Introduction
Pregnancy is a time of tremendous stress on the body
of the mother, involving a substantial increase in the workload of
various different organs (1).
Therefore, organ systems that are well controlled in the
non-pregnant state may become dysregulated during pregnancy, which
may result in metabolic diseases, including diabetes mellitus
(2). The dysregulation of organ
systems during pregnancy may cause significant harm to the mother
and/or the fetus (2). The late
stages of pregnancy are catabolic and associated with the elevation
of blood glucose. Gestational diabetes mellitus (GDM) is a common
metabolic complication during pregnancy. Hyperglycemia has
prominent effects on the mother, the course of the pregnancy and
the fetus (3). Patients with
gestational and pre-GDM have a greater risk for preeclampsia (PE),
which is a hypertensive syndrome associated with proteinuria
(4). Since a long-term consequence
of diabetes mellitus is the damage to small blood vessels, it is
hypothesized that PE results from endothelial cell injury as a
consequence of ischemia (5).
Abnormalities in the process of implantation appear to set in
motion events that lead to the occurrence of PE from gestation week
20 (6). Clinical and experimental
studies have suggested that GDM and PE share pathophysiological
features, including systemic endothelial inflammation and
dyslipidemia. These conditions are associated with augmented risks
for cardiovascular morbidity and mortality later in life (7–9).
However, few studies have assessed risk factors for concurrent GDM
and PE (DPE).
In recent years, there has been an increased focus
on the role of the maternal immune system in inflammation and
endothelial dysfunction, which is associated with the development
of DPE (10,11). The immune system has a major role in
normal pregnancy (12). During
pregnancy, the systemic maternal immune system is altered, and
maternal T cells acquire a transient state of tolerance that is
specific for paternal alloantigens (13). CD4+ T, type 17 T-helper
(Th17) and regulatory T (Treg) cells have an important role in
maintaining self-tolerance. The identification of Th17 and Treg
cells has opened up a broad field of research into the potential
roles of these cells in controlling disorders during pregnancy. An
important characteristic of PE is an alteration in the prevalence
of Th17 and Treg cells, which may contribute to the development of
maternal systemic inflammation (14,15). The
interleukin (IL)-17 cytokine is produced by Th17 cells, which are
potent inflammatory factors for activating the adaptive immune
response. Treg cells are a major component in establishing immune
tolerance (16,17). IL-35 is a newly identified
immunosuppressive cytokine secreted from Treg cells, and consists
of p35 and Epstein-Barr virus-induced gene 3 (EBI3) subunits
(18,19). C-reactive protein (CRP) is a systemic
inflammatory marker primarily synthesized in the liver (20). In recent years, the role of immune
activation and inflammation in the pathogenesis of DPE has gained
increasing attention.
Recent studies have reported that an abnormal
maternal lipid profile, including total cholesterol (TC),
triglyceride (TG), low-density lipoprotein (LDL), very LDL (VLDL)
and high-density lipoprotein (HDL), increase the health risks
associated with pregnancy (21,22). The
accumulation of cholesterol and TGs may cause damage to endothelial
function. It has been reported that lipid profile abnormalities in
maternal patients with GDM increase the risk of vascular injury,
which may lead to endothelial dysfunction, a pathogenic factor of
PE (23). Lipids constitute a large
part of the arterial wall, as do plasma and intracellular
membranes; therefore, lipid abnormalities may lead to vascular
damage, which has a critically important role in the pathogenesis
of GDM (24). Therefore, DPE may be
associated with lipid profile abnormalities and vascular
dysfunction.
Obesity and excessive gestational weight gain (GWG)
are important preventable risk factors for adverse maternal and
neonatal outcomes (25). Excessive
maternal GWG and obesity are associated with an increased risk of
metabolic and immunological dysfunction (26). Previous studies have indicated that
excessive GWG and obesity during pregnancy are associated with
increased secretion of adipokines and inflammatory cytokines
(27,28). However, the pathophysiological
mechanisms that underlie an increased body mass index (BMI) remain
to be fully elucidated. Abnormal lipid levels and immunological
dysfunction are likely to be important factors that contribute to
disorders during pregnancy. At present, there is limited
information on the effects of the immune mechanisms that link lipid
metabolism and high BMI to the pathogenesis of DPE. The present
study aimed to investigate the association between the levels of
maternal lipids, blood glucose, IL-17/IL-35 and BMI in order to
examine the risk factors for DPE.
Materials and methods
Study population
The present study comprised 30 patients with DPE, 33
patients with GDM, 33 patients with PE and 33 subjects with normal
pregnancy were enrolled from regular checkups performed in the
Department of Obstetrics of the Maternity and Child Health Hospital
of Zhenjiang (Jiangsu, China) between January 2013 and August 2016.
PE was considered when hypertension (≥140/90 mmHg) was detected on
two separate occasions with an interval of >6 h. Proteinuria was
considered to be present when the 24-h total urinary protein
excretion was ≥300 mg/24 h. PE was categorized as severe when the
systemic blood pressure was ≥160/110 mmHg, or if there was
consistent proteinuria (>5 g/24 h). GDM was diagnosed using the
criteria of the American Diabetes Association (ADA), where,
following a 75-g oral glucose tolerance test during 24–28 weeks of
gestation, one of the three following categories was fulfilled:
Based on the one-step approach recommended by the ADA, the pregnant
women were defined as having GDM if they had at least one abnormal
high glucose value out of three 75 g OGTT, fasting blood glucose
(FBG), ≥5.1 mmol/l, 1 h post 75-g oral glucose load, ≥10.0 mmol/l,
and 2 h post 75-g oral glucose load, ≥8.5 mmol/l (27). Patients with cardiovascular, renal,
metabolic disease and an abortion history were excluded from the
study. None of the patients were undergoing treatment or received
any medication within 2 months prior to sample collection. The
ethics committee of the Maternity and Child Health Hospital
(Zhenjiang, China) approved the study protocol, and all
participants provided written informed consent. Their clinical
characteristics are listed in Table
I.
 | Table I.Demographic data of the pregnant
subjects. |
Table I.
Demographic data of the pregnant
subjects.
| Parameter | NP (n=33) | GDM (n=33) | PE (n=33) | DPE (n=30) |
|---|
| Maternal age
(years) | 28.70±1.16 | 29.20±1.03 | 28.20±6.97 | 29.90±1.37 |
| Gestational age
(weeks) | 38.57±0.98 | 38.70±1.40 | 36.47±1.60 | 38.10±1.20 |
| Nulliparous | 23 (69.7) | 20 (60.6) | 19 (57.6) | 21 (70.0) |
| Smokers | 0 (0.0) | 1 (3.0) | 1 (3.0) | 1 (3.3) |
| Proteinuria | 0.080±0.01 |
0.92±0.61 |
4.27±1.04a,b |
2.91±0.64a |
Blood sample preparation
Venous blood samples (10 ml) were obtained by
venipuncture from patients with GDM, PE, DPE and normal pregnancy.
Following centrifugation for 10 min at 3,000 × g at 20°C, the serum
was stored at −80°C for later analysis of the cytokines. Peripheral
blood mononuclear cells (PBMCs) were isolated using the standard
Ficoll-Hypaque method (11).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from PBMCs using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Complementary (c)DNA was synthesized using the High-Capacity
cDNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
mRNA levels of forkhead box P3 (FoxP3), P35, EBI3, IL-17 and
β-actin were determined using the SYBR Green two-step RT-qPCR kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's protocol and as previously described (11). β-actin was used as a housekeeping
gene. The primer sequences for FoxP3, P35, EBI3, IL-17 and β-actin
are listed in Table II.
Quantification was performed using the 2−∆∆Cq method
(29).
 | Table II.Primers for polymerase chain
reaction. |
Table II.
Primers for polymerase chain
reaction.
| Gene | Forward primer | Reverse primer |
|---|
| P35 |
5′-TCCTCCCTTGAAGAACCGGA-3′ |
5′-TGACAACGGTTTGGAGGGAC-3′ |
| EBI3 |
5′-TCCTTCATTGCCACGTACAG-3′ |
5′-GCTCTGTTATGAAAGGCACG-3′ |
| FoxP3 |
5′-TGAGAAGGACAGGGAGCCAA-3′ |
5′-GAGAAGCTGAGTGCCATGCA-3′ |
| IL-17 |
5′-CTCCAGAAGGCCCTCAGACTAC-3′ |
5′-GGGTCTTCATTGCGGTGG-3′ |
| β-actin |
5′-TGGCACCCAGCACAATGAA-3′ |
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ |
Analysis of cytokines and CRP using
ELISA
The concentrations of serum IL-35, IL-17 and CRP
were analyzed using a commercial ELISA 3 plex sample kit according
to the manufacturer's protocols (IL-17 cat. no. KAC1591; CRP cat.
no. 88-7502-28; IL-35 cat. no. 439508 LEGEND MAX™ Human;
Thermo Fisher Scientific, Inc, Waltham, MA, USA). All samples were
analyzed in duplicate.
Biochemical analysis
The levels of serum TG, TC and HDL were measured
using an automatic biochemistry analyzer (AU5800; Beckman Coulter,
Inc., Brea, CA, USA). The levels of LDL and VLDL were calculated
using the Friedewald equation (30).
The glucose levels in maternal blood were determined using an
enzymatic system (TMRUSGT15-0901; Beckman Coulter, Inc.).
BMI
The BMI was calculated using the Friedewald formula
(kg/m2) and according to the Institute of Medicine
classification and the published recommended guidelines for
gestational weight gain (30), the
patients were categorized as follows: Underweight, <18.5
kg/m2; normal weight, 18.5–24.9 kg/m2;
overweight, 25.0–29.9 kg/m2 and obese, ≥30.0
kg/m2. Maternal patients with missing data regarding
height, weight prior to pregnancy or time of delivery were excluded
from the sample.
Statistical analysis
The statistical significance of differences among
groups was assessed with GraphPad Prism (version 5.0; GraphPad
Software, Inc., La Jolla, CA, USA). Values are expressed as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference. One-way analysis of variance
with a post-hoc Turkey's test was performed to determine the
differences in the means among multiple groups and was used for
assessment of differences between two groups. Pearson's correlation
coefficients were calculated for the assessment of correlations
between variables.
Results
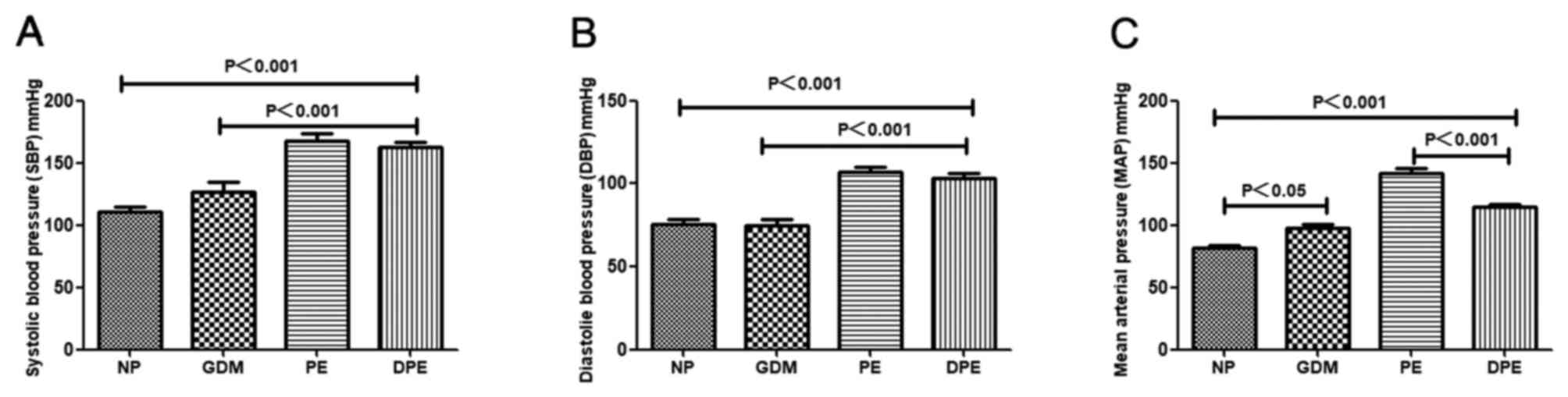
Elevated blood pressure in patients
with DPE
In the present study, it was observed that the SBP
and DBP in patients with PE and DPE were significantly higher
compared with NP and patients with GDM. In addition, it was
identified that the mean arterial pressure (MAP) were significantly
higher in patients with GDM, PE and DPE compared with NP. These
differences in MAP were significantly higher in patients with PE
compared to patients with DPE. Of note, the MAP of patients with
GDM was 97.50±10.34 mmHg, and an MAP of ≥85 mmHg is indicative of a
tendency to develop PE (1) (Fig. 1).
Elevated levels of FBG in patients
with DPE
The glucose levels in maternal blood were determined
by ELISA. As indicated in Fig. 2,
elevated levels of FBG were detected in patients with GDM, PE and
DPE compared with NP. Furthermore, patients with DPE and GDM also
exhibited elevated levels of FBG compared with PE. These results
suggest that DPE and GDM have similar metabolic disorder
characteristics by high level of blood glucose. It has been
reported that elevated blood glucose levels of GDM have effects on
other tissues (31). Therefore, the
resultant hypertrophy and proliferation of vascular smooth muscle
may contribute to hypertension.
Hyperlipidaemia and high BMI in
patients with DPE
As presented in Fig.
3, significantly higher levels of serum TG were identified in
patients with GDM, PE and DPE compared with NP and the difference
in TG were also significantly higher in GDM compared with PE and
DPE. There were significantly higher levels of serum TC in patients
with GDM, PE and DPE compared with NP and this difference of TC
were also significantly higher in DPE compared with PE and GDM. In
addition, there were significantly higher levels of serum LDL in
patients with GDM, PE and DPE compared with NP and this difference
LDL were also significantly higher in PE compared with DPE and GDM.
Significantly higher levels of serum VLDL in patients with GDM, PE
and DPE were observed when compared with NP and VLDL levels were
also significantly higher in PE compared with GDM. In addition,
higher BMI values were identified in patients with GDM, PE and DPE.
Of note, lower concentrations of HDL were detected in patients with
GDM, PE and DPE compared with those in subjects with normal
pregnancy.
 | Figure 3.Lipid profile and BMI of patients
with GDM, PE and DPE. Levels of (A) TG, (B) TC, (C) LDL, (D) VLDL
and (E) Serum HDL of patients in each group. (F) The BMI of
patients in the GDM, PE and DPE group upon enrollment. BMI body
mass index; DPE, GDM with PE; GDM, gestational diabetes mellitus;
PE, preeclampsia; TG, triglyceride; TC, total cholesterol; LDL,
low-density lipoprotein; VLDL, very LDL; HDL, high-density
lipoprotein; NP, normal pregnant subjects. |
mRNA expression of FoxP3, P35, EBI3
and IL-17 in patients with DPE
As previous studies have indicated a role for the
IL-17/IL-35 imbalance in the pathogenesis of PE (11), possible alterations of the mRNA
expression of FoxP3, P35, EBI3 and IL-17 were examined in the
patients with GDM, PE and DPE. As indicated in Fig. 4, the mRNA expression of P35, EBI3 and
FoxP3, which is characteristic of Treg cells, was decreased, while
the expression of IL-17 was elevated in patients with GDM, PE and
DPE compared with NP. These results suggested a potential
involvement of the IL-17/IL-35 imbalance in GDM, PE and DPE.
 | Figure 4.mRNA expression of FoxP3, P35, EBI3
and IL-17 in patients with GDM, PE and DPE. mRNA Levels of (A)
IL-17, (B) P35, (C) EB13 and (D) FoxP3 in each group. FoxP3,
forkhead box protein 3; EBI3, Epstein-Barr virus-induced gene 3;
IL, interleukin; GDM, gestational diabetes mellitus; PE,
preeclampsia; DPE, GDM with PE; NP, normal pregnant subjects. |
Serum cytokine and CRP
concentration
The production of the Th17-associated cytokines
IL-17 and IL-35 by Tregs in patients with GDM, PE and DPE are
indicated in Fig. 5. Significantly
higher serum levels of IL-17 were observed in patients with GDM, PE
and DPE compared with NP. This difference in IL-17 was also
significantly higher in PE and DPE compared with GDM (Fig. 5A). By contrast, lower levels of IL-35
were observed in patients with GDM, PE and DPE compared with NP and
levels of IL-35 were also significantly lower in PE and DPE
compared with GDM (Fig. 5B). The
levels of CRP, a systemic inflammatory marker, were significantly
elevated in patients with GDM, PE and DPE. CRP was also
significantly higher in PE and DPE compared with GDM (Fig. 5C). These results suggest an abnormal
immune response in patients with GDM, PE and DPE, which is
characteristic of a shift to Th17-type immunity and systemic
inflammation.
Correlation of DBP with IL-17, BMI and
TG, as well as IL-17 with proteinuria and BMI in patients with
DPE
As indicated in Fig.
6, positive correlations were identified between the DBP and
the levels of IL-17 (r=0.8718 and P<0.001), the BMI (r=0.6575
and P<0.05) and TG (r=0.8058 and P<0.001). In addition,
positive correlations between IL-17 levels and the BMI as well as
between IL-17 levels and the presence of proteinuria were
identified in patients with DPE (r=0.9020 and r=0.7666,
respectively; P<0.01 for each). Based on these observations, it
was hypothesized that elevated IL-17, BMI and TG are probably
involved in the development of endothelial dysfunction with major
effects, including elevated DBP and proteinuria.
Discussion
GDM is a group of metabolic disorders characterized
by hyperglycemia. GDM is 6–10 times more common than
pre-gestational diabetes mellitus. GDM occurs in 4.3–17.5% of all
pregnancies in China (32).
Hyperglycemia has prominent effects on the mother, the course of
the pregnancy and the fetus (33).
In the present study, elevated levels of FBG were detected in
patients with GDM, PE and DPE compared with those in subjects with
normal pregnancy. Furthermore, the levels of FBG were also
increased in patients with DPE and GDM compared with those in
patients with PE. These results suggest that high blood glucose
levels during pregnancy are common in patients with GDM. Of note,
it was reported that elevated blood glucose levels in GDM patients
affect other tissues, particularly small blood vessels (34). Therefore, the resultant hypertrophy
and proliferation of vascular smooth muscle may contribute to the
association between GDM and the subsequent development of PE
(35). Although the mechanisms
remain to be fully elucidated, several studies have suggested that
hyperglycemia has a role in the etiology of these diseases
(36,37). A hypothesis for this observation is
that when blood glucose levels remain excessively high for extended
periods of time, this induces damage to blood vessels, particularly
small blood vessels (38).
Furthermore, the present study identified that MAP values were
significantly higher in patients with GDM, PE and DPE compared with
those in normal pregnancy. In particular, the MAP of patients with
GDM (97.50±10.34) was ≥85 mmHg, indicative of a tendency to develop
PE (9). These results suggest that
hyperglycemia in GDM may lead to vascular dysfunction and the
subsequent complication of PE.
In the present study, significantly higher levels of
serum TG, TC, LDL and VLDL were identified in patients with GDM, PE
and DPE compared with those in subjects with normal pregnancies. By
contrast, decreased HDL concentrations were identified in patients
with GDM, PE and DPE. These results indicate that high TG, TC, LDL
and VLDL, as well as low HDL levels, may be associated with DPE.
The HDL concentration generally increases throughout pregnancy
(39); HDL is a vasodilator, which
may reverse cholesterol transport by carrying excess, potentially
harmful cholesterol to the liver for excretion (40). A number of HDL constituents may
interact with the vascular endothelium (41,42).
Various of studies have reported that elevated TG, TC, LDL and VLDL
levels and decreased HDL levels may serve a role in the development
of endothelial damage and vascular dysfunction (43,44).
Obesity, which is primarily caused by intake and storage of
nutritional substrates, is an important public health issue
(45). In the present study, the
patients with DPE had an elevated BMI. In pregnant women who are
obese, the plasma levels of TG and VLDL are higher, and the
concentration of HDL is lower compared with those in non-obese
pregnant women (46). It is possible
that in pregnant women who are obese, the HDL concentration may be
insufficient to fully protect the maternal vascular endothelium.
Obese pregnant women are at a higher risk of endothelial
dysfunction, thereby increasing the risk of developing DBP
(47). Furthermore, positive
correlations between DBP and BMI, and between DBP and TG were
observed in patients with DPE. The present study indicated that
abnormal lipid metabolism and elevated BMI are also risk factors
for DBP.
Normal pregnancy is a state of immune tolerance
(48). Maternal T lymphocytes
acquire a transient state of tolerance for paternal alloantigens. T
cells are a major component of the immune response and have an
important role at the maternal-fetal interface (49). The impaired recruitment of T cells in
the peripheral blood has been associated with disorders during
pregnancy and the overactivation of the immune system (50). Treg cells may have an important role
in avoiding maternal immune self-reactivity and establishing immune
tolerance during pregnancy (51).
The expression of FoxP3 is regarded as a characteristic of Treg
cells, which distinguishes Treg cells from activated
CD4+ T cells (51). IL-35
is only produced by Treg cells; the biological functions of IL-35
include the direct suppression of the proliferation and development
of Th17 cells (52). A previous
study has demonstrated that Treg cells and Th17 significantly
increase and decrease, respectively, in a balanced state (Th17 and
Treg cells form a complex and dynamic network to maintain
homeostasis during normal pregnancy (53). On the other hand, Th17 plays a
critical role in the induction of inflammation and Treg cells are
essential for maintaining pregnancy and regulating inflammation. A
previous study by our group demonstrated a decreased number of Treg
cells in pregnancies with complications, including PE and
miscarriage (54). In the present
study, it was observed that the expression of FoxP3 and IL-35
declined in patients with GDM and DPE, suggesting that a reduced
production of Treg cells may lead to inflammation. IL-17 and CRP
are involved in low-grade inflammation (55). IL-17 is a characteristic inflammatory
cytokine expressed by Th17 cells. IL-17 is hypothesized to serve as
a link between somatic tissues and the immune response (56). The present study revealed that the
levels of IL-17 and CRP are elevated in patients with DPE, whereas
these factors are downregulated in a normal pregnancy. However, the
levels of IL-17 and CRP are also significantly lower in normal
pregnancy compared with non-pregnant individuals. Similar to other
gestation-associated diseases (including PE and GDM), DPE was
associated with a decreased expression of FoxP3 and IL-35, and an
increased expression of IL-17. Therefore, maternal immune tolerance
that is present in normal pregnancies may be less effective with
concurrent DPE. The results of the present study suggest that an
imbalance between pro-inflammatory factors (e.g., IL-17 and CRP)
and anti-inflammatory factors (e.g., IL-35) is implicated in the
pathogenesis of GDM, PE and DPE. Th17 cells may serve important
roles in the pathogenesis of numerous inflammatory conditions,
which are associated with complications during pregnancy. IL-35 is
a newly identified immunosuppressive cytokine exclusively produced
by Treg cells. It has been demonstrated that lymphocytes are
endogenously polarized to IL-17 to induce a decrease in IL-35
(57), in patients with DPE. These
data indicate that the blood environment characterized by increased
Th17 and decreased Treg may contribute to the imbalance of
circulating Th17/Treg in patients with DPE. CRP is a systemic
inflammatory marker and a component of the innate immune system.
Elevated levels of CRP may reflect an inflammatory state in women
with DPE (58). Previous studies
observed that the cytokine balance between Th1 cytokines (e.g.,
IL-2 and interferon-γ) and Th2 cytokines (e.g., IL-4) was broken in
pregnant women with diabetes. Several studies have reported on this
Th1/Th2 (IL-4) imbalance, also known as the
pro-inflammatory/anti-inflammatory cytokine imbalance, in diabetes
(59). Therefore, the role of immune
activation and inflammation in the pathogenesis of DPE is worthy of
further investigation. Furthermore, the present study indicated
that enhanced levels of IL-17 in patients with DPE were positively
correlated with increased proteinuria, DBP and BMI. These results
suggested that a systemic inflammatory state may lead to damage to
blood vessels and endothelial dysfunction. It appears that an
abnormal maternal lipid profile, high BMI and an IL-17/IL-35
imbalance are involved in the development of endothelial
dysfunction. These factors have major effects that lead to elevated
blood pressure and proteinuria, as those parameters reflect
endothelial function (60).
Of note, the present study has certain limitations.
First, all of the study participants were recruited at one
hospital. Furthermore, the number of recruited patients was
relatively small. Therefore, further studies with a large sample
size should be performed in the future.
In summary, the present study demonstrated that the
DPE group was similar to the PE group with regard to systemic
inflammation, as indicated by the increased levels of IL-17 and
CRP, as well as the decreased levels of FoxP3 and IL-35. It was
indicated that an abnormal maternal lipid profile, high BMI,
hyperglycemia and low HDL may be involved in the endothelial injury
that is associated with the pathogenesis of DPE. In addition,
positive correlations of the DBP with the BMI, TG and IL-17 levels
were observed. There were also positive correlations between IL-17
levels and BMI, as well as between IL-17 levels and proteinuria.
These results suggest that systemic inflammation and endothelial
dysfunction may be involved in the pathogenesis of DPE. Further
studies are required to link systemic inflammation and endothelial
dysfunction with the mechanisms of DPE.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Bureau of Zhenjiang Mandatory Research Projects (grant
no. SH2016054) and the Maternal and Child Health Research Projects
of Jiangsu Province (grant nos. F201502, F201604 and F201435).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WC and XW conceived the study, designed the
experiments, analyzed the data and prepared the manuscript. WC was
a major contributor in writing the manuscript. TC, BX and YH
selected the subject and obtained samples for the present study. WX
and FF performed the experiments. SZ and ZW collected the clinical
data. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The ethics committee of the Maternity and Child
Health Hospital (Zhenjiang, China) approved the study protocol, and
all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PE
|
preeclampsia
|
|
GDM
|
gestational diabetes mellitus
|
|
DPE
|
GDM with PE
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
Th
|
T helper
|
|
IL-17
|
interleukin-17
|
|
EBI3
|
Epstein-Barr virus-induced gene 3
|
|
Treg cells
|
regulatory T cells
|
|
FoxP3
|
forkhead box protein 3
|
|
BP
|
blood pressure
|
|
MAP
|
mean arterial pressure
|
|
BMI
|
body mass index
|
|
CRP
|
C-reactive protein
|
References
|
1
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Catalano PM: Trying to understand
gestational diabetes. Diabet Med. 31:273–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cundy T, Ackermann E and Ryan EA:
Gestational diabetes new criteria may tripli the prevalence but
effect on outcomes is unclear. BMJ. 348:g15672014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steegers EA, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Preeclampsia. Lancet. 376:631–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Committee Opinion No. 638: First-trimester
risk assessment for early-onset preeclampsia. Obstet Gynecol.
126:e25–e27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lo JO, Mission JF and Caughey AB:
Hypertensive disease of pregnancy and maternal mortality. Curr Opin
Obstet Gynecol. 25:124–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen CW, Jaffe IZ and Karumanchi SA:
Preeclampsia and cardiovascular disease. Cardiovasc Res.
101:579–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Engeland A, Bjørge T, Daltveit AK,
Skurtveit S, Vangen S, Vollset SE and Furu K: Risk of diabetes
after gestational diabetes and preeclampsia. A registry-based study
of 230,000 women in Norway. Eur J Epidemiol. 26:157–163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiwani A, Marseille E, Lohse N, Damm P,
Hod M and Kahn JG: Gestational diabetes mellitus: Results from a
survey of country prevalence and practices. J Matern Fetal Neonatal
Med. 25:600–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pantham P, Aye IL and Powell TL:
Inflammation in maternal obesity and gestational mellitus.
Placenta. 36:709–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao W, Wang X, Chen T, Zhu H, Xu W, Zhao
S, Cheng X and Xia L: The expression of Notch/Notch Ligand, IL-35,
IL-17, and Th17/Treg in preeclampsia. Dis Markers. 2015:3161822015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gaffen SL, Jain R, Garg AV and Cua DJ: The
IL-23-IL-17 immune axis: From mechanisms to therapeutic testing.
Nat Rev Immunol. 14:585–600. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito S: Th17 cells and regulatory T
cells: New light on pathophysiology of preeclampsia. Immunol Cell
Biol. 88:615–617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laresgoiti-Servitje E: A leading role for
the immune system in the pathophysiology of preeclampsia. J Leukoc
Biol. 94:247–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gratz IK, Rosenblum MD, Maurano MM, Paw
JS, Truong HA, Marshak-Rothstein A and Abbas AK: Self antigen
controls the balance between effector and regulatory T cells in
peripheral tissues. J Immunol. 192:1351–1355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanidaizar D and Koulmanda M: Inflammation
and the balance of Treg and Th17 cells in transplant rejection and
tolerance. Curr Opin Organ Transplant. 15:411–415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Erlebacher A: Mechanisms of T cell
tolerance towards the allogeneic fetus. Nat Rey Immunol. 13:23–33.
2013. View
Article : Google Scholar
|
|
18
|
Collison LW, Workman CJ, Kuo TT, Boyd K,
Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS and Vignali DA:
The inhibitory cytokine IL-35 contributes to regulatory T-cell
function. Nature. 450:566–569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Collison LW, Chaturvedi V, Henderson AL,
Glacomin PR, Guy C, Bankorti J, Finkelstein D, Forbes K, Workman
CJ, Brown SA, et al: IL-35-mediated induction of a potent
regulatory T cell population. Nat Immunol. 11:1093–1101. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kashanian M, Aghbali F and Mahahi N:
Evaluation of the diagnostic value of the first trimester maternal
serum high-sensitivity C-reactive protein level for prediction of
pre-eclampsia. J Obstet Gynaecol Res. 39:1549–1554. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeb A: Triglyceride composition, oxidation
and oxidation compounds in camellia oil using liquid
chromatography-mass spectrometry. Chem Phys Lipids. 165:608–614.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gohil JT, Patel PK and Gupta P: Estimation
of lipid profile in subjects of pre-eclampsia. J Obstet Gynaecol
India. 61:399–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gaillard R, Durmuş B, Hofman A, Mackenbach
JP, Steegers EA and Jaddoe VW: Risk factors and outcomes of
maternal obesity and excessive weight gain during pregnancy.
Obesity (Silver Spring). 21:1046–1055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flegal K, Graubard B, Williamson D and
Gail M: Excess deaths associated with underweight, overweight, and
obesity. JAMA. 293:1861–1867. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kotsis V, Stabouli S, Papakatsika S, Rizos
Z and Parati G: Mechanisms of obesity-induced hypertension.
Hypertens Res. 33:386–393. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
American Diabetes Association: Standards
of medical care in diabetes-2009. Diabetes Care. 32 Suppl
1:S13–S61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Far Arjmandi M, Ziaei S and Kazemnejad A:
The impact of maternal age, pre-pregnancy body mass index, weight
gain and parity on glucose challenge test (GCT). Int J Fertil
Steril. 5:207–2010. 2012.PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Friedewald WT, Levy RI and Fredrickson DS:
Estimation of the concentration of low density lipoprotein
cholesterol in plasma, without use of the preparative
ultracentrifuge. Clin Chem. 18:499–502. 1972.PubMed/NCBI
|
|
31
|
International Diabetes Federation:
Recommended protocol for screening, management and follow up of
women with GDMImplementation Protocol Guidelines for Healthcare
Professionals. International Diabetes Federation; Brussels:
2015
|
|
32
|
Zhu WW, Fan L, Yang HX, Kong LY, Su SP,
Wang ZL, Hu YL, Zhang MH, Sun LZ, Mi Y, et al: Fasting plasma
glucose at 24–28 weeks to screen for gestational diabetes mellitus:
New environment from China. Diabetes Care. 36:2038–2040. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lindsay KL, Brennan L, Kennelly MA, Curran
S, Coffey M, Smith TP, Foley ME, Hatunic M and McAuliffe FM:
Maternal metabolic response to dietary treatment for
impairedglucose tolerance and gestational diabetes mellitus. Ir J
Med Sci. 2018.Doi: 10.1007/s11845-018-1744-y. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smith GN, Pudwell J, Walker M and Wen SW:
Risk estimation of metabolic syndrome at one and three years after
a pregnancy complicated by preeclampsia. J Obstet Gynaecol Can.
34:836–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Teodoro JS, Gomes AP, Varela AT, Duarte
FV, Rolo AP and Palmeira CM: Uncovering the beginning of diabetes:
The cellular redox status and oxidative stress as starting players
in hyperglycemic damage. Mol Cell Biochem. 376:103–110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Warrington JP, Gceorge EM, Palei AC,
Spradley FT and Granger JP: Recent advances in the understanding if
the pathophysiology of preeclampsia. Hypertension. 62:666–673.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Coustan DR: Diagnosis of gestational
diabetes. Scan J Clin Lab Invest Suppl. 244:27–33. 2014. View Article : Google Scholar
|
|
38
|
Popova P, Tkachuk A, Dronova A, Gerasimov
A, Kravchuk E, Bolshakova M, Rozdtestvenskaya O, Demidova K,
Nikolaeva A and Grineva E: Fasting glycemia at the first prenatal
visit and pregnancy outcomes in Russian women. Minerva Endocrinol.
