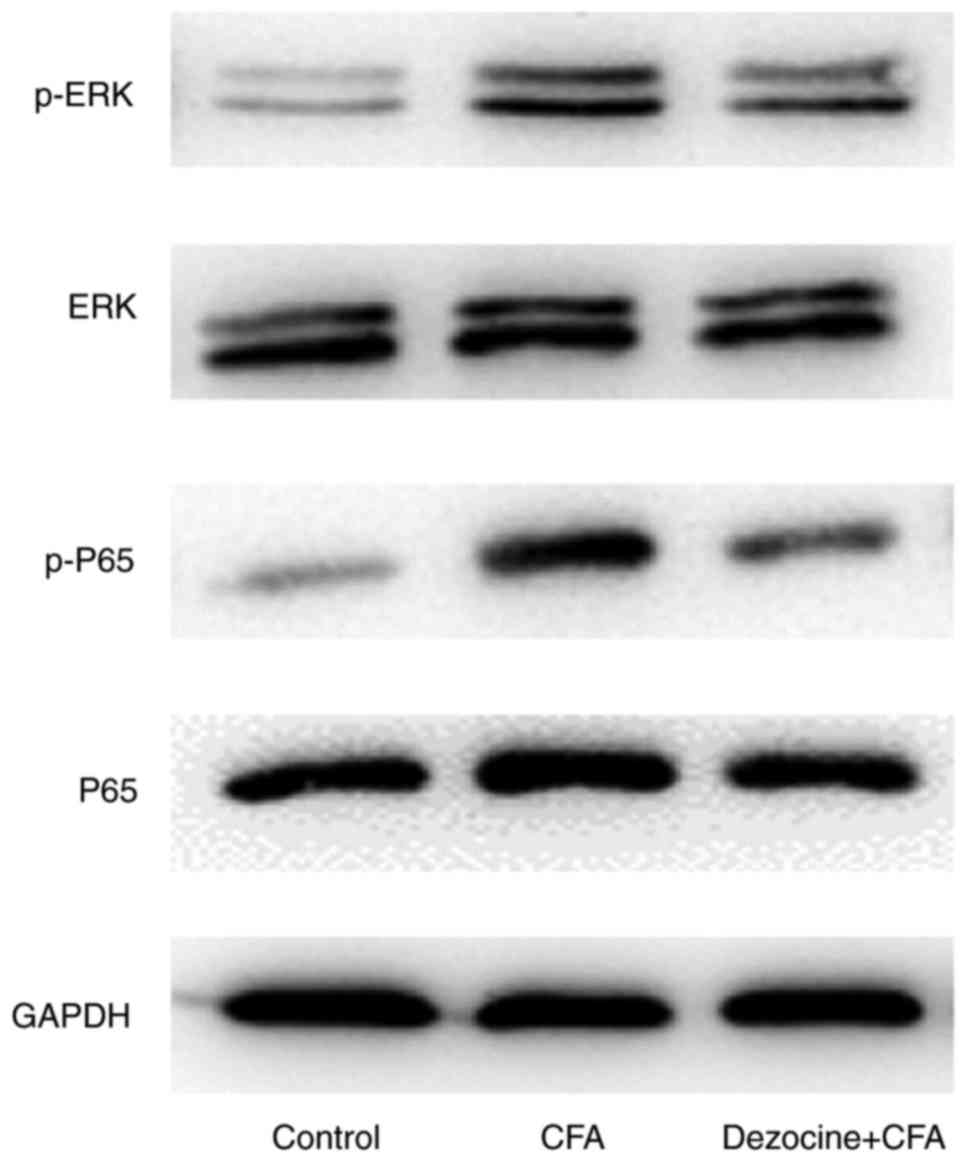
Effect of dezocine on the
phosphorylated (p)-ERK1/2 and p-p65 protein levels
Compared with rats in the control group, the
peripheral inflammation in rats that was generated by injection
with CFA led to the induction of p-ERK1/2 and p-p65 levels.
