Introduction
Epilepsy is a common neurological syndrome, the
recurrence of which is apt to induce damages to brain tissues in
varying degrees, including cognitive dysfunction or damage to the
structure and functions of mitochondria (1,2). At
present, anti-epilepsy drugs in clinical practice are only designed
for certain specific targets, and long-term administration may make
patients more susceptible to a series of serious adverse reactions,
including hematopoietic system damage, Stevens-Johnson syndrome,
severe hepatic dysfunction (3) or
aggravate cognitive dysfunction (3).
Therefore, identifying a novel drug that can effectively improve
cognitive functions without side-effects or toxicity has been
considered as a priority.
Oxidative stress refers to the oxidative damage
caused by reactive oxygen species (ROS). The excessive generation
of free radicals or the dysfunction of in vivo
anti-oxidation systems result in imbalance in the metabolism of
oxygen radicals, which may damages tissues and thus the body as a
whole (4). ROS may also act on the
mitochondria (5), leading to changes
in permeability of the mitochondrial membrane, which can contribute
to the apoptosis of neurons and make patients more susceptible to
the onset of epilepsy (6,7). Thus, cell apoptosis has an important
role in the pathogenesis of epilepsy.
Mitogen-activated protein kinase (MAPK) pathways are
important transmembrane signal transduction pathways, primarily
including three major MAPK cascade reactions: p38, extracellular
signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK)
(8). These cascade reactions are not
only associated with cell growth, proliferation and apoptosis, but
also have key roles in these processes. p38, ERK1/2 and JNK are all
sensitive to the stimulation of ROS (9,10).
Fructus corni polysaccharide (PFC), obtained from
the Traditional Chinese Medicine Fructus corni, is one of the most
common Traditional Chinese Medicines (11). Previous pharmacological studies have
suggested that PFC has a wide range of biological activities,
including: Antioxidative, anti-inflammatory, immuno-modulatory,
antidiabetic, and hypoglycemic (12,13).
Thus, the antioxidant activity of PFC may have potential benefits
in protecting against epilepsy.
In the present study, an epileptic rat model was
established through the induction of lithium chloride-pilocarpine
to investigate the potential effect of PFC on mitochondrial damage
in hippocampal tissues and the potential underlying mechanism,
thereby identifying novel pathways and theoretical evidence for the
clinical treatment of epilepsy.
Materials and methods
Reagents
PFC (95%) was provided by Ningbo Dekang Biological
Products Co., Ltd. (Ningbo, China). Lithium chloride and
pilocarpine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), were
used to establish the epileptic rat model. Malondialdehyde (MDA;
cat. no. JC201712) content and superoxide dismutase (SOD; cat. no.
JC201722) activity in hippocampal tissue were detected with ELISA
detection kits provided by Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). ROS level was detected by flow
cytometry (BD FACSAria™ II; BD Biosciences, San Jose,
CA, USA) using the oxidation sensitive fluorescent dye,
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; CAS:D6883;
Sigma-Aldrich; Merck KGaA).
Study design
Rats were randomly assigned into the following five
groups: I: Control group (n=15), which was administered saline
(vehicle) via gavage for 44 days (6 ml/kg/day); II:
Lithium-pilocarpine (LP) group (n=15), which was administered
saline (6 ml/kg/day) for 24 days followed by lithium-pilocarpine
treatment for 20 days (model establishment); III, IV and V (n=15
per group): LP+PFC groups that were administered PFC (100, 200 or
300 mg/kg/day) for 24 days followed by lithium-pilocarpine
treatment for 20 days. The mortality and seizure stages of the rats
were recorded throughout.
Model establishment and screening
A total of 120 male Sprague Dawley rats (age, 6–8
weeks; weight, 180–220 g) were used in the present study; they were
purchased from the Shandong Province Animal Research Center
(Shandong, China). The rats had free access to food and water and
were housed in a temperature controlled room (21±2°C) in a 12 h
light/dark cycle. Rats received an intraperitoneal injection of 3
mmol/kg lithium chloride (127 mg/kg), and after 18–24 h, atropine
sulfate (1 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was administered to rats to antagonize the peripheral cholinergic
effect of pilocarpine, which was initially administered following
another 30 min (20 mg/kg). The behavior of rats was observed and
graded based on the Racine classification system (14). After 30 min if no epileptic seizures
were observed, pilocarpine (10 mg/kg) was administered to the rats
without the onset of status epilepticus (SE) every 30 min until the
onset of SE was successfully induced. Rats that failed in the model
establishment were excluded. For rats with SE lasting for 1 h,
intraperitoneal administration of 10% chloral hydrate (350 mg/kg)
(Sigma-Aldrich; Merck KGaA) was performed to terminate the seizure,
and additional doses were administered if a poor effect was
observed 30 min following the previous injection. Surviving rats
would enter the quiet phase 24 h after modeling as previously
described (15), the toxic stage
consisted of the initial 2–4 days. Rats would suffer spontaneous
epilepsy 7–20 days after modeling. A total of 60 rats with seizures
above Racine grade IV were considered as successful spontaneous
epileptic models, and those that failed to meet the criterion were
excluded. Animal protocols were approved by the Animal Ethics
Committee of Weifang People's Hospital Animal Center (Weifang,
China).
Behavioral assessment
Behavioral assessment was performed during the model
establishment and every day following successful modeling in
accordance with Racine's level 6 evaluation standards (14), and scored as follows: Stage 0, no
response; stage 1, vibrissae twitching and hyperactivity; stage 2,
clonus, head nodding and myoclonic jerks of the head; stage 3,
unilateral forelimb clonus; stage 4, rearing with bilateral
forelimb clonus; and stage 5, generalized tonic-clonic seizure with
loss of writing reflex.
Detection of MDA content and SOD
activity
Rats were sacrificed after the 44 day treatment
period and the hippocampus was immediately removed and homogenized
in ice-cold normal saline. Following cooling, the samples were
centrifuged at 2,500 × g at 4°C for 20 min. Supernatants were taken
to determine the levels of MDA and SOD using ELISA kits according
to the manufacturer's protocols.
Detection of mitochondrial ROS
production
As described previously (16), dichlorohydrofluorescein diacetate
(DCFH-DA) was used for accurate measurement of production of
mitochondrial ROS. Rats were sacrificed and hippocampus tissues
were rapidly obtained and homogenized. DCFH-DA fluorescence was
used for measurement at 488 nm excitation and 530 nm emission.
Detection of mitochondrial membrane
potential
Rhodamine 123 (Rh-123) staining was used to
determine the mitochondrial membrane potential in rats according to
a previous study (17). Mitochondria
were then pelleted by centrifugation at 8,600 × g at 4°C for 15 min
and fluorescence of the supernatant was measured at 503 nm
excitation and 527 nm emission using a spectrophotometer.
Nissl staining
Following sacrifice, rat brains were immediately
harvested, fixated with 4% paraformaldehyde at 20°C for 24 h, and
then embedded in paraffin. Subsequently, the paraffin-embedded
brains were cut into 10-µm thick sections for Nissl staining with
toluidine blue to evaluated general neuronal morphology. After
being flushed with distilled water, the sections were placed in a
37°C water bath and incubated for 10 min with 0.5% thionine. The
staining results were observed under a light microscope
(magnification, ×400). The neuronal density in the CA1 region of
the hippocampus was calculated as previously described (18,19).
Western blot analysis
Hippocampi were collected and homogenized in ice
using homogenization buffer, then centrifuged at 13,200 × g for 15
min at 4°C. Supernatants were collected, and a bicinchoninic acid
protein assay kit was used to calculate the protein concentrations.
Cytoplasmic and mitochondrial proteins were each isolated via
differential centrifugation with a mitochondrial fractionation kit
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) according to the
manufacturer's instructions. Protein samples (50 µg) were subjected
to 10% SDS-PAGE. Following electrophoresis, proteins were
transferred to polyvinylidene difluoride membranes and blocked with
5% (vol/vol) non-fat dry milk in Tris-buffered saline with Tween-20
(TBST; 10 mM Tris-HCl, 150 mM NaCl and 0.1% Tween-20; pH 7.6) at
room temperature for 2 h, followed by incubation overnight with
1:1,000 dilutions of cleaved caspase-3 (cat. no. 9661S), p38 MAPK
(cat. no. 8690S), phosphorylated (p-)p38 MAPK (cat. no. 4511S), ERK
(cat. no. 4695S), p-ERK (cat. no. 4370S), JNK (cat. no. 9252S),
p-JNK (cat. no. 9255S) (all Cell Signaling Technology, Inc.,
Danvers, MA, USA) and cytochrome C (cat. no. sc-13561; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) primary antibodies. β-actin
(dilution, 1:2,000; cat. no. sc-517582; Santa Cruz Biotechnology,
Inc.) served as the internal control. The membrane was washed in
TBST 3 times for 5 min each. The membrane was subsequently
incubated with horseradish peroxidase-conjugated secondary antibody
(cat. no. 5571S; 1:1,000; Cell Signaling Technology, Inc.) at room
temperature for an additional 2 h, and washed an additional 3 times
in TBST. The membrane was subsequently incubated with enhanced
chemiluminescence substrate solution at 20°C for 5 min according to
the manufacturer's protocol (cat. no. orb90504; Biorbyt, Cambridge,
UK) and visualized using autoradiography film. The imaging program
Quantity One 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
was used for quantification.
Statistical analysis
Data are presented as the mean + standard deviation.
The statistical analyses were performed using GraphPad Prism 5.01
(GraphPad Software, Inc., La Jolla, CA, USA) and PASW 18.0 (SPSS,
Inc., Chicago, IL, USA). One-way analysis of variance was used to
compare differences among multiple groups, followed by the least
significant difference post hoc test. Three independent repeats
were performed for each experiment. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of PFC on SOD activity and MDA
content
Compared with the control group, SOD activities
significantly decreased (P<0.01) and the MDA content was
significantly increased (P<0.001) in the LP group. Gavage with
200 mg/kg PFC (P<0.05) and 300 mg PFC (P<0.01) significantly
reduced the content of MDA and upregulated the activity of SOD
compared with LP group. These findings suggest that PFC treatment
may ameliorate oxidative stress (Fig. 1A
and B).
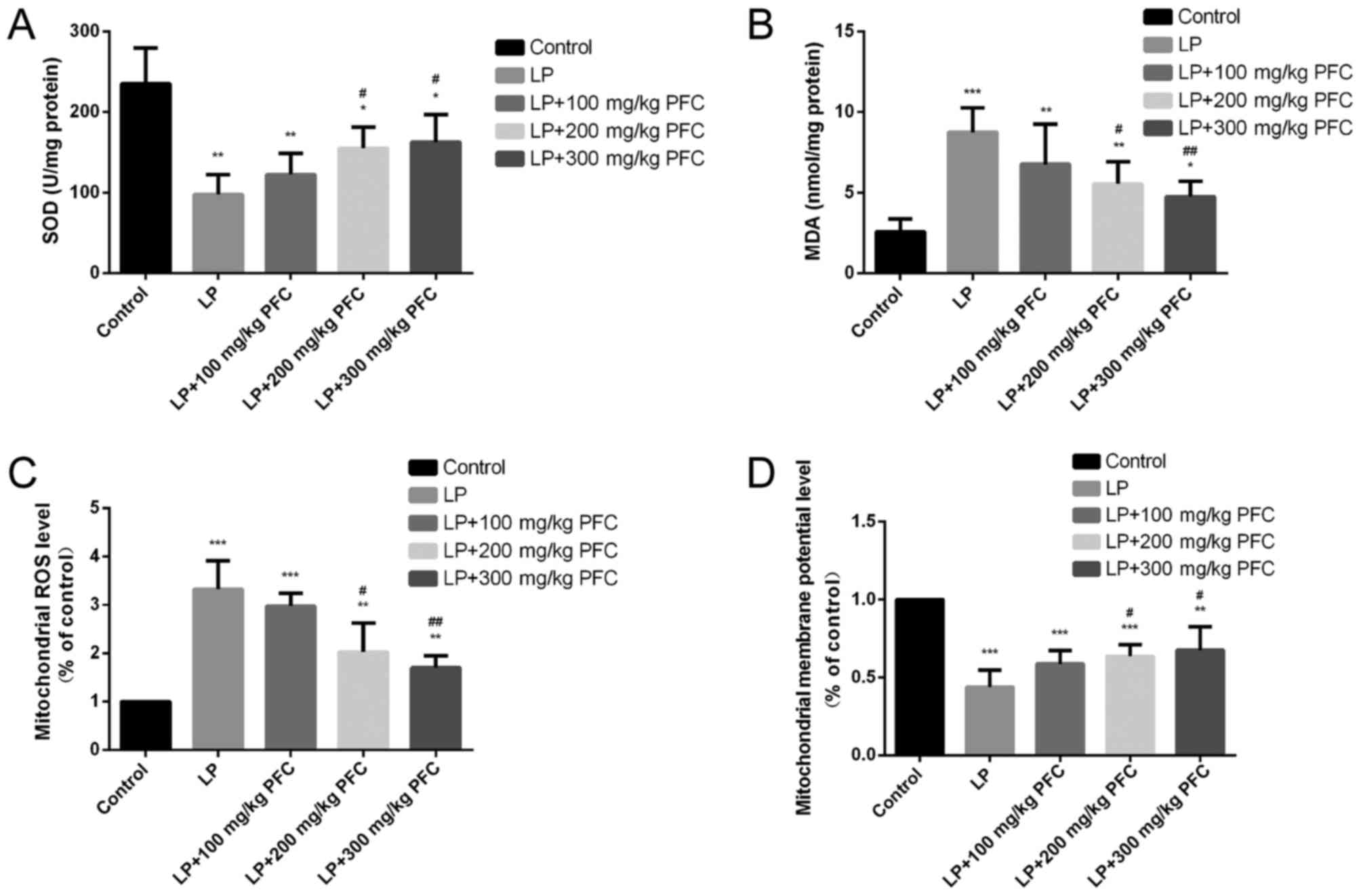 | Figure 1.Effects of PFC on hippocampal MDA
content, SOD activity, mitochondrial ROS generation and the
mitochondrial membrane potential. (A) Effect of PFC on SOD
activity. Compared with the control group, SOD activity was
significantly decreased in the LP group. Compared with the LP
injury group, SOD activity was increased in the LP+PFC groups. (B)
Effect of PFC on MDA content. Compared with control group, MDA
content was significantly increased in LP group. Compared with the
LP injury group, MDA content was significantly decreased in the
LP+PFC groups. (C) Effect of PFC on mitochondrial ROS production.
Compared with the control group, mitochondrial ROS production was
significantly increased in the LP group. Compared with the LP
injury group, the mitochondrial ROS production was decreased in the
LP+PFC groups. (D) Effect of PFC on mitochondrial membrane
potential. Compared with the control group, the mitochondrial
membrane potential was significantly decreased in the LP group.
Compared with the LP group, the mitochondrial membrane potential
was increased in the LP+PFC groups. *P<0.05, **P<0.01,
***P<0.001 vs. control group; #P<0.05,
##P<0.01 vs. LP group. Data are presented as the mean
+ standard deviation (n=5). PFC, Fructus corni polysaccharide; SOD,
superoxide dismutase; MDA, malondialdehyde; ROS, reactive oxygen
species; LP, lithium-pilocarpine. |
Effects of PFC on mitochondrial ROS
production induced by epilepsy
The present results demonstrated that the level of
mitochondrial ROS formation was significantly increased in LP group
compared with control (P<0.001). However, treatment with 200
mg/kg PFC (P<0.05) and 300 mg PFC (P<0.01) significantly
decreased the levels of mitochondrial ROS production, compared with
the LP group (Fig. 1C).
Effect of PFC on mitochondrial
membrane potential modification induced by epilepsy
In the LP group the mitochondrial membrane potential
was significantly reduced compared with the control group
(P<0.001). Treatment with PFC (200, 300 mg/kg) significantly
ameliorated this decline in mitochondrial membrane potential
(P<0.05; Fig. 1D).
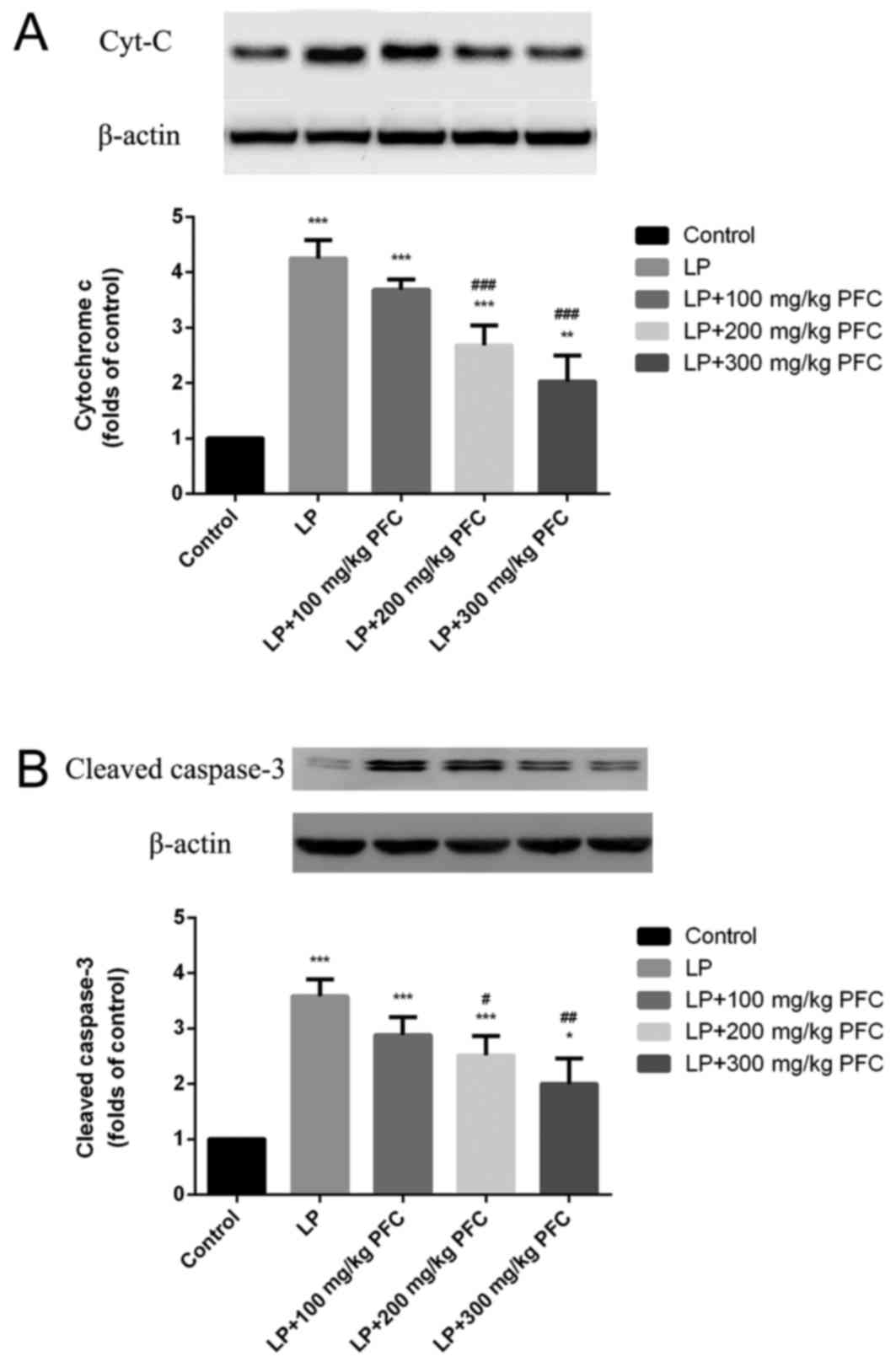
Effect of PFC on cytochrome C and
activation of cleaved-caspase-3 following epilepsy
Via western blotting, the release of cytochrome C
was demonstrated to significantly increase in the LP group compared
with control (P<0.001; Fig. 2A).
Cleaved caspase-3 is a characteristic sign of apoptosis (20). It was also demonstrated that cleaved
caspase-3 production significantly increased in LP groups
(P<0.001; Fig. 2B). Treatment
with 200 mg/kg PFC (P<0.001) and 300 mg PFC (P<0.001)
significantly ameliorated this cytochrome C release. Treatment with
200 mg/kg PFC (P<0.05) and 300 mg PFC (P<0.01) significantly
reduced cleaved-caspase-3 activation.
Effect of PFC on MAPK phosphorylation
in epileptic rats
The MAPK family comprises the following 3 primary
subfamilies: ERK1/2, JNK, and p38. The phosphorylation of p38 and
JNK levels were significantly increased in LP groups compared with
control (P<0.001; Fig. 3A and B).
However, induction of epilepsy had no significant effect in
inducing the phosphorylation of ERK (Fig. 3C). The administration of 200 mg/kg
PFC (P<0.05) and 300 mg PFC (P<0.01) significantly
ameliorated the increases in p38 and JNK phosphorylation, compared
with the LP group.
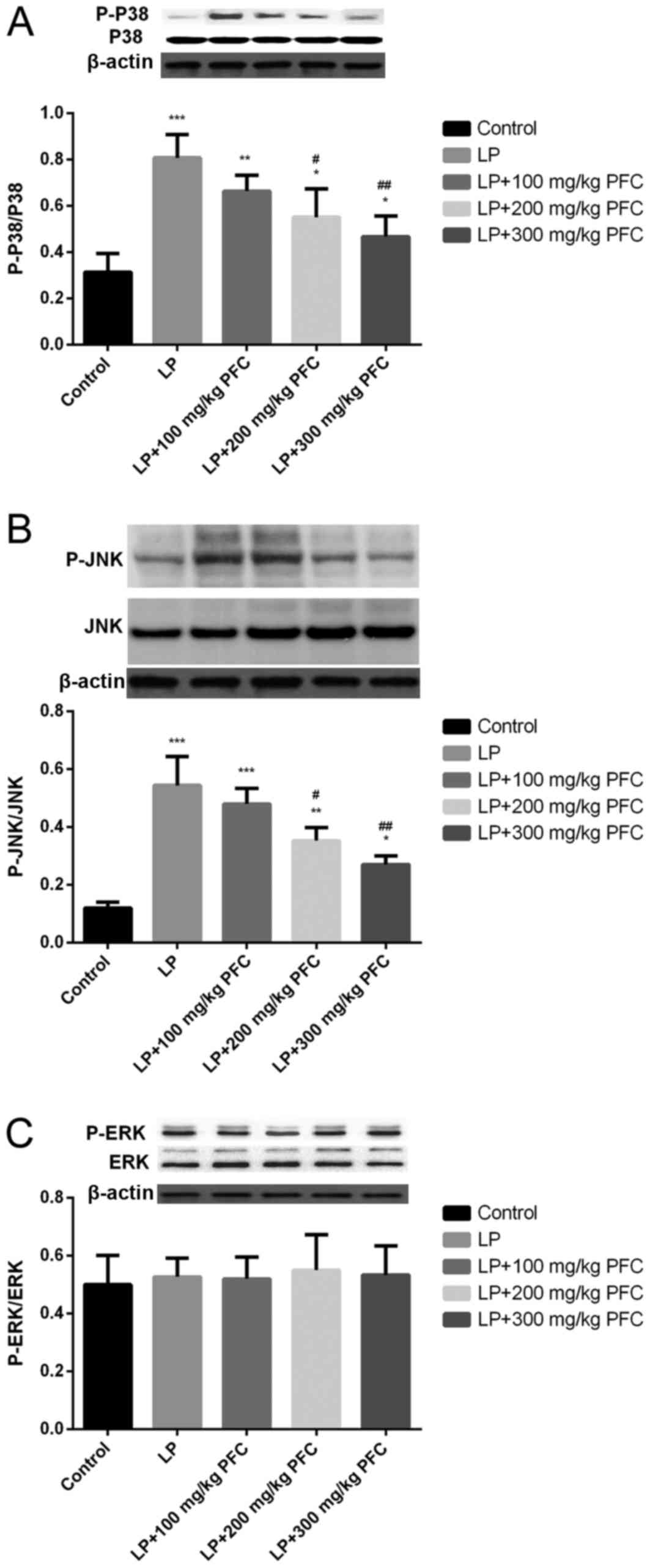 | Figure 3.Effects of PFC on the phosphorylation
of kinase in mitogen-activated protein kinase signal pathways,
namely P-38, JNK and ERK. Representative western blotting and
densitometry data for the levels of (A) p-p38/p38, (B) p-JNK/JNK
and (C) p-ERK/ERK in each group. PFC administration prevented the
increases in p38 and JNK phosphorylation. Induction of epilepsy did
not induce the phosphorylation of ERK. *P<0.05, **P<0.01,
***P<0.001 vs. control group; #P<0.05,
##P<0.01 vs. LP group. Data are presented as the mean
+ standard deviation (n=5). PFC, Fructus corni polysaccharide; JNK,
c-Jun N-terminal kinase; ERK, extracellular signal-regulated
kinase; p, phosphorylated; LP, lithium-pilocarpine. |
PFC retrieved hippocampal neurons in
CA1 area
As presented in Fig.
4, neurons in the control group (Fig. 4A) appeared as light blue, regularly
shaped cell bodies with palely stained nuclei in the Nissl-stained
sections. However, neurons in the LP group (Fig. 4B) exhibited blurred cell membranes
and pyknotic nuclei. Furthermore, the mean density of intact
surviving neurons was significantly lower compared with control
(P<0.001; Fig. 4F). These
morphological abnormalities were alleviated in PFC groups (Fig. 4C-F), which indicates that PFC
treatment has a beneficial influence on damaged neurons in the CA1
region.
 | Figure 4.PFC rescues CA1 pyramidal neurons
from seizure-induced damage as revealed by Nissl staining. (A)
control group, (B) LP group, (C) LP + 100 mg/kg PFC group, (D) LP +
200 mg/kg PFC group, (E) LP + 300 mg/kg PFC group (magnification,
×400). (F) Compared with the LP group, treatment with PFC
significantly ameliorated the number of surviving neurons.
*P<0.05, **P<0.01, ***P<0.001 vs. control group;
##P<0.01 vs. LP group. Data are presented as the mean
+ standard deviation (n=5). PFC, Fructus corni polysaccharide; LP,
lithium-pilocarpine. |
Discussion
Epilepsy is frequently characterized by complicated
pathogenesis. It has been observed that oxidative stress and
apoptosis have a vital role in the pathological process of epilepsy
in previous studies (21,22), and that a causal relation exists
between them. Under normal circumstances, the body can
physiologically generate a small amount of oxygen free radicals
(4). The ROS generated in the
electron transfer of the respiratory chain in mitochondria can be
eradicated by the endogenous anti-oxidation system in cells to
sustain the balance in the body (23). The anti-oxidation system in the brain
tissues, which is relatively inferior to that in other tissues or
organs, can be affected by various factors, such as ischemia or
hypoxia (4). The brain is
susceptible to the imbalance between the oxidation system and
anti-oxidation system in cells (24). Excessive ROS in the body may give
rise to the massive accumulation of free radicals, resulting in a
further marked increase of ROS (4).
Meanwhile, ROS may act on the unsaturated fatty acids found on
cellular membrane and mitochondrial membrane, inducing lipid
peroxidation and cell apoptosis (25). In the present study, it was
demonstrated that ROS level the MDA content in hippocampal tissues
were significantly increased, and the activity of SOD (an important
indicator of endogenous anti-oxidation system in cells) was
significantly decreased in the LP group compared with controls.
This confirmed that the epileptic seizure induced by lithium
chloride-pilocarpine induced stress and damaged the hippocampal
tissues.
Mitochondria not only serve as the main source of
endogenous ROS, but also the action target of ROS (26,27).
Mitochondrial dysfunction can increase the generation of ROS and
aggravate cell apoptosis induced by oxidant (28). Oxidative stress injuries can lead to
an imbalance between the oxidative effect and the anti-oxidative
effect in the body, thereby massively increasing the generation of
ROS, which will cause the mitochondrial permeability transition
pore to open (29). As a result,
cytochrome-C from the mitochondria will be delivered into the
cytoplasm, where caspase-3, the apoptotic executioner, will be
activated to induce cell apoptosis (30,31).
ROS, as a kind of signal molecule, can affect the
activities of a variety of signal transduction pathways, such as
the Janus kinase/signal transducer and activator of transcription
pathway, the protein kinase C pathway and the MAPK pathway. Among
those pathways, MAPK is a key transmembrane signal transduction
pathway (32). In the present study,
it was further investigated whether MAPK signal transduction
pathway was activated in neural apoptosis induced by ROS. It was
observed that the phosphorylation levels of p38 and JNK signal
transduction pathways in hippocampus tissues were significantly
augmented. Following treatment with PFC, phosphorylation levels
were significantly decreased, suggesting that the protection
mechanism of PFC may be associated with the decreased
phosphorylation of kinase in the MAPK signal pathway.
In recent years, neuronal apoptosis has received
increasing attention in the field of epilepsy research. It has been
argued that the prevention and treatment of neuronal apoptosis
following epilepsy has important clinical significance. Following
epileptic seizure, neurons in the brain are damaged and lost, of
which the hippocampal neurons are lost most significantly (33,34). It
has been suggested that neuronal loss occurs in the hippocampal
dentate gyrus region, and CA1 and CA3 regions in different degrees
in model rats, which may be in the forms of either neuronal
necrosis or apoptosis. It has typically been suggested that cell
apoptosis is the primary form of brain neuronal death following
epilepsy (35,36). The occurrence of apoptosis is
regulated by genes, and ROS may cause apoptosis through a variety
of ways. The MAPK cascade pathway is an important example (37,38). It
has been demonstrated that MAPK phosphorylation can lead to the
release of mitochondrial cytochrome C and then cause the activation
of caspase (39). Fructus corni, a
kind of Traditional Chinese Medicine, is one of 40 different types
of commonly used medicinal materials in China, and is widely used
in the traditional and clinical medication. In recent years,
progress has been made in the study on PFC (11,40).
Pharmacological studies have demonstrated that the PFC is an
important component of the bio-active material of Fructus corni
(41). Previous studies have
demonstrated that PFC exhibits many pharmacological effects,
including anti-aging, anti-tumor, anti-oxidation,
immuno-enhancement and memory improvement (42,43).
The present study demonstrated that PFC serves as a
novel agent for anti-epilepsy treatment. It decreases the
alteration in mitochondrial membrane potential, cytochrome C
leakage and activation of cleaved caspase-3 through reducing the
activation of hippocampus ROS and the MAPK cascade pathway
following epilepsy, thereby alleviating the apoptosis of neurons
and having a neuroprotective effect on epilepsy. In this way, PFC
has therapeutic and protective effects on epilepsy, but the
specific mechanism of PFC for epileptic seizure remains to be
elucidated. The present study was limited to observing the changes
in the PFC protein, further investigation is required to study the
potential mechanisms by which PFC may have an effect.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during the present
study are included in this article.
Authors' contributions
XS, LK and LZ contributed to the study design. XS
performed data collection, XS performed data analysis and LK
performed data interpretation. XS and LK were responsible for
manuscript preparation and LK performed literature search. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Weifang People's Hospital Animal Center (Weifang,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen J, Liu XM, Yue X and Chen SZ: The
clinical efficacy and safety of levetiracetam add-on therapy for
child refractory epilepsy. Eur Rev Med Pharmacol Sci. 20:2689–2694.
2016.PubMed/NCBI
|
|
2
|
Azakli H, Gurses C, Arikan M, Aydoseli A,
Aras Y, Sencer A, Gokyigit A, Bilgic B and Ustek D: Whole
mitochondrial DNA variations in hippocampal surgical specimens and
blood samples with high-throughput sequencing: A case of mesial
temporal lobe epilepsy with hippocampal sclerosis. Gene.
529:190–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehla J, Reeta KH, Gupta P and Gupta YK:
Protective effect of curcumin against seizures and cognitive
impairment in a pentylenetetrazole-kindled epileptic rat model.
Life Sci. 87:596–603. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hass DT and Barnstable CJ: Uncoupling
protein 2 in the glial response to stress: Implications for
neuroprotection. Neural Regen Res. 11:1197–1200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baille V, Clarke PG, Brochier G, Dorandeu
F, Verna JM, Four E, Lallement G and Carpentier P: Soman-induced
convulsions: The neuropathology revisited. Toxicology. 215:1–24.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sfaello I, Baud O, Arzimanoglou A and
Gressens P: Topiramate prevents excitotoxic damage in the newborn
rodent brain. Neurobiol Dis. 20:837–848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee KY, Bae ON, Weinstock S, Kassab M and
Majid A: Neuroprotective effect of asiatic acid in rat model of
focal embolic stroke. Biol Pharm Bull. 37:1397–1401. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petrosillo G, Ruggiero FM, Di Venosa N and
Paradies G: Decreased complex III activity in mitochondria isolated
from rat heart subjected to ischemia and reperfusion: role of
reactive oxygen species and cardiolipin. FASEB J. 17:714–716. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu B, Tewari AK, Zhang L, Green-Church
KB, Zweier JL, Chen YR and He G: Proteomic analysis of protein
tyrosine nitration after ischemia reperfusion injury: Mitochondria
as the major target. Biochim Biophys Acta. 1794:476–485. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng Q, Wei Z and Lau BH: Fructus corni
enhances endothelial cell antioxidant defenses. Gen Pharmacol.
31:221–225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CC, Hsu CY, Chen CY and Liu HK:
Fructus Corni suppresses hepatic gluconeogenesis related gene
transcription, enhances glucose responsiveness of pancreatic
beta-cells, and prevents toxin induced beta-cell death. J
Ethnopharmacol. 117:483–490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Wang X, Shen B, Kang L and Fan E:
Extraction, structure and bioactivities of the polysaccharides from
Fructus corni. Recent Pat Food Nutr Agric. 5:57–61. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Racine RJ: Modification of seizure
activity by electrical stimulation. II. Motor seizure.
Electroencephalogr Clin Neurophysiol. 32:281–294. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Ren M, Guo J, Yang G, Long X, Hu
R, Shen W, Wang X and Zeng K: The inhibitory effects of Npas4 on
seizures in pilocarpine-induced epileptic rats. PLoS One.
9:e1158012014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thummasorn S, Kumfu S, Chattipakorn S and
Chattipakorn N: Granulocyte-colony stimulating factor attenuates
mitochondrial dysfunction induced by oxidative stress in cardiac
mitochondria. Mitochondrion. 11:457–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi J, Hong ZY, Xin H and Zhu YZ:
Neuroprotective effects of leonurine on
ischemia/reperfusion-induced mitochondrial dysfunctions in rat
cerebral cortex. Biol Pharm Bull. 33:1958–1964. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buchan A and Pulsinelli WA: Hypothermia
but not the N-methyl-D-aspartate antagonist, MK-801, attenuates
neuronal damage in gerbils subjected to transient global ischemia.
J Neurosci. 10:311–316. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kirino T, Tamura A, Tomukai N and Sano K:
Treatable ischemic neuronal damage in the gerbil hippocampus. No To
Shinkei. 38:1157–1163. 1986.(In Japanese). PubMed/NCBI
|
|
20
|
Xie N, Li H, Wei D, LeSage G, Chen L, Wang
S, Zhang Y, Chi L, Ferslew K, He L, et al: Glycogen synthase
kinase-3 and p38 MAPK are required for opioid-induced microglia
apoptosis. Neuropharmacology. 59:444–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cardenas-Rodriguez N, Huerta-Gertrudis B,
Rivera-Espinosa L, Montesinos-Correa H, Bandala C, Carmona-Aparicio
L and Coballase-Urrutia E: Role of oxidative stress in refractory
epilepsy: Evidence in patients and experimental models. Int J Mol
Sci. 14:1455–1476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Henshall DC: Apoptosis signalling pathways
in seizure-induced neuronal death and epilepsy. Biochem Soc Trans.
35:421–423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bielli A, Scioli MG, Mazzaglia D, Doldo E
and Orlandi A: Antioxidants and vascular health. Life Sci.
143:209–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vishnoi S, Raisuddin S and Parvez S:
Glutamate excitotoxicity and oxidative stress in epilepsy:
Modulatory role of melatonin. J Environ Pathol Toxicol Oncol.
35:365–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong H, Lu J, Xia L, Zhu M and Yin H:
Formation of electrophilic oxidation products from mitochondrial
cardiolipin in vitro and in vivo in the context of apoptosis and
atherosclerosis. Redox Biol. 2:878–883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Crouser ED and Parinandi N: Mitochondrial
mechanisms are at the ‘heart’ of novel ischemia-reperfusion
therapies. Crit Care Med. 39:593–595. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao K, Ye P, Zhang L, Tan J, Tang X and
Zhang Y: Epigallocatechin gallate protects against oxidative
stress-induced mitochondria-dependent apoptosis in human lens
epithelial cells. Mol Vis. 14:217–223. 2008.PubMed/NCBI
|
|
28
|
Ganie SA, Dar TA, Bhat AH, Dar KB, Anees
S, Zargar MA and Masood A: Melatonin: A potential anti-oxidant
therapeutic agent for mitochondrial dysfunctions and related
disorders. Rejuvenation Res. 19:21–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ralph SJ, Pritchard R, Rodríguez-Enríquez
S, Moreno-Sánchez R and Ralph RK: Hitting the bull's-eye in
metastatic cancers-NSAIDs elevate ROS in mitochondria, inducing
malignant cell death. Pharmaceuticals (Basel). 8:62–106. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brenner D and Mak TW: Mitochondrial cell
death effectors. Curr Opin Cell Biol. 21:871–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
32
|
Alvarez-Jaimes L, Feliciano-Rivera M,
Centeno-González M and Maldonado-Vlaar CS: Contributions of the
mitogen-activated protein kinase and protein kinase C cascades in
spatial learning and memory mediated by the nucleus accumbens. J
Pharmacol Exp Ther. 314:1144–1157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
DeGiorgio CM, Tomiyasu U, Gott PS and
Treiman DM: Hippocampal pyramidal cell loss in human status
epilepticus. Epilepsia. 33:23–27. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mikati MA, Abi-Habib RJ, El Sabban ME,
Dbaibo GS, Kurdi RM, Kobeissi M, Farhat F and Asaad W: Hippocampal
programmed cell death after status epilepticus: evidence for
NMDA-receptor and ceramide-mediated mechanisms. Epilepsia.
44:282–291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spragg DD, Akar FG, Helm RH, Tunin RS,
Tomaselli GF and Kass DA: Abnormal conduction and repolarization in
late-activated myocardium of dyssynchronously contracting hearts.
Cardiovasc Res. 67:77–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gard JJ, Yamada K, Green KG, Eloff BC,
Rosenbaum DS, Wang X, Robbins J, Schuessler RB, Yamada KA and
Saffitz JE: Remodeling of gap junctions and slow conduction in a
mouse model of desmin-related cardiomyopathy. Cardiovasc Res.
67:539–547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito
H and Takekawa M: Formation of stress granules inhibits apoptosis
by suppressing stress-responsive MAPK pathways. Nat Cell Biol.
10:1324–1332. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Son Y, Cheong YK, Kim NH, Chung HT, Kang
DG and Pae HO: Mitogen-activated protein kinases and reactive
oxygen species: How can ROS activate MAPK pathways? J Signal
Transduct. 2011:7926392011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ledirac N, Antherieu S, D'Uby AD, Caron JC
and Rahmani R: Effects of organochlorine insecticides on MAP kinase
pathways in human HaCaT keratinocytes: Key role of reactive oxygen
species. Toxicol Sci. 86:444–452. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shao X, Luo Q, Qin Q, Qiu G and Li Z:
Effect of fructus corni polysaccharides on damaged sexual function
of male rats. Zhongguo Zhong Yao Za Zhi. 35:772–775. 2010.(In
Chinese). PubMed/NCBI
|
|
41
|
Yamabe N, Kang KS, Goto E, Tanaka T and
Yokozawa T: Beneficial effect of Corni Fructus, a constituent of
Hachimi-jio-gan, on advanced glycation end-product-mediated renal
injury in Streptozotocin-treated diabetic rats. Biol Pharm Bull.
30:520–526. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Duan Y, Pei K, Cai H, Tu S, Zhang Z, Cheng
X, Qiao F, Fan K, Qin K, Liu X and Cai B: Bioactivity
evaluation-based ultra high-performance liquid chromatography
coupled with electrospray ionization tandem
quadrupole-time-of-flight mass spectrometry and novel distinction
of multi-subchemome compatibility recognition strategy with
Astragali Radix-Fructus Corni herb-pair as a case study. J Pharm
Biomed Anal. 129:514–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang X, Liu J, Jin NA, Xu D, Wang J, Han Y
and Yin N: Fructus Corni extract-induced neuritogenesis in PC12
cells is associated with the suppression of stromal interaction
molecule 1 expression and inhibition of Ca2+ influx. Exp
Ther Med. 9:1773–1779. 2015. View Article : Google Scholar : PubMed/NCBI
|