Introduction
Glioblastoma is the most aggressive cancer of the
brain (1). Despite advances in
recent decades on the treatment of glioblastoma, including surgical
resection combined with radiotherapy and chemotherapy, the survival
time of glioblastoma patients remains unsatisfactory, with a median
survival period of ≤12 months (1–3).
Increasing evidence has demonstrated that the poor prognosis of
glioblastoma patients is largely attributed to the intrinsic
molecular changes, and several oncogenes and tumor suppressors have
been identified in the process of glioblastoma progression, certain
of which may be potential therapeutic targets (2–4).
RWD domain containing 3 (RWDD3), also known as RWD
domain-containing sumoylation enhancer), was previously reported to
be highly expressed in the cerebellum, pituitary, heart, kidney,
liver, pancreas, adrenal gland and prostate (5). RWDD3 has been demonstrated to enhance
glucocorticoid receptor sumoylation and transcriptional activity
(6,7). It interacts with the small
ubiquitin-like modifier (SUMO) conjugating-enzyme Ubc9 and
consequently increases Ubc9 thioester formation, thus favoring
sumoylation of specific targets (5).
During cellular stress, such as that induced by hypoxia and heat
shock, RWDD3 was observed to inhibit the nuclear factor-κB
signaling through increasing the IκB levels, and to stabilize
hypoxia-inducible factor (HIF)-1α via suppressing HIF-1 and −2α
ubiquitination and degradation (5,8).
Recently, Wu et al (9)
reported that loss of RWDD3 is crucial for pancreatic
neuroendocrine tumorigenesis, particularly in metastasis formation.
In addition, the expression of RWDD3 was demonstrated to be
increased in pituitary tumors, glioma and von Hippel-Lindau tumors,
suggesting that it may contribute to the development and
progression of these tumors (5,10–12).
Recently, Fan et al (13)
reported that inhibition of RWDD3 expression reduced glioblastoma
U251 cells proliferation, migration and invasion, as well as
induced cell apoptosis. However, the clinical significance of RWDD3
expression and the regulatory mechanism of RWDD3 in glioblastoma
remain largely unknown.
Therefore, the present study investigated the
expression of RWDD3 in glioblastoma tissues and cell lines, as well
as its clinical significance. Furthermore, the study examined the
exact roles of RWDD3 in regulating the proliferation, cell cycle
progression, apoptosis, migration and invasion of glioblastoma
cells in vitro, as well as the involved molecular
mechanism.
Materials and methods
Clinical tissue samples
The present study was approved by the Ethics
Committee of the Brain Hospital of Hunan Province (also known as
the Second People's Hospital of Hunan Province, Changsha, China). A
total of 63 glioblastoma tissues and 13 normal brain tissues were
collected during surgical resection at the Second People's Hospital
of Hunan Province between April 2011 and March 2014. The
glioblastoma patients included 41 male and 32 female, with an age
ranging between 32 and 62 years, and mean age of 43.2 years. These
normal tissues were obtained from 13 patients (8 male and 5 female
patients), with an age ranging between 38 and 57 years (mean age of
45.3 years). In addition, these glioblastoma patients were all at
stage IV, and divided into a high RWDD3 expression group and a low
expression group based on the calculated mean expression value
(1.81 was the cut-off value). Written informed consents were
obtained from all patients. The survival of these patients was
recorded, and patients were followed up for 32 months. The tissue
samples were frozen in liquid nitrogen immediately following
surgery and stored at −80°C until further use.