However, the phosphorylation levels were reduced by dezocine
pretreatment in dezocine+CFA rats as compared with those in the CFA
group (Fig. 6).
Discussion
In the present study, the effects of dezocine on
CFA-induced inflammatory pain in rats and the possible underlying
molecular mechanisms were investigated. It was observed that CFA
induced peripheral inflammatory pain in rats and downregulated the
PWT and PWL values; however, these were significantly alleviated
upon dezocine pretreatment. In addition, the p-p65, p-ERK1/2,
COX-2, PGE2, IL-1β and TNF-α levels were markedly upregulated
following CFA injection, and this upregulation was suppressed by
dezocine pretreatment. Taken together, the analgesic effect of
dezocine on inflammatory pain induced by CFA may be associated with
the inhibition of the spinal ERK1/2-COX-2 pathway.
A rat model of inflammatory plain was established in
the present study subcutaneous injection with 100 µl CFA, while 1
ml dezocine (0.4 µg/kg) was injected at 30 min prior to CFA
administration in order to examine the effects of dezocine on
CFA-induced inflammatory pain in rats. At 1 day before the
establishment of the rat model and 6 h after CFA injection at days
1, 3, 7 and 10, the PWT and PWL were measured with a dynamic
plantar esthesiometer. The results revealed that the PWL and PWT
values of rats in the CFA group were significantly higher in
comparison with those of the control group, while
pre-administration of dezocine significantly increased these values
in the dezocine+CFA group (Figs. 1
and 2). These observations suggested
that dezocine reduced the CFA-induced inflammatory pain in rats.
However, the molecules that were responsible for these alterations
required further investigation.
The inflammatory process involves the accumulation
of pro-inflammatory cytokines, prostaglandins and COX-2 (6,7). A large
number of studies have indicated that certain cytokines directly
stimulated nociceptors (14–16). Furthermore, elevated mRNA and protein
levels of IL-1β/TNF-α were identified in the anterior cingulate
cortex (16), which area was
indispensable for formalin-induced pain (17). Thus, in the current study, the
ipsilateral sides of the lumbar spinal cord were harvested for
detection of the expression levels of COX-2, IL-1β and TNF-α. It
was identified that, as compared with the control group, CFA
significantly increased the mRNA and protein expression levels of
COX-2, IL-1β and TNF-α, while dezocine pretreatment inhibited the
upregulation of these factors (Figs.
3 and 4).
Under pathological conditions, COX-2 produces PGE2
at a 10–20-times higher rate in comparison with the physiological
levels (18). The ELISA results in
the current study further revealed a significant increase of the
PGE2 protein level in the CFA group as compared with the control
group, while prior exposure of CFA-injected rats to dezocine led to
a clear decrease in PGE2 protein as compared with the untreated
CFA-injected rats (Fig. 5).
The MAPK family kinases are expressed in the spinal
cord and have been reported to regulate inflammatory pain (4). A previous study revealed that ERK1/2
was activated in CFA-induced inflammatory pain (5). Furthermore, ERK1/2 activation followed
by COX-2 expression was observed to serve a pivotal role in
generating inflammatory pain (8).
Nuclear factor (NF)-κB is a heterodimeric nuclear factor (p50 and
p65) that is widely expressed in the central nervous system
(19,20). In addition, the cytokines IL-1β and
TNF-α, which are released after stress, have been reported to also
induce the activation of NF-κB (6).
In the present study, compared with rats in the control group, the
peripheral inflammation in CFA-injected rats was observed to lead
to the induction of p-ERK1/2 and p-p65 in the ipsilateral lumbar
4–6 spinal cord, whereas these levels were reduced by dezocine
pretreatment as compared with those in the CFA group (Fig. 6).
In conclusion, the analgesic effect of dezocine on
inflammatory pain induced by CFA was associated with the activation
inhibition of the spinal ERK1/2-COX-2 signaling pathway. Taken
together, the current study demonstrated a novel treatment for
inflammatory pain in rats, which may be helpful for the treatment
of inflammatory pain in clinical patients in the future.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Woolf CJ, Bennett GJ, Doherty M, Dubner R,
Kidd B, Koltzenburg M, Lipton R, Loeser JD, Payne R and Torebjork
E: Towards a mechanism-based classification of pain? Pain.
77:227–229. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woolf CJ: What is this thing called pain?
J Clin Investig. 120:3742–3744. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pearson G, Robinson F, Gibson Beers T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endoc Rev. 22:153–183. 2001. View Article : Google Scholar
|
|
4
|
Ji RR, Kohno T, Moore KA and Woolf CJ:
Central sensitization and LTP: Do pain and memory share similar
mechanisms? Trends Neurosci. 26:696–705. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adwanikar H, Karim F and Gereau RW IV:
Inflammation persistently enhances nocifensive behaviors mediated
by spinal group I mGluRs through sustained ERK activation. Pain.
111:125–135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allan SM and Rothwell NJ: Inflammation in
central nervous system injury. Philos Trans R Soc Lond B Biol Sci.
358:1669–1677. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lucas SM, Rothwell NJ and Gibson RM: The
role of inflammation in CNS injury and disease. Br J Pharmacol. 147
Suppl 1:S232–S240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park JW, Choi YJ, Suh SI and Kwon TK:
Involvement of ERK and protein tyrosine phosphatase signaling
pathways in EGCG-induced cyclooxygenase-2 expression in Raw 264.7
cells. Biochem Biophys Res Commun. 286:721–725. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Brien JJ and Benfield P: Dezocine. A
preliminary review of its pharmacodynamic and pharmacokinetic
properties, and therapeutic efficacy. Drugs. 38:226–248.
1989.PubMed/NCBI
|
|
10
|
Liu R, Huang XP, Yeliseev A, Xi J and Roth
BL: Novel molecular targets of dezocine and their clinical
implications. Anesthesiology. 120:714–723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li NN, Huang YQ, Huang LE, Guo SH, Shen
MR, Guo CL, Zhu SM and Yao YX: Dezocine antagonizes morphine
analgesia upon simultaneous administration in rodent models of
acute nociception. Pain Physician. 20:E401–E409. 2017.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu FX, Pan RR, Yu WF and Liu R: The
anti-nociception effect of dezocine in a rat neuropathic pain
model. Transl Perioper Pain Med. 1:5–8. 2014.PubMed/NCBI
|
|
14
|
Opree A and Kress M: Involvement of the
proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta,
and IL-6 but not IL-8 in the development of heat hyperalgesia:
Effects on heat-evoked calcitonin gene-related peptide release from
rat skin. J Neurosci. 20:6289–6293. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Obreja O, Rathee PK, Lips KS, Distler C
and Kress M: IL-1 beta potentiates heat-activated currents in rat
sensory neurons: Involvement of IL-1RI, tyrosine kinase, and
protein kinase C. FASEB J. 16:1497–1503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Y, Zhu L and Gao YJ: Pain-related
aversion induces astrocytic reaction and proinflammatory cytokine
expression in the anterior cingulate cortex in rats. Brain Res
Bull. 84:178–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johansen JP, Fields HL and Manning BH: The
affective component of pain in rodents: Direct evidence for a
contribution of the anterior cingulate cortex. Proc Natl Acad Sci
USA. 98:8077–8082. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seibert K, Masferrer J, Zhang Y, Leahy K,
Hauser S, Gierse J, Koboldt C, Anderson G, Bremer M, Gregory S, et
al: Expression and selective inhibition of constitutive and
inducible forms of cyclooxygenase. Adv Prostaglandin Thromboxane
Leukot Res. 23:125–127. 1995.PubMed/NCBI
|
|
19
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: New discoveries and insights. Ann Rev Immunol.
14:649–683. 1996. View Article : Google Scholar
|
|
20
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Ann Rev Immunol. 16:225–260. 1998. View Article : Google Scholar
|