41:477–485. 2016.PubMed/NCBI
|
|
39
|
Barrett HL, Nitert Dekker M, Mclntyre HD
and Callaway LK: Normalizing metabolism in diabetic pregnancy: Is
it time to target lipids? Diabetes Care. 37:1484–1493. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kingwell BA and Chapman MJ: Future of
high-density lipoprotein infusion therapies: Potential for clinical
management of vascular disease. Circulation. 128:1112–1121. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song GJ, Kim SM, Park KH, Kim J, Choi I
and Cho KH: SR-BI mediates high density lipoprotein (HDL)-induced
anti-inflammatory effect in macrophages. Biochem Biophys Res
Commun. 457:112–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Demirci O, Tuğrul AS, Dolgun N, Sözen H
and Eren S: Serum lipids level assessed in early pregnancy and risk
of pre-eclampsia. J Obstet Gynaecol Res. 37:1427–1432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matsumoto S, Gotoh N, Hishinuma S, Abe Y,
Shimizu Y, Katano Y and Ishihata A: The role of
hypertriglyceridemia in the development of atherosclerosis and
endothelial dysfunction. Nutrients. 6:1236–1250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Flood-Nichols SK, Stallings JD, Gotkin JL,
Tinnemore D, Napolitano PG and Ippolito DL: Elevated ratio of
maternal plasma ApoCIII to ApoCII in preeclampsia. Reprod Sci.
18:493–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Punthumapol C and Kittichotpanich B:
Comparative study of serum lipid concentrations in preeclampsia and
normal pregnancy. J Med Assoc Thai. 91:957–961. 2008.PubMed/NCBI
|
|
46
|
Kabiru W and Raynor B: Obstetric outcomes
associated with increase in BMI category during pregnancy. Am J
Obstet Gynecol. 191:928–932. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gallos I, Sivakumar K, Kilby M,
Coomarasamy A, Thangaratinam S and Vatish M: Pre-eclampsia is
associated with, and preceded by, hypertriglyceridaemia: A
meta-analysis. BJOG. 20:1321–1332. 2013. View Article : Google Scholar
|
|
48
|
Barrdel E, Larousserie F, Charlot-Rabiega
P, Coulomb-L'Herminé A and Devergne O: Human CD4+ CD25+ FoxP3+
regulatory T cells do not constitutively express IL-35. J Immunol.
181:6898–6905. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rasanen J, Girsen A, Lu X, Lapidus JA,
Standley M, Reddy A, Dasari S, Thomas A, Jacob T, Pouta A, et al:
Comprehensive maternal serum proteomic profiles of preclinical and
clinical preeclampsia. J Proteome Res. 9:4274–4281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ozkan ZS, Simsek M, Ilhan F, Deveci D,
Godekmerdan A and Sapmaz E: Plasma IL-17, IL-35, Interferon-γ,
Socs3 and TGF-β levels in pregnant women with preeclampsia, and
their relation with severity of disease. J Matern Fetal Neonatal
Med. 27:1513–1517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kassan M, Wecker A, Kadowitz P, Trebak M
and Matrogui K: CD4+CD25+FoxP3 regulatory T cells and vascular
dysfunction in hypertension. J Hypertens. 31:1939–1943. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sasaki S, Darmochwal-Kolarz D, Suzuki D,
Sakai M, Ito M, Shima T, Shiozaki A, Rolinski J and Saito S:
Proportion of peripheral blood and decidual CD4(+) CD25(bright)
regulatory T cells in pre-eclampsia. Clin Exp Immunol. 149:139–145.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Darmochwal-Kolarz D, Kludka-Sternik M,
Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B and
Oleszczuk J: The predominance of Th17 lymphocytes and decreased
number and function of Treg cells in preeclampsia. J Reprod
Immunol. 93:75–81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cao W, Xu W, Chen T, Wang X, Wang X, Qiu
J, Chen N and Mao Y:
CD4+CD25+FoxP3+ regulatory T cells
and cytokines interact with estradiol in case missed abortion. Exp
Ther Med. 7:417–422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Glebezdina NS, Olina AA, Nekrasova IV and
Kuklina EM: Role of endogenous melatonin in the regulation of
Th17/Treg balance during pregnancy. Bull Exp Biol Med. 164:462–465.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Figueiredo AS and Schummacher A: The T
helper type 17/regulatory T cells paradigm in pregnancy.
Immunology. 148:13–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Onuegbu AJ, Olisekodiaka JM, Udo JU,
Umeononihu O, Amah UK, Okwara JE and Atuegbu C: Evaluation of
high-sensitivity C-reactive protwin and serum lipid profile in
southeastern nigerlan women with pre-ealampsia. Med Princ Pract.
24:276–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Khan NA: Role of T-cells in diabetic
pregnancy and macrosomia. Indian J Biochem Biophys. 44:344–349.
2007.PubMed/NCBI
|
|
59
|
Di Mario U, Dotta F, Gargiulo P,
Sutherland J, Andreani D, Guy K, Pachi A and Fallucca F: Immunology
in diabetes pregnancy: Activated Tcells in diabetic mother and
neonates. Diabetologia. 30:66–71. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lima VJ, Andrade CR, Ruschi GE and Sass N:
Serum lipid levels in pregnancies complicated by preeclampsia. Sao
Paulo Med J. 129:73–76. 2011. View Article : Google Scholar : PubMed/NCBI
|