Cell culture and transfection
Human glioblastoma cell lines, including A172
(CRL-1620™), U87 (HTB-14™), U251 (accession number not found),
M059J (CRL-2366™) and T98G (CRL-1690™), and normal human astrocytes
were purchased from the American Type Culture Collection
(Rockville, MD, USA). Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified incubator containing 5%
CO2. Cell transfection was conducted using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Briefly, RWDD3-specific
small interfering RNA (RWDD3 siRNA, Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) was transfected into U87 and U251 cells to
knockdown the RWDD3 expression. Besides, non-specific siRNA (NC
siRNA, Santa Cruz Biotechnology, Inc.) was transfected into U87 and
U251 cells as the control group. Following transfection for 48 h,
the expression levels of RWDD3 were examined. To clarify whether
phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling was
involved in RWDD3-mediated glioblastoma cells, 740Y-P (Fanbiotech,
Beijing, China), an agonist of PI3K/AKT signaling, was used to
treat RWDD3 siRNA-transfected U87 and U251 cells for 20 min at room
temperature. Subsequent to the treatment, cell proliferation and
invasion were evaluated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the glioblastoma
tissues and cells using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) The RNA concentration was measured by using
NanoDrop2000 (Thermo Fisher Scientific, Inc.), and then converted
into cDNA using a Reverse Transcription kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. For
mRNA expression detection, qPCR was conducted using a One-Step qPCR
kit (Toyobo Co., Ltd., Tokyo, Japan) on a 7500 thermocycler system
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. The primer sequences used were as follows: RWDD3,
5′-AGACAGATGGGACCGTGTTCA-3′ (forward) and
5′-CTGCTTGCTCAAGTAACTTCTCT-3′ (reverse); GAPDH,
5′-GGAGCGAGATCCCTCCAAAAT-3′ (forward) and
5′-GGCTGTTGTCATACTTCTCATGG-3′ (reverse). The PCR conditions
involved initial denaturation at 95°C for 5 min, followed by 35
cycles of denaturation at 95°C for 15 sec, and annealing/elongation
at 60°C for 30 sec. The experiment was repeated three times. The
relative mRNA expression was analyzed by the 2−ΔΔCq
method (14).
Western blot analysis
Cells were solubilized in cold
radioimmunoprecipitation lysis buffer (Thermo Fisher Scientific,
Inc.), and the concentration of protein was examined using a BCA
Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Next, 60 µg protein per
lane was separated by 12% SDS-PAGE and then transferred onto a
polyvinylidene difluoride membrane (Thermo Fisher Scientific,
Inc.). The membrane was subsequently incubated with
phosphate-buffered saline (PBS) containing 5% milk at room
temperature for 3 h. Following three washes with PBS, the membrane
was incubated at room temperature for 3 h with the following rabbit
primary antibodies: Polyclonal anti-RWDD3 (1:50; ab91555),
polyclonal anti-matrix metalloproteinase 2 (MMP2; 1:100; ab37150),
polyclonal anti-MMP9 (1:100; ab38898), polyclonal anti-B-cell
lymphoma 2 (Bcl-2; 1:100; ab59348), polyclonal
anti-Bcl-2-associated X protein (Bax; 1:100; ab53154), monoclonal
anti-total PI3K (1:50; ab40755), polyclonal anti-phosphorylated
PI3K (1:50; ab182651), polyclonal anti-total AKT (1:50; ab8805),
polyclonal anti-phosphorylated AKT (1:50; ab38449) and polyclonal
anti-GAPDH (1:50; ab9485; all purchased from Abcam, Cambridge, MA,
USA). Subsequent to three washes with PBS, the membrane was
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:5,000; ab6721; Abcam) at room temperature for
40 min. After a further three washes with PBS, chemiluminescent
detection was conducted using an Enhanced Chemiluminescence kit
(Pierce; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The relative protein expression was
analyzed using Image-Pro plus software 5.0 (Media Cybernetics,
Inc., Rockville, MD, USA).
Cell proliferation assay
U87 and U251 cells (20,000 cells/well) were seeded
in a 96-well plate, and 100 µl DMEM containing 0.5 g/l MTT (Thermo
Fisher Scientific, Inc.) was added. Subsequent to culture at 37°C
for 12, 24, 48 or 72 h, the medium was removed, and 50 µl dimethyl
sulfoxide (Beyotime Institute of Biotechnology, Shanghai, China)
was added. Following incubation at 37°C for 10 min, the absorbance
was measured at a wavelength of 570 nm using the Varioskan LUX
multimode microplate reader (Thermo Fisher Scientific, Inc.).
Cell apoptosis assay
Cell apoptosis was examined using the Alexa
Fluor® 488 Annexin V/Dead Cell Apoptosis kit (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Briefly, U87 and U251 cells were harvested and re-suspended in 200
µl binding buffer. Next, cells (1×106 cells/ml) were
incubated with 10 µl Annexin V and 5 µl propidium iodide (PI) in
the dark for 15 min. Subsequently, 300 µl binding buffer was added,
and the cells were analyzed using BD Accuri C6 flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
Cell cycle progression analysis
U87 and U251 cells (1×106) were washed
twice with PBS, resuspended in 70% ethanol and fixed overnight at
−20°C. The cells were washed twice in PBS with 3% bovine serum
albumin (BSA) and then incubated at room temperature for 30 min in
PBS containing 40 µg/ml PI, 3% BSA and 0.2 mg/ml RNase. The DNA
content was analyzed using the BD Accuri C6 flow cytometer.
Cell invasion assay
The cell invasion ability was evaluated using BD
Falcon Cell Culture Inserts (BD Biosciences) pre-coated with
Matrigel (BD Biosciences), according to the manufacturer's
protocol. Briefly, the U87 and U251 cell suspension
(2×105 cells/ml) was prepared in DMEM, and 300 µl of the
suspension was added into the upper chamber of the 24-well plates,
while 300 µl DMEM supplemented with 10% FBS was added into the
lower chamber. Following incubation at 37°C for 24 h, cells that
did not invade through the membrane in the filter were carefully
wiped out using a cotton-tipped swab. Next, the filter was fixed in
90% alcohol at room temperature for 10 min and then stained with
0.1% crystal violet (Thermo Fisher Scientific, Inc.) at room
temperature for 10 min. The invading cells were counted under a
BX53 microscope (Olympus Corp., Tokyo, Japan).
Cell migration assay
U87 and U251 cells were cultured to 100% confluence.
In order to terminate cell proliferation, mitomycin C (10 µg/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to treat
the cells at 37°C for 2 h. The cells were then washed with DMEM
three times, and wounds with a width of ~1 mm were created with a
plastic scriber. Subsequent to washing with PBS, the cells were
incubated in DMEM supplemented with 10% FBS at 37°C for 48 h.
Finally, the cells were observed under the BX53 microscope.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three samples. SPSS version 17.0 software (SPSS, Inc.,
Chicago, IL, USA) was used to conduct statistical analyses.
Differences were examined using Student's t-test for two-group
comparison or analysis of variance for comparison of more than two
groups. The Kaplan-Meier method was used for survival analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
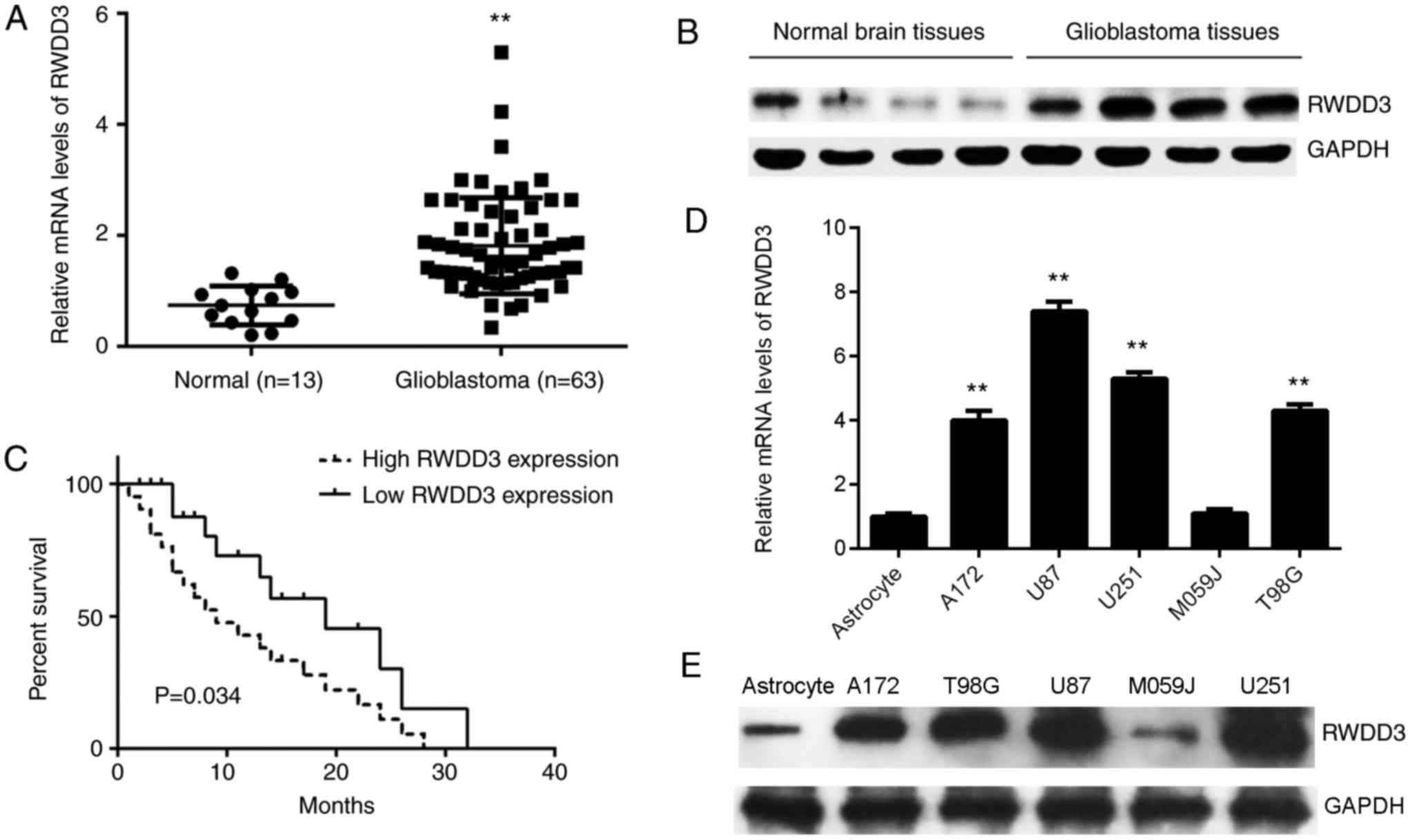
Upregulation of RWDD3 in human
glioblastoma tissues
To preliminarily investigate the role of RWDD3 in
glioblastoma, the current study first examined the mRNA and protein
expression levels of RWDD3 in glioblastoma and normal brain
tissues. As shown in Fig. 1A and B,
the mRNA and protein expression levels of RWDD3 were significantly
higher when compared with those in normal brain tissues. These
patients were all at stage IV, and divided into a high RWDD3
expression group and a low expression group based on the mean
expression value (1.81 was the cut-off value). In addition, the
patients with high RWDD3 levels presented a shorter overall
survival time when compared with those with low RWDD3 levels
(Fig. 1C). Subsequently, the
expression of RWDD3 in certain common glioblastoma cell lines was
examined, including in A172, U87, U251, M059J and T98G cells. As
shown in Fig. 1D and E, the mRNA and
protein levels of RWDD3 were also increased in glioblastoma cells
compared with the normal human astrocyte cells. Therefore, it is
suggested that the increased expression of RWDD3 may be associated
with the malignancy of glioblastoma.
Knockdown of RWDD3 suppresses
glioblastoma cell proliferation and induces cell cycle arrest at G1
phase
As U87 and U251 cells exhibited the highest
expression of RWDD3, these two cell lines were used in the
subsequent experiments in vitro. In order to downregulate
the RWDD3 levels, U87 and U251 cells were transfected with RWDD3
siRNA. Following transfection, the mRNA and protein levels of RWDD3
were significantly reduced in RWDD3 siRNA group compared with
control group (Fig. 2A and B).
However, transfection with negative control (NC) siRNA exerted no
evident effect on the expression of RWDD3 (Fig. 2A and B).
Next, an MTT assay was conducted to determine the
effects of RWDD3 downregulation on the proliferation of U87 and
U251 cells. As shown in Fig. 2C,
knockdown of RWDD3 significantly inhibited U87 at 48 and 72 h and
U251 cell proliferation at 72 h when compared with the NC siRNA
group. The cell cycle distribution was also further examined, and
it was observed that inhibition of RWDD3 expression led to a cell
cycle arrest at G1 and G2 phase (Fig. 2D). Therefore, these findings
suggested that knockdown of RWDD3 suppressed glioblastoma cell
proliferation, at least partly, via inducing a cell cycle arrest at
G1 phase.
RWDD3 downregulation promotes
glioblastoma cell apoptosis
The effect of RWDD3 knockdown on glioblastoma cell
apoptosis was further analyzed. As shown in Fig. 3A, the cell apoptosis was
significantly increased in the RWDD3-downregulated U87 and U251
cells, when compared with the NC siRNA group. The present study
then examined the expression of apoptotic-associated genes,
including Bcl-2 and Bax. The data indicated that the protein
expression of the pro-apoptotic Bax was significantly upregulated,
while the protein expression of anti-apoptotic Bcl-2 was markedly
downregulated, in U87 and U251 cells following the knockdown of
RWDD3 expression by siRNA transfection (Fig. 3B). These data suggest that RWDD3 may
inhibit glioblastoma cell apoptosis via affecting the expression
levels of Bax and Bcl-2.
Downregulation of RWDD3 reduces U87
and U251 cell invasion and migration
The invasion and migration capacities of U87 and
U251 cells following the knockdown of RWDD3 were also examined. As
shown in Fig. 4A and B, transfection
with RWDD3 siRNA caused a significant reduction in the invasion and
migration of U87 and U251 cells, when compared with the NC siRNA
group. Further investigation revealed that the protein levels of
MMP2 and MMP9 were also markedly decreased following the inhibition
of RWDD3 in U87 and U251 cells (Fig.
4C). These findings suggest that RWDD3 serves a promoting role
in glioblastoma cell invasion and migration via mediating the MMP2
and MMP9 expression levels.
Inhibition of RWDD3 expression
downregulates PI3K/AKT signaling in U87 and U251 cells
The PI3K/AKT signaling pathway has been demonstrated
to be crucial for the survival, proliferation and motility of
multiple types of cells (15,16).
Thus, the present study subsequently examined the activity of the
PI3K/AKT signaling pathway in U87 and U251 cells following
inhibition of RWDD3 expression. As shown in Fig. 5, the phosphorylation levels of PI3K
and AKT were significantly downregulated in U87 and U251 cells in
the RWDD3 siRNA group, when compared with those in the NC siRNA
group. These finding suggest that the promoting effects of RWDD3 on
the malignant phenotypes of glioblastoma cells were exerted, partly
at least, via affecting the activity of the PI3K/AKT signals.
To further confirm these findings, 740Y-P was used,
which is an agonist of PI3K/AKT signaling, to further investigate
whether the cell proliferation or migration could be restored. The
findings demonstrated that the proliferation and migration were
significantly upregulated in the RWDD3 siRNA + 740Y-P group, when
compared with the RWDD3 siRNA group (Fig. 6A and B), suggesting that the
activation of PI3K/AKT signaling rescued the suppressive effects of
RWDD3 downregulation on glioblastoma cell proliferation and
migration. Consistently, the MMP2 and MMP9 protein levels were also
increased in the RWDD3 siRNA + 740Y-P group, when compared with the
RWDD3 siRNA group (Fig. 6C).
Discussion
Limited evidence exists regarding the clinical
significance of RWDD3 expression and the regulatory mechanism of
RWDD3 in glioblastoma. Thus, this was examined in the present
study. It was initially observed that RWDD3 was significantly
upregulated in glioblastoma tissues, and a high expression of RWDD3
was associated with poor prognosis of patients. Subsequently,
knockdown of RWDD3 in glioblastoma cell lines significantly reduced
the cell proliferation, cell cycle progression, migration and
invasion, whereas it promoted cell apoptosis, accompanied with
reduced activity of PI3K/AKT signaling. Furthermore, activation of
PI3K/AKT signaling rescued the suppressive effects of RWDD3
downregulation on glioblastoma cell proliferation and migration,
concurrent with increased protein levels of MMP2 and MMP9.
During the development and malignant progression of
glioblastoma, metabolic reorganization is driven by HIF-1α, which
makes use of aerobic glycolysis as the main source of energy and
biosynthetic molecules (17,18). It has been demonstrated that HIF-1α
promoted the expression of numerous genes associated with tumor
growth and metastasis (19–21). The expression and activity of HIF-1α
is controlled mainly by oxygen levels (8). Under normoxia, HIF-1α rapidly binds
oxygen-dependent ubiquitin, which leads to subsequent degradation
of HIF-1α (8). However, SUMO-1,
which has a similar structure to that of ubiquitin, competes with
ubiquitin for HIF-1α and thus protects HIF-1α from degradation
(22). Previous studies have
demonstrated that RWDD3 is able to enhance the stabilization and
transcriptional activity of HIF-1α during hypoxia via promoting the
sumoylation of HIF-1α, through which RWDD3 may serve a promoting
role in tumor growth and metastasis (8,10). In
addition, it has been reported that RWDD3 was upregulated in
glioma, as well as in pituitary tumors (10,23);
however, the clinical significance of RWDD3 expression and the
regulatory mechanism of RWDD3 in glioblastoma requires further
elucidation. In the present study, it was initially demonstrated
that RWDD3 was upregulated in glioblastoma tissues, and this
upregulation may predicate poor prognosis in glioblastoma patients.
Consistent with the clinical data, the expression levels of RWDD3
were also significantly increased in several common glioblastoma
cell lines, when compared with normal human astrocyte cells.
Therefore, the current study reported for the first time an
upregulation of RWDD3 in glioblastoma.
The present study further examined the function of
RWDD3 in the regulation of the malignant phenotypes of glioblastoma
cells in vitro using U87 and U251 cells, as they exhibited
the highest expression of RWDD3. It was observed that knockdown of
RWDD3 caused a significant reduction in U87 and U251 cell
proliferation. This reduced cell proliferation caused by RWDD3
downregulation may be attributed to the cell cycle arrest at G1
phase and cell apoptosis. To further confirm these findings, the
study examined the expression levels of apoptosis-associated
proteins, Bax and Bcl-2. It was observed that the pro-apoptotic Bax
was significantly upregulated following RWDD3 knockdown, while the
anti-apoptotic Bcl-2 was markedly downregulated following
inhibition of RWDD3 expression in glioblastoma cells.
MMP2 and MMP9 are zinc-dependent enzymes capable of
cleaving type IV and V collagens (24,25).
Through this function, they participate in the breakdown of the
extracellular matrix in physiological processes, including
embryonic development, reproduction and tissue remodeling, as well
as in pathological processes, such as tumor cell invasion and
migration (26,27). In the present study, it was
identified that inhibition of RWDD3 expression led to reduced
glioblastoma cell migration and invasion, accompanied with
decreased protein levels of MMP2 and MMP9. These findings suggest
that RWDD3 was able to mediate the protein expression of MMP2 and
MMP9, and thus served a promoting role in glioblastoma cell
migration and invasion.
PI3K/AKT signaling has a central role in the
regulation of tumor cell survival, growth, motility, angiogenesis
and metabolism (28). Previous
studies have demonstrated that the PI3K/AKT signaling is frequently
hyperactivated in various types of human cancer, including
glioblastoma, and is thus suggested to be a potential therapeutic
target for cancer treatment (29,30).
Indeed, several PI3K inhibitors have been used in phase I/II
clinical trials for glioblastoma treatment (30). In the current study, it was observed
that knockdown of RWDD3 caused a decreased activity of PI3K/AKT
signaling in U87 and U251 cells, while activation of PI3K/AKT
signaling rescued the suppressive effects of RWDD3 downregulation
on glioblastoma cell proliferation and migration, suggesting that
the PI3K/AKT signaling is involved in the RWDD3-mediated malignant
phenotypes of glioblastoma cells.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that RWDD3 is
significantly upregulated in glioblastoma, and that it serves an
oncogenic role in the regulation of the malignant phenotypes of
glioblastoma cells, at least partly, via mediating the activity of
PI3K/AKT signaling. Therefore, the current study suggests that
RWDD3 may be a novel promising target for the treatment of
glioblastoma. A limitation of the present study is the lack of
in vivo experiments, and animal experiments should be
conducted in future studies to examine the role of RWDD3 in
glioblastoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XC collected clinical tissues and wrote the
manuscript. WK designed the study and revised the manuscript. HH,
BL, YZ, BZ and LY performed the in vitro experiments.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Brain Hospital of Hunan Province (also known as
the Second People's Hospital of Hunan Province, Changsha, China).
Written informed consent was obtained from all patients.
Consent for publication
Written informed consents were obtained from all
patients involved in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Z, Guo J, Ma Y, Lin Z and Zhang L:
Oncogenic role of MicroRNA-30b-5p in glioblastoma through targeting
proline-rich transmembrane protein 2. Oncol Res. May 17–2017.(Epub
ahead of print).
|
|
3
|
Ding B, Cui B, Gao M, Li Z, Xu C, Fan S
and He W: Knockdown of ras-related protein 25 (Rab25) inhibits the
in vitro cytotoxicity and in vivo antitumor activity of human
glioblastoma multiforme cells. Oncol Res. 25:331–340. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Li W, Liu S, Zheng X, Shi L, Zhang
W and Yang H: Knockdown of collagen triple helix repeat containing
1 (CTHRC1) inhibits epithelial-mesenchymal transition and cellular
migration in glioblastoma cells. Oncol Res. 25:225–232. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arciuch Antico VG, Tedesco L, Fuertes M
and Arzt E: Role of RSUME in inflammation and cancer. FEBS Lett.
589:3330–3335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liberman AC, Druker J, Garcia FA, Holsboer
F and Arzt E: Intracellular molecular signaling. Basis for
specificity to glucocorticoid anti-inflammatory actions. Ann N Y
Acad Sci. 1153:6–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Druker J, Liberman AC, Antunica-Noguerol
M, Gerez J, Paez-Pereda M, Rein T, Iñiguez-Lluhí JA, Holsboer F and
Arzt E: RSUME enhances glucocorticoid receptor SUMOylation and
transcriptional activity. Mol Cell Biol. 33:2116–2127. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carbia-Nagashima A, Gerez J, Perez-Castro
C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F and Arzt E:
RSUME, a small RWD-containing protein, enhances SUMO conjugation
and stabilizes HIF-1alpha during hypoxia. Cell. 131:309–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Y, Tedesco L, Lucia K, Schlitter AM,
Garcia JM, Esposito I, Auernhammer CJ, Theodoropoulou M, Arzt E,
Renner U and Stalla GK: RSUME is implicated in tumorigenesis and
metastasis of pancreatic neuroendocrine tumors. Oncotarget.
7:57878–57893. 2016.PubMed/NCBI
|
|
10
|
Fuertes M, Gerez J, Haedo M, Giacomini D,
Páez-Pereda M, Labeur M, Stalla GK and Arzt E: Cytokines and genes
in pituitary tumorigenesis: RSUME role in cell biology. Front Horm
Res. 38:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shan B, Gerez J, Haedo M, Fuertes M,
Theodoropoulou M, Buchfelder M, Losa M, Stalla GK, Arzt E and
Renner U: RSUME is implicated in HIF-1-induced VEGF-A production in
pituitary tumour cells. Endocr Relat Cancer. 19:13–27. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerez J, Tedesco L, Bonfiglio JJ, Fuertes
M, Barontini M, Silberstein S, Wu Y, Renner U, Páez-Pereda M,
Holsboer F, et al: RSUME inhibits VHL and regulates its tumor
suppressor function. Oncogene. 34:4855–4866. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan YH, Zhu XG, WU MJ, Chai Y, YE MH,
Xi-Ao B and Wu L: Effects of lentivirus-mediated RWDD3 silencing on
proliferation and invasion of human glioma U251 cells. Chin J
Pathophysiol. 31:1550–1556. 2015.
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katase N, Nishimatsu SI, Yamauchi A,
Yamamura M, Terada K, Itadani M, Okada N, Hassan NMM, Nagatsuka H,
Ikeda T, et al: DKK3 overexpression increases malignant properties
of head and neck squamous cell carcinoma cells. Oncol Res.
26:45–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao P, Guan HT, Dai ZJ, Ma YG, Liu XX and
Wang XJ: Knockdown of SPOCK1 inhibits the proliferation and
invasion in colorectal cancer cells by suppressing the PI3K/Akt
pathway. Oncol Res. 24:437–445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu W, Hu Q, Nie E, Yu T, Wu Y, Zhi T,
Jiang K, Shen F, Wang Y, Zhang J and You Y: Hypoxia induces H19
expression through direct and indirect Hif-1α activity, promoting
oncogenic effects in glioblastoma. Sci Rep. 7:450292017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gabriely G, Wheeler MA, Takenaka MC and
Quintana FJ: Role of AHR and HIF-1α in glioblastoma metabolism.
Trends Endocrinol Metab. 28:428–436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu M, Wang D and Li N: MicroRNA-20b
downregulates HIF-1α and Inhibits the proliferation and invasion of
osteosarcoma cells. Oncol Res. 23:257–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y, Yang C, Wang K, Liu X and Liu Q:
MicroRNA-33b inhibits the proliferation and migration of
osteosarcoma cells via targeting hypoxia-inducible factor-1α. Oncol
Res. 25:397–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu F, Lin B, Liu X, Zhang W, Zhang E, Hu
L, Ma Y, Li X and Tang X: ERK signaling pathway is involved in
HPV-16 E6 but not E7 oncoprotein-induced HIF-1α protein
accumulation in NSCLC cells. Oncol Res. 23:109–118. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, Cai F, Shi R, Wei JN and Wu XT:
Hypoxia regulates sumoylation pathways in intervertebral disc
cells: Implications for hypoxic adaptations. Osteoarthritis
Cartilage. 24:1113–1124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gerez J, Fuertes M, Tedesco L, Silberstein
S, Sevlever G, Paez-Pereda M, Holsboer F, Turjanski AG and Arzt E:
In silico structural and functional characterization of the RSUME
splice variants. PLoS One. 8:e577952013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue Q, Cao L, Chen XY, Zhao J, Gao L, Li
SZ and Fei Z: High expression of MMP9 in glioma affects cell
proliferation and is associated with patient survival rates. Oncol
Lett. 13:1325–1330. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu Y, Zhang X, Wang L, Ji Z, Xie M, Zhou
X, Liu Z, Shi H and Yu R: Loss of SH3GL2 promotes the migration and
invasion behaviours of glioblastoma cells through activating the
STAT3/MMP2 signalling. J Cell Mol Med. 21:2685–2694. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miao Y, Lu M, Yan Q, Li S and Feng Y:
Inhibition of proliferation, migration, and invasion by knockdown
of pyruvate kinase-M2 (PKM2) in ovarian cancer SKOV3 and OVCAR3
cells. Oncol Res. 24:463–475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amar S, Minond D and Fields GB: Clinical
implications of compounds designed to inhibit ECM-modifying
metalloproteinases. Proteomics. 17(23–24)2017.PubMed/NCBI
|
|
28
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramezani S, Vousooghi N, Kapourchali
Ramezani F and Joghataei MT: Perifosine enhances
bevacizumab-induced apoptosis and therapeutic efficacy by targeting
PI3K/AKT pathway in a glioblastoma heterotopic model. Apoptosis.
22:1025–2034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao HF, Wang J, Shao W, Wu CP, Chen ZP,
To ST and Li WP: Recent advances in the use of PI3K inhibitors for
glioblastoma multiforme: Current preclinical and clinical
development. Mol Cancer. 16:1002017. View Article : Google Scholar : PubMed/NCBI
|