Introduction
Renal cell carcinoma (RCC) accounts for 3% of all
malignant tumors; ~270,000 new cases are diagnosed and ~116,000
RCC-associated mortalities occur worldwide per annum (1,2).
Furthermore, 25–30% of patients have already developed metastatic
disease at the time-point of diagnosis (3). The 5-year survival rate of RCC patients
at stage I is ~95%, but that of patients at stage IV is only 20%
(4). Thus, early detection and
treatment of RCC are important. However, RCC is resistant to
conventional chemotherapy and radiotherapy, and there is a lack of
effective biomarkers for screening (4,5).
Therefore, novel therapeutic methods and biomarkers should be
developed to improve RCC diagnosis and treatment.
MicroRNAs (miRNAs/miRs), a class of RNAs of ~20
nucleotides in length, regulate gene expression at the
post-transcriptional level and are involved not only in normal
biological processes, but also in tumorigenesis (6). Increasing evidence has indicated that
the expression of various miRNAs is aberrantly regulated in
different cancer types (7). Previous
studies have demonstrated that certain miRNAs are involved in RCC.
However, the role of miR199b-5p in RCC has remained elusive. In the
present study, a series of experiments was performed to investigate
the effect of miR-199b-5p on RCC cell lines.
Materials and methods
Specimens and cell lines
RCC tissues and paired adjacent normal tissues
(located 2.0 cm outside of the visible RCC lesions) were collected
from 42 patients undergoing surgery at Peking University Shenzhen
Hospital (Shenzhen, China). None of the patients had received any
anti-cancer treatment prior to the surgery. All selected cases have
been pathologically diagnosed. All of above tissues were stored at
−80°C until the RNA was extracted. Patient characteristics are
presented in Table I.
 | Table I.Clinicopathological characteristics of
patients with RCC. |
Table I.
Clinicopathological characteristics of
patients with RCC.
| Characteristic | Number of cases |
|---|
| Mean age, range
(year) | 53 (27–72) |
| Sex
(male/female) | 33/9 |
| Histological type
(clear cell/papillary) | 35/7 |
| Fuhrman grade
(I/II/III/IV) | 29/10/2/1 |
| AJCC clinical stage
(I/II/III+IV) | 32/9/1 |
The ACHN and 786-O RCC cell lines and the 293T
reference cell line were obtained from the American Type Culture
Collection (Manassas, VA, USA) and the Type Culture Collection of
the Chinese Academy of (Shanghai, China), respectively. The human
RCC cell lines (786-O and ACHN) were originally obtained from the
American Type Culture Collection (Manassas, VA, USA). The human
embryo kidney cell line 293T (293T) was purchased from the Type
Culture Collection of the Chinese Academy of Medical Sciences
(Shanghai, China). All of them were cultured in Dulbecco's modified
Eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), with 10% fetal bovine serum (FBS; GE Healthcare,
Little Chalfont, UK), 1% glutamine and 1% antibiotics (100 U/ml
penicillin and 100 mg/ml streptomycin; Gibco; Thermo Fisher
Scientific, Inc.). All cells were cultured in an incubator at 37%
in a humidified atmosphere with 5% CO2.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from tissues and cells was isolated with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
purified with the RNeasy Maxi kit (Qiagen, Hilden, Germany)
according to the manufacturer's instructions. After determining the
RNA concentration with a NanoDrop 2000c (Thermo Fisher Scientific,
Inc.), the miScript II RT kit (Qiagen) was applied to
reverse-transcribe miRNA to complementary (c)DNA. The expression
levels of miR-199b-5p were measured with a miScript
SYBR® Green PCR Kit (Qiagen) by real-time qPCR on the
Roche Lightcycler 480 Real-Time PCR System (Roche Diagnostics,
Basel, Switzerland). The 10-µl reaction mixture contained 5 µl 2X
QuantiTect SYBR Green PCR Master mix, 3.7 µl RNase-free water, 1 µl
cDNA template, 0.4 µl specific miRNA primer and 10X miScript
Universal Primer. The forward primer of miR-199b-5p had the
sequence 5′-CCCAGUGUUUAGACUAUCUGUUC-3′ and the reverse primer was a
universal primer, provided with the miScript SYBR® green
PCR Kit. U6 was used as an internal control. The forward primer of
U6 was 5′-CTCGCTTCGGCAGCACA-3′ and the reverse primer was
5′-ACGCTTCACