Introduction
Abdominal aortic aneurysm (AAA) is a serious disease
with high morbidity and high mortality, and its incidence has
increased with lifestyle changes and aging. Many factors affect the
occurrence and development of AAA, such as hypertension, peripheral
arterial disease, and smoking, among others. Thus, early detection
and early treatment as well as joint screening play an important
role in the occurrence and development of AAA (1,2).
However, no drugs or methods have been developed to inhibit the
formation and development of AAAs in an early stage. AAA is
pathologically characterized by chronic inflammation of the aortic
wall, degradation of the extracellular matrix (3), extensive destruction and re-formation
of the medial layer during neovascularization, and attenuation of
vascular smooth muscle cells (4).
These changes destroy medial membranes and promote aortic aneurysm
expansion. Thus, the aorta gradually expands and eventually breaks
down.
Early growth response factor 1 (Egr-1) is a zinc
finger transcription factor and member of the early gene family.
Egr-1 participates in various pathophysiologic processes, such as
cell proliferation, differentiation, and apoptosis (5), inflammatory reaction (6), thrombosis (7), and extracellular matrix degradation and
synthesis (8). Egr-1 expression can
be rapidly induced by various stimulating factors, such as growth
factors, proinflammatory cytokines, lipopolysaccharides, hypoxia,
shear stress, and vascular damage. Once activated, Egr-1 controls
the expression of several genes implicated in the pathogenesis of
AAA, indicating that this transcription factor represents a key
molecular target for controlling the formation of AAA.
Bioinformatics analysis showed that Egr-1 is a key transcription
factor in the formation and pathogenesis of AAA (9). Egr-1 has been also observed to be
involved in thrombus formation and the inflammatory pathogenesis of
AAA (10). The role of Egr-1 as a
mechanical stress-response gene causing aneurysm formation was also
demonstrated (11). Thus, Egr-1 is
considered as a target for AAA prevention and control.
Early growth response factor-1 DNA enzyme (EDRz) is
a small single-stranded DNA fragment with enzymatic activity that
can specifically cut Egr-1, inhibit the protein expression of
Egr-1, and block the expression of other Egr-1-regulated genes. As
a biological tool, EDRz can specifically block gene expression.
However, it is unclear whether EDRz inhibits AAA or the expression
of other genes related to Egr-1.
In the present study, EDRz was introduced into rats
with AAA, and the effect of EDRz on AAA formation was examined. In
addition, the expression of Egr-1 and matrix metalloproteinase
(MMP)-2, MMP-9 were examined after introducing EDRz into vessels.
This study aimed to examine the inhibitory effects of EDRz on the
formation of AAA and verify the feasibility of this enzyme for AAA
gene therapy.
Materials and methods
Construction of EDRz
The DNA enzyme EDRz was synthesized by Sangon
Biotech Co. (Shanghai, China) according to the sequence published
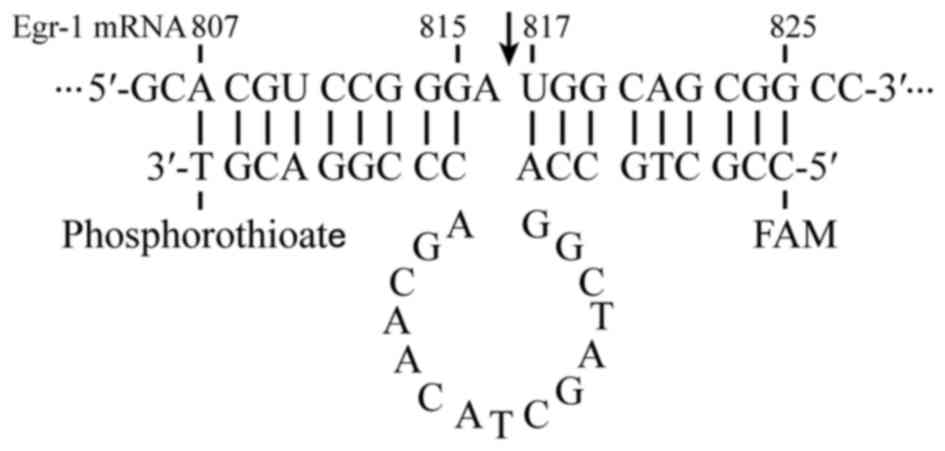
in GenBank. Fig. 1 shows the EDRz
sequence as follows: 5′-CCGCTGCCAGGCTAGCTACAACGACCCGGACGT-3′. The
3′ termini of the oligonucleotides were protected from exonucleases
by phosphorothioate linkage. Fluorescence microscopy was applied to
determine the nucleotide distribution by using marked 5′-end
carboxyl fluorescein. A total of 495 µg of EDRz was added to 80 µl
of diethyl pyrocarbonate solution (1:1,000) and mixed after
centrifugation. Next, 120 µl of jetPRIME (Polyplus-transfection SA,
Illkirch, France), 32 µl of 1 mM MgCl2, and 568 µl of
30% F-127 pluronic gel (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) were added. This 800 µl solution was shock-mixed and
stored at 4°C (7).
Ninety male SD rats (Liaoning Changsheng
Biotechnology, Dalian, China) weighing 250–300 g were used in this
study. This study was carried out in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was been reviewed and approved by the Institutional Animal
Care and Use Committee (IACUC) of Jiamusi University. The rats were
randomly divided into three groups: control group (only elastase
perfusion), jetPRIME group (elastase perfusion + jetPRIME
transfection reagent), and EDRz group (elastase perfusion +
jetPRIME transfection reagent + Egr-1 DNA enzyme). Thirty rats were
included in each group. The rats were anesthetized with 3%
pentobarbital sodium (40 mg/kg, intraperitoneal injection). The
skin was prepared and disinfected, and then the rodents were fixed
on an operating table in the supine position. A SXP-1C-type
microscope (×10 magnification; Smoif, Shanghai, China) was used for
sterile surgery. The inferior vena cava and abdominal aorta were
separated (Fig. 2). The abdominal
aortic trunk with a length of about approximately 1 cm located
inferior to the left renal vein was separated and the infrarenal
aortic diameter was measured. To block the blood flow on the upper
part of this segment, we inserted a PE10 catheter (Smiths Medical,
Minneapolis, MN, USA) into the left common iliac artery to the
separated abdominal aorta. The lower part of the aorta was ligated
temporarily to create a closed lumen, and 1 ml (20 µ) of elastase
(Beijing Solarbio Science and Technology Co., Ltd., Beijing, China)
was perfused for 20 min. For the EDRz group, 20 µl of the mixture
containing DEPC, jetPRIME, MgCl2, EDRz, and F-127
pluronic gel was used and evenly coated on the peri-adventitial of
the elastase perfused abdominal aorta. The specimens were collected
after 28 days.
Detecting transfection of EDRz
The specimens were fixed and dehydrated in a sucrose
gradient. The sections were embedded and sliced into 5-µm-thick
sections with a constant freezing microtome. Transfection of EDRz
was observed under a fluorescence microscope.
Ultrastructural analysis
Animal pressure perfusion was fixed with 3%
glutaraldehyde solution in 0.1 M of phosphate buffer (pH 7.4) via a
catheter for 30 min at 20°C with a pressure of 100 mmHg. Specimens
were dehydrated with alcohol and embedded in EPON. The sections
were stained with lead citrate and uranyl acetate and observed with
a transmission electron microscope.
Hematoxylin and eosin (H&E)
staining
AAA specimens were fixed in 4% polyformaldehyde for
at least 24 h, and the conventional paraffin-embedded sections were
cut into 5-µm-thick slices. Changes in the morphological
characteristics of the aortic wall were observed by H&E
staining.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) from
the medial membrane of AAA, and the purity and concentration of RNA
were determined using a NanoDrop1000 (NanoDrop; Thermo Fisher
Scientific, Inc.; Wilmington, DE, USA). The RNA was
reverse-transcribed into cDNA using the PrimeScript RT reagent Kit
(Takara Bio, Inc., Otsu, Japan). qPCR was performed using SYBR
Premix Ex Taq™ II (Takara Bio, Inc.) on an ABI 7500 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The qPCR parameters were as follows: 95°C of
denaturation for 30 sec, 95°C for 5 sec, and 60°C for 30 sec for a
total of 40 times. The sequences of the primers were as follows:
Egr-1: 5′-CAGGAGTGATGAACGCAAGA-3′ (forward) and
5′-GGGGATGGGTAGGAAGAGAG-3′ (reverse). MMP-2:
5′-GATACAGGTGTGCCAAGGTG-3′ (forward) and 5′-AAAGGGCAAACAAAGCAAAC-3′
(reverse). MMP-9: 5′-CTGCAGTGCCCTTGAACTAA-3′ (forward) and
5′-TATCCGGCAAACTAGCTCCT-3′ (reverse); β actin:
5′-TGTCACCAACTGGGACGATA-3′ (forward) and 5′-GGGGTGTTGAAGGTCTCAAA-3′
(reverse). The expression of the target gene was determined using
β-actin as a reference using the 2−ΔΔCq method. Samples
were examined in triplicate.
Western blotting
Total protein was extracted from the frozen medial
membrane of the AAA with Lysis Buffer RIPA (Beyotime Institute of
Biotechnology, Beijing, China). The protein concentration was
determined using the BCA (Science Fdbiao, China) protein
concentration assay kit. Protein extracts were separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis in 12 sodium
dodecyl sulfate and transferred to a polyvinylidene fluoride
membrane. Skim milk or bovine serum albumin was added to
Tris-buffered saline, blocked for 1 h, and then incubated with
Egr-1 antibody [EGR1 (15F7) Rabbit mAb, cat. no. 4153, 1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA], MMP-2 (MMP-2
Polyclonal Antibody, cat. no. A6247, 1:1,000; ABclonal Technology,
Woburn, MA, USA), MMP-9 (MMP-9 Polyclonal Antibody, cat. no.
A11147, 1:1,000; ABclonal Technology), and β-actin (cat. no. TA-09,
1:2,000; ZSGB-BIO, Beijing, China) antibody overnight at 4°C.
Horseradish peroxidase-labeled goat antirabbit IgG
(Peroxidase-Conjugated Goat anti-Rabbit IgG, cat. no. ZB-2301,
1:2,000; ZSGB-BIO) was used. Image analysis was conducted using
Image lab software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Immunohistochemistry
The specimens were fixed with 4% formalin. The
slices were immersed in dewaxing xylene and ethanol. The sections
were incubated in 3% H2O2 for 10 min to
inhibit endogenous peroxidase activity. These sections were blocked
with 5% bovine serum albumin or milk powder. Egr-1 antibody [EGR1
(15F7) Rabbit mAb, cat. no. 4153, 1:1,000; Cell Signaling
Technology, Inc.], MMP-2 (MMP-2 Polyclonal Antibody, cat. no.
A6247, 1:1,000; ABclonal Technology), and MMP-9 (MMP-9 Polyclonal
Antibody, cat. no. A11147, 1:1,000; ABclonal Technology) were
incubated at 4°C overnight. After washing 3 times, biotin-labeled
secondary antibody was added and incubated for 30 min. The three
membranes were washed and incubated with
biotin-horseradish-peroxidase complex at room temperature for 30
min. The films were then washed with PBS and DAB, counterstained
with hematoxylin, and mounted with neutral balata. As a negative
control, the primary antibody was substituted with PBS.
Statistical analysis
Data were expressed as the mean ± standard deviation
or χ2 test for the difference between groups. Three or
more groups were compared by one-way analysis of variance and
Bonferroni post hoc test for multiple comparisons. A Student's
t-test was used for comparisons between 2 groups. Data were
analyzed with Prism GraphPad 6 (GraphPad, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
EDRz transfection
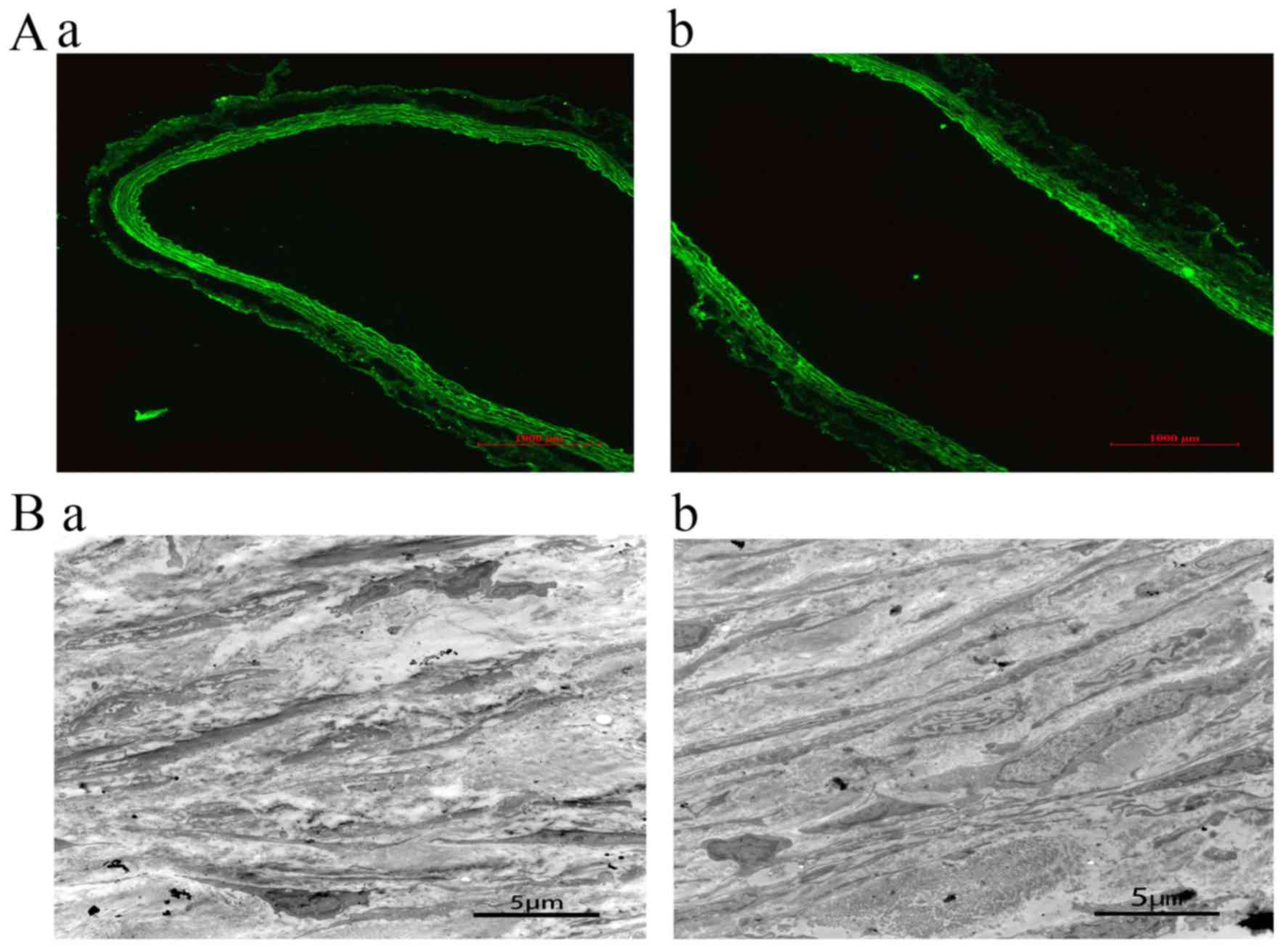
Blood vessels were collected 24 h and 28 days after
transfection, and transfection was confirmed by fluorescence
microscopy. In the control group and jetPRIME group, no
fluorescence was detected. A wide range of green fluorescence was
observed in the intima and media of the blood vessels in EDRz the
group. This result demonstrates that EDRz was transfected into the
inner and media (Fig. 3Aa). After 28
days of transfection, green fluorescence was observed in the vessel
wall, indicating that EDRz was a persistent transfection process
(Fig. 3Ab).
Transmission electron microscopy
Transmission electron microscopy showed that the
medial layer of the vessels was disordered and that elastic fibers
were broken and arranged irregularly. The phenotype of smooth
muscle cells was secretory. Moreover, smooth muscle cells were
significantly reduced and irregularly arranged in the control group
(Fig. 3Ba) compared to in the EDRz
group (Fig. 3Bb).
EDRz inhibits the formation of
AAA
AAA is a dilatation of the abdominal aorta,
typically above the normal diameter of the artery by more than 50%.
By measuring the diameter of the abdominal aorta, all arteries
reached the diagnostic criteria for aneurysms. After 28 days,
elastase was perfused into the abdominal aorta in rats to determine
the inhibitory effect of EDRz on AAA development by observing the
aortic diameter in the EDRz group.
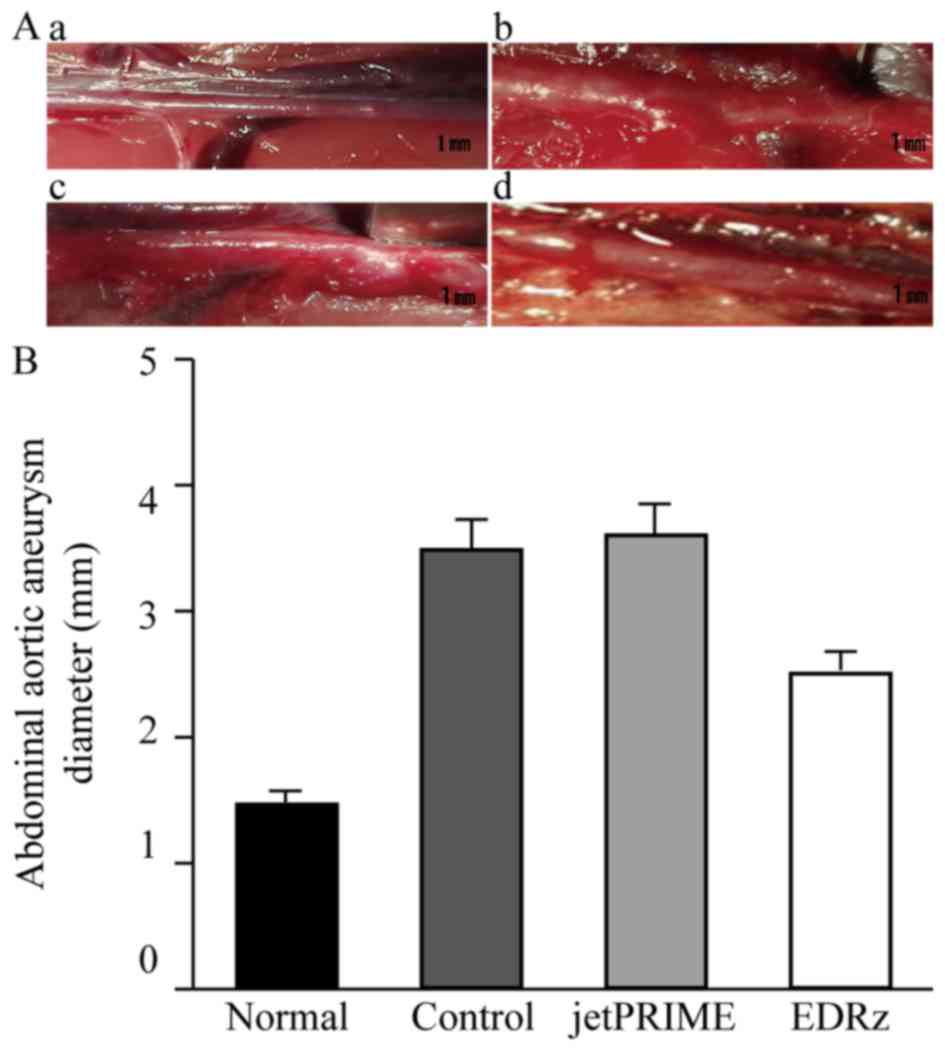
Aneurysm formation in the abdominal aorta at 28 days
was observed in each group. Representative macroscopic images
immediately before AAA induction (Normal: Fig. 4Aa) and 28 days after AAA induction in
the groups are shown in Fig. 4A. The
EDRz group (Fig. 4Ad) showed minimal
aneurysm formation compared to the control (Fig. 4Ab) and transfection reagent group
(Fig. 4Ac). The abdominal aortic
diameter in the three groups gradually increased after AAA
induction, with significantly smaller diameters in the EDRz group
(2.5±0.1 mm) than in the control group (3.5±0.1 mm) and
transfection reagent group (3.6±0.1 mm). No significant difference
was observed between the control and transfection reagent group
(P>0.05; Fig. 4B).
H&E staining
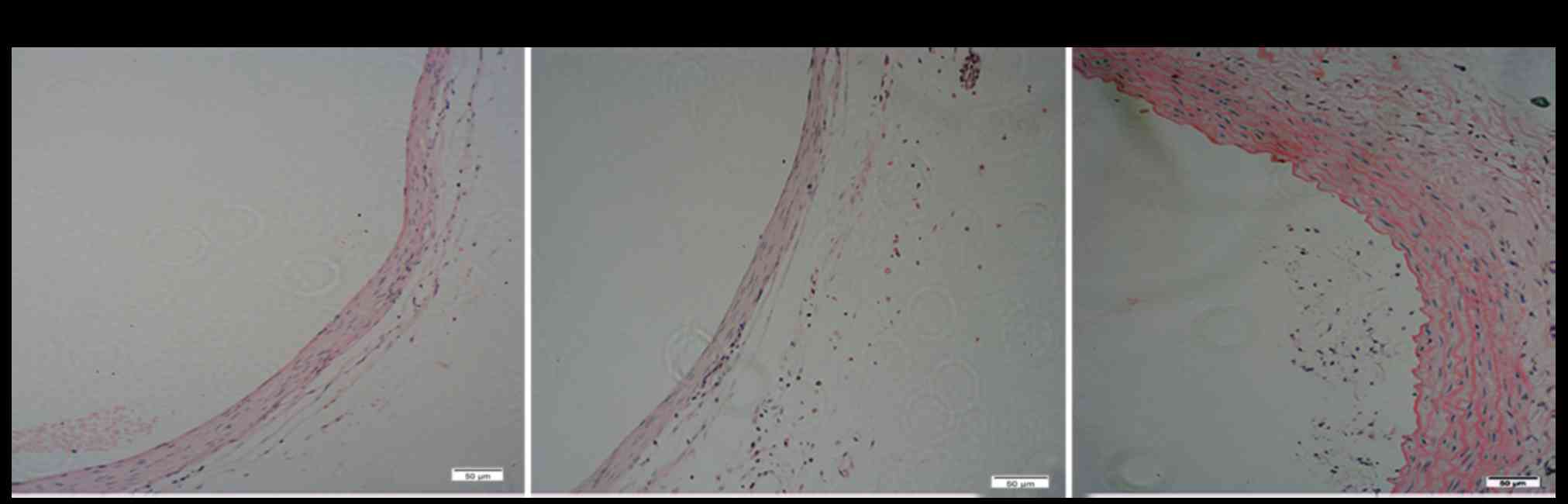
We histologically examined the thickness of the
medial membrane of the artery (Fig.
5). H&E staining showed that the number of smooth muscle
cells per unit area decreased in the control group (Fig. 5A) and jetPRIME group (Fig. 5B), and the medial was obviously
thinner than in the EDRz treatment group (Fig. 5C). The media layer thickness of the
control group was slightly lower in the EDRz group (control group:
72.8±18.3 µm, EDRz group: 125.2±20.8 µm; P<0.05). No significant
difference was observed between the control and transfection
reagent group (control group: 72.8±18.3 µm, transfection reagent
group: 76.3±22.8 µm; P>0.05).
Egr-1, MMP-2, and MMP-9 mRNA
levels
Compared to the control group, the EDRz group showed
significantly lower mRNA expression levels of Egr-1 (control group:
1.00±0.23, EDRz group: 0.46±0.09, P<0.01) and MMP-2 (control
group: 1.01±0.09, EDRz group: 0.39±0.17; P<0.01) and MMP-9
(control group: 1.00±0.08, EDRz group: 0.34±0.53; P<0.01).
Compared to the jetPRIME group, the EDRz group showed significantly
lower mRNA expression levels of Egr-1 (jetPRIME group: 1.09±0.24,
EDRz group: 0.46±0.09, P<0.01) and MMP-2 (jetPRIME group:
1.08±0.24, EDRz group: 0.39±0.17, P<0.01) and MMP-9 (jetPRIME
group: 0.95±0.15, EDRz group: 0.34±0.53, P<0.01). The expression
levels of Egr-1, MMP-2, and MMP-9 in the control and transfection
reagent group did not differ significantly (P>0.05; Fig. 6).
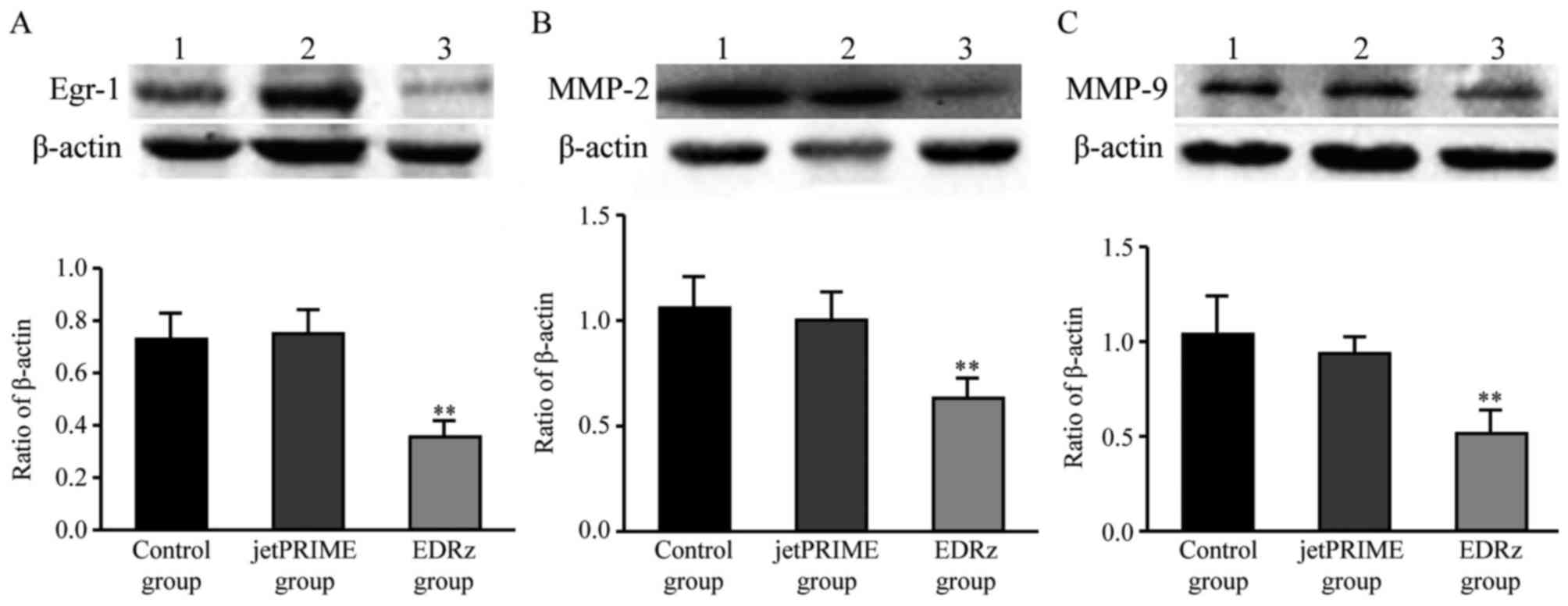
Western blotting analysis
Based on the western blotting results, Egr-1 protein
expression levels in the control group and jetPRIME group were
significantly higher than in the EDRz group (control group:
0.72±0.10, jetPRIME group: 0.75±0.09, EDRz group: 0.35±0.06)
(Fig. 7A). MMP-2 protein expression
levels in the control and jetPRIME group were significantly higher
than in the EDRz group (control group: 1.05±0.17, jetPRIME group:
1.00±0.13, EDRz group: 0.63±0.10) (Fig.
7B). MMP-9 protein expression levels in the control and
jetPRIME group were significantly higher than in the EDRz group
(control group: 1.04±0.18, jetPRIME group: 0.93±0.08, EDRz group:
0.53±0.11) (Fig. 7C). However, the
expression levels of Egr-1, MMP-2, and MMP-9 in the control group
and jetPRIME group did not differ significantly (P>0.05;
Fig. 7).
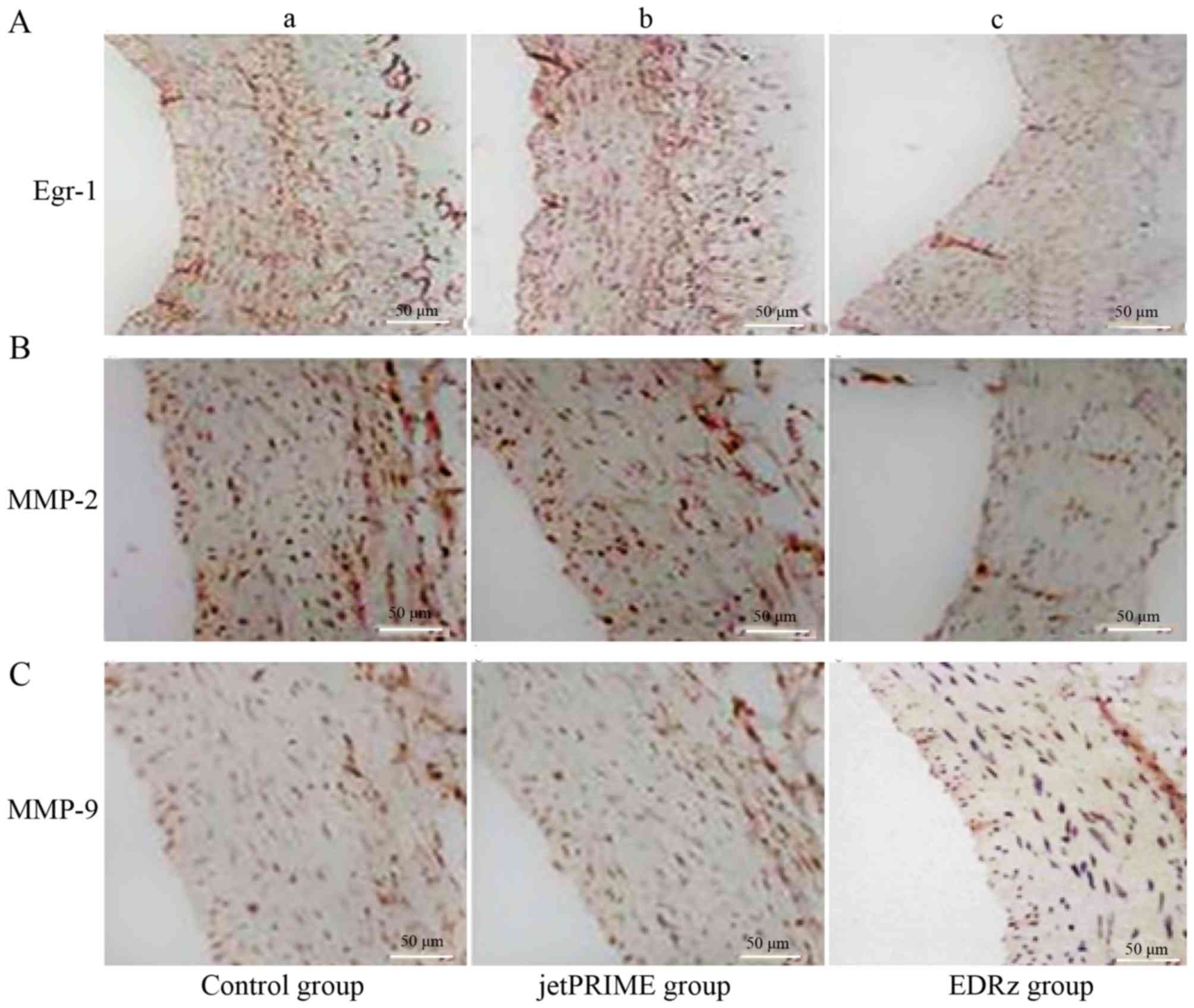
Immunohistochemistry
Immunostaining for Egr-1 revealed a significantly
higher proportion of positive area in the control group (29.3±4.3%;
Fig. 8Aa) and jetPRIME group
(27.1±5.0%; Fig. 8Ab) than in the
EDRz group (14.3±3.3%, P<0.05; Fig.
8Ac). MMP-2 showed a significantly higher proportion of
positive area in the control group (24.2±5.8%; Fig. 8Ba) and jetPRIME group (24.9±6.2%;
Fig. 8Bb) than in the EDRz group
(11.3±2.6%; P<0.05; Fig. 8Bc).
MMP-9 showed a significantly higher proportion of positive area in
the control group (20.8±3.3%; Fig.
8Ca) and jetPRIME group (21.1±5.3%; Fig. 8Cb) than in the EDRz group (14.1±6.3%,
P<0.05; Fig. 8Cc). However, the
expression levels of Egr-1, MMP-2, and MMP-9 in the control and
jetPRIME group did not differ significantly (P>0.05; Fig. 8).
Discussion
Egr-1, a zinc finger early transcription factor, is
a member of the early gene family. In the study of AAA, Egr-1 has
attracted much attention. Egr-1 is poorly expressed in the normal
artery wall. However, it is induced by acute mechanical injury,
other vascular stresses, growth factors, and proinflammatory
cytokines (12,13). Egr-1 also regulates the expression of
genes related to the development of vascular diseases (14). Bioinformatics analysis showed that
Egr-1 is a key transcription factor in the formation and
pathogenesis of AAA (15). Egr-1 was
found to be involved in thrombus formation and the inflammatory
pathogenesis of AAA. The role of Egr-1 as a mechanical
stress-response gene that causes aneurysm formation was also
demonstrated (11). Thus, Egr-1 is
being considered as a new target for AAA prevention and
control.
DNA enzymes (deoxyribozyme, DRz) are DNA molecules
with catalytic capabilities that cleave specific RNA strands. The
‘10–23’ DNA enzyme is the 23rd clone of the 10th cycle of in
vitro selection. Therefore, the enzyme activity center is a
‘10–23 motif’ (16,17). AUG (816–818 sequence) is a selected
target of Egr-1 mRNA. A phosphorothioate modification was made in
the 3′ end to resist nuclease degradation, and the 5′ end was
labeled with carboxy fluorescein for detection purposes. Base 816
(A) of the Egr-1 mRNA did not base pair with EDRz. However, the
remaining EDRz sites base paired with Egr-1 mRNA, followed by
conformational changes. Moreover, nucleophilic attack occurred on
the adjacent phosphate. The Egr-1 mRNA molecular structure was
dissociated by two transesterification reactions. Our previous
study demonstrated the specificity and efficacy of synthetic EDRz
for inhibiting Egr-1 (7,18,19).
However, whether the development of AAA can be suppressed by
inhibiting Egr-1 expression remains unknown.
Synthetic DNA enzymes that specifically degrade
Egr-1 mRNA prevent the induction of Egr-1 protein expression in
human and rat aortic vascular smooth muscle cells (20–22). In
previous studies, Egr-1 DNA enzyme reduced the mRNA and protein
expression of Egr-1 in aortic smooth muscle cells and inhibited
cell proliferation and migration (23). This property has been successfully
applied to suppress gene expression. In our study, green
fluorescence was observed in the vessel wall after 24 h and 28
days, indicating that EDRz was a persistent transfection process
and that expression of Egr-1 was continuously inhibited. EDRz can
effectively inhibit the formation and development of AAA. AAA is a
dilatation of the abdominal aorta, typically above the normal
diameter of the artery by more than 50%. By measuring the diameter
of the abdominal aorta, all arteries reached the diagnostic
criteria for aneurysms. The EDRz group showed minimal aneurysm
formation compared to the control and transfection reagent group.
These results suggest that EDRz inhibits AAA by inhibiting Egr-1
expression.
Elastase perfusion degrades the elastic fiber in the
media and forms elastic fragments. In our study, H&E staining
and transmission electron microscopy showed that the medial layer
of the vessels was disordered and that elastic fibers were broken
and arranged irregularly in the control group and jetPRIME group.
In the control group, the media was significantly thinner than in
the EDRz group.
Moreover, the number of smooth muscle cells was
significantly reduced and these cells were irregularly arranged in
the control group compared to in the EDRz group. This causes a
local inflammatory reaction and produces proinflammatory factors,
MMPs, endogenous and elastic proteases, among others. These
processes promote elastase and collagen fiber degradation. Arterial
wall inflammation can cause vascular smooth muscle cell apoptosis,
MMP expression, and inflammation. Therefore, MMPs promote the
degradation of the extracellular matrix, and inflammation
participates in the occurrence and development of AAA (24,25).
Moreover, the suppression of MMP expression (26) can effectively inhibit AAA.
Destruction of the elastic plate in blood vessels also contributes
to cell proliferation, migration, and relocalization in the
extracellular matrix, which promotes arterial expansion and causes
matrix shrinkage and aneurysm formation and rupture. MMPs are
proteases that can promote extracellular matrix degradation and an
inflammatory response, both of which contribute to aneurysm
development. Particularly, MMP-2 and MMP-9 can significantly
degrade elastin and participate in inflammatory responses (27).
Egr-1, MMP-2, and MMP-9 levels were evaluated to
determine the effectiveness of EDRz in inhibiting the AAA response.
In the present study, the mRNA and protein expression levels of
Egr-1, MMP-2, and MMP-9 were determined along with the inhibition
of AAA genesis in rats transfected with EDRz. The results indicate
that EDRz inhibits AAA development and reduces MMP-9 and MMP-2
expression.
Previous studies showed that Egr-1 promotes the
expression of MMP-9 and MMP-2 and the migration and metastasis of
inflammatory and cancer cells. Moreover, this phenomenon causes the
formation and rupture of aneurysms (28). Shin et al (29) found that MMP2 and MMP-9 promote the
degradation of elasticity and extracellular matrix and
inflammation. MMP-9 transcription caused by tumor necrosis factor
is closely related to Egr-1. MMP-9 expression activity is enhanced
by Egr-1 binding to its promoter. Harja et al (30) observed that the MMP-2 expression
level in apo E−/− knockout mice was higher than that in
C56BL6 mice with the same genetic background. Although it remains
unclear whether Egr-1 can bind to MMP-2, a previous study
demonstrated that stimulation of MMP-2 is related to the expression
of MT1-MMP. Egr-1 can be combined with the MT1-MMP binding site
(31). Sho et al (32) reported that mechanical and shear
stresses can induce Egr-1 expression, whereas the transcription of
MT1-MMP regulated by Egr-1 can activate MMP-2. MT1-MMP is found in
endothelial and smooth muscle cells, which may be involved in
long-term maintenance of MMP-2 activation and further contribute to
dilatation of the aorta. Therefore, EDRz may inhibit the
development of AAA by inhibiting MMP-2 and MMP-9 expression.
However, the specific mechanism of this inhibitory effect requires
further investigation.
This study has some limitations. The rat model of
elastase-induced AAA used in our study is pathologically different
from human AAAs. Human AAAs often exhibit atherosclerosis and
intramural thrombosis such as in diabetic or hyperlipidemia rats.
Whether the dose of EDRz used in this study was appropriate is
unclear. We selected the EDRz dose based on our previous report,
which showed that EDRz prevents stenosis and occlusion of
autogenous vein graft in vivo. How to apply this information
in humans also remains unclear. Whether EDRz can delay or treat AAA
progression when administrated after the onset of AAA is unknown,
which will be the focus of our future studies.
In conclusion, we found that Egr-1 plays an
important role in AAA formation. EDRz inhibits the mRNA and protein
expression of Egr-1 and regulates the expression of MMP-2 and
MMP-9. Thus, the development of AAA in rats was inhibited. EDRz may
be useful as a new drug for preventing or treating AAA.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Nature
Science Foundation of China (grant nos. 30801123 and 81070254) and
the Reserve Talents of Universities Overseas Research Program of
Heilongjiang and Project Sponsored by the Scientific Research
Foundation for the Returned Overseas Chinese Scholars, State
Education Ministry [grant no. (2014) 1685].
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contribution
SW and HD contributed equally to this paper. SW, HD,
CL, XH and YF were involved in the study conception and design. SW,
HD, GX and CO were involved in analysis and interpretation of data.
SW, HD, GX and LC collected data. SW, HD, CL, GX, XH, CO and LC
wrote the article. CL, XH, CO and YF performed critical revision of
the article. SW, HD, CL, GX, XH and CO approved the final article.
SW, HD, GX, LC and YF performed statistical analysis. CL and XH
obtained funding. CL had overall responsibility.
Ethics approval and consent to
participate
The animal use protocol was been reviewed and
approved by the Institutional Animal Care and Use Committee (IACUC)
of Jiamusi University (Heilongjiang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare there they have no competing
interest.
References
|
1
|
Grøndal N, Søgaard R and Lindholt JS:
Baseline prevalence of abdominal aortic aneurysm, peripheral
arterial disease and hypertension in men aged 65–74 years from a
population screening study (VIVA trial). Br J Surg. 102:902–906.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benson RA, Poole R, Murray S, Moxey P and
Loftus IM: Screening results from a large United Kingdom abdominal
aortic aneurysm screening center in the context of optimizing
United Kingdom national abdominal aortic aneurysm screening
programme protocols. J Vasc Surg. 63:301–304. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shin S, Cho YP, Jun H, Park H, Hong HN and
Kwon TW: Transglutaminase type 2 in human abdominal aortic aneurysm
is a potential factor in the stabilization of extracellular matrix.
J Vasc Surg. 57:1362–1370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang L, Cong Z, Hao S, Li P, Huang H, Shen
Y, Li K and Jing H: Protective effect of melatonin on the
development of abdominal aortic aneurysm in a rat model. J Surg
Res. 209:266–278.e1. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pagel JI and Deindl E: Early growth
response 1-a transcription factor in the crossfire of signal
transduction cascades. Indian J Biochem Biophys. 48:226–235.
2011.PubMed/NCBI
|
|
6
|
Wang NP, Pang XF, Zhang LH, Tootle S,
Harmouche S and Zhao ZQ: Attenuation of inflammatory response and
reduction in infarct size by postconditioning are associated with
downregulation of early growth response 1 during reperfusion in rat
heart. Shock. 41:346–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu C, Zhang X, Wang S, Cheng M, Liu C,
Wang S, Hu X and Zhang Q: Transfected early growth response gene-1
DNA enzyme prevents stenosis and occlusion of autogenous vein graft
in vivo. Biomed Res Int. 2013:3104062013.PubMed/NCBI
|
|
8
|
Ha YM, Lee DH, Kim M and Kang YJ: High
glucose induces connective tissue growth factor expression and
extracellular matrix accumulation in rat aorta vascular smooth
muscle cells via extracellular signal-regulated kinase 1/2. Korean
J Physiol Pharmacol. 17:307–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Charolidi N, Pirianov G, Torsney E, Pearce
S, Laing K, Nohturfft A and Cockerill GW: Pioglitazone identifies a
new target for aneurysm treatment: Role of Egr1 in an experimental
murine model of aortic aneurysm. J Vasc Res. 52:81–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin IS, Kim JM, Kim KL, Jang SY, Jeon ES,
Choi SH, Kim DK, Suh W and Kim YW: Early growth response factor-1
is associated with intraluminal thrombus formation in human
abdominal aortic aneurysm. J Am Coll Cardiol. 53:792–799. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamashiro Y, Papke CL, Kim J, Ringuette
LJ, Zhang QJ, Liu ZP, Mirzaei H, Wagenseil JE, Davis EC and
Yanagisawa H: Abnormal mechanosensing and cofilin activation
promote the progression of ascending aortic aneurysms in mice. Sci
Signal. 8:ra1052015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong L, Shen X, Lin L, Leitges M, Rosario
R, Zou YS and Yan SF: PKCβ promotes vascular inflammation and
acceleration of atherosclerosis in diabetic ApoE null mice.
Arterioscler Thromb Vasc Biol. 33:1779–1787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morawietz H, Ma YH, Vives F, Wilson E,
Sukhatme VP, Holtz J and Ives HE: Rapid induction and translocation
of Egr-1 in response to mechanical strain in vascular smooth muscle
cells. Circ Res. 84:678–687. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu M, Zhu X, Zhang J, Liang J, Lin Y, Zhao
L, Ehrengruber MU and Chen YE: Egr-1 target genes in human
endothelial cells identified by microarray analysis. Gene.
315:33–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan K, Liang W and Zhang J: A
comprehensive analysis of differentially expressed genes and
pathways in abdominal aortic aneurysm. Mol Med Rep. 12:2707–2714.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Wang N, Luo Q and Wan L: The 10–23
DNA enzyme generated by a novel expression vector mediate
inhibition of taco expression in macrophage. Oligonucleotides.
20:61–68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lam CH and Perrin DM: Introduction of
guanidinium-modified deoxyuridine into the substrate binding
regions of DNAzyme 10–23 to enhance target affinity: Implications
for DNAzyme design. Bioorg Med Chem Lett. 20:5119–5122. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pagel JI, Ziegelhoeffer T, Heil M, Fischer
S, Fernández B, Schaper W, Preissner KT and Deindl E: Role of early
growth response 1 in arteriogenesis: impact on vascular cell
proliferation and leukocyte recruitment in vivo. Thromb Haemost.
107:562–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dickinson MG, Kowalski PS, Bartelds B,
Borgdorff MA, van der Feen D, Sietsma H, Molema G, Kamps JA and
Berger RM: A critical role for Egr-1 during vascular remodelling in
pulmonary arterial hypertension. Cardiovasc Res. 103:573–584. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang M, Satchell L, Duhadaway JB,
Prendergast GC and Laury-Kleintop LD: RhoB links PDGF signaling to
cell migration by coordinating activation and localization of Cdc42
and Rac. J Cell Biochem. 112:1572–1584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Uchida K, Sasahara M, Morigami N, Hazama F
and Kinoshita M: Expression of platelet-derived growth factor
B-chain in neointimal smooth muscle cells of balloon injured rabbit
femoral arteries. Atherosclerosis. 124:9–23. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iyoda T, Zhang F, Sun L, Hao F,
Schmitz-Peiffer C, Xu X and Cui MZ: Lysophosphatidic acid induces
early growth response-1 (Egr-1) protein expression via protein
kinase Cδ-regulated extracellular signal-regulated kinase (ERK) and
c-Jun N-terminal kinase (JNK) activation in vascular smooth muscle
cells. J Biol Chem. 287:22635–22642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Y, Han W and Liu GN: A DNA enzyme
targeting Egr-1 inhibits rat vascular smooth muscle cell
proliferation by down-regulation of cyclin D1 and TGF-beta1. Braz J
Med Biol Res. 43:17–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Longo GM, Xiong W, Greiner TC, Zhao Y,
Fiotti N and Baxter BT: Matrix metalloproteinases 2 and 9 work in
concert to produce aortic aneurysms. J Clin Invest. 110:625–632.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan YF, Pei JF, Zhang Y, Zhang R, Wang F,
Gao P, Zhang ZQ, Wang TT, She ZG, Chen HZ and Liu DP: The
paraoxonase gene cluster protects against abdominal aortic aneurysm
formation. Arterioscler Thromb Vasc Biol. 37:291–300. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chabasse C, Siefert SA, Chaudry M,
Hoofnagle MH, Lal BK and Sarkar R: Recanalization and flow regulate
venous thrombus resolution and matrix metalloproteinase expression
in vivo. J Vasc Surg Venous Lymphat Disord. 3:64–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tanaka A, Hasegawa T, Chen Z, Okita Y and
Okada K: A novel rat model of abdominal aortic aneurysm using a
combination of intraluminal elastase infusion and extraluminal
calcium chloride exposure. J Vasc Surg. 50:1423–1432. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pezet M, Jacob MP, Escoubet B, Gheduzzi D,
Tillet E, Perret P, Huber P, Quaglino D, Vranckx R, Li DY, et al:
Elastin haploinsufficiency induces alternative aging processes in
the aorta. Rejuvenation Res. 11:97–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin SY, Kim JH, Baker A, Lim Y and Lee
YH: Transcription factor Egr-1 is essential for maximal matrix
metalloproteinase-9 transcription by tumor necrosis factor alpha.
Mol Cancer Res. 8:507–519. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harja E, Chang JS, Lu Y, Leitges M, Zou
YS, Schmidt AM and Yan SF: Mice deficient in PKCbeta and
apolipoprotein E display decreased atherosclerosis. FASEB J.
23:1081–1091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haas TL, Stitelman D, Davis SJ, Apte SS
and Madri JA: Egr-1 mediates extracellular matrix-driven
transcription of membrane type 1 matrix metalloproteinase in
endothelium. J Biol Chem. 274:22679–22685. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sho E, Sho M, Singh TM, Nanjo H, Komatsu
M, Xu C, Masuda H and Zarins CK: Arterial enlargement in response
to high flow requires early expression of matrix metalloproteinases
to degrade extracellular matrix. Exp Mol Pathol. 73:142–153. 2002.
View Article : Google Scholar : PubMed/NCBI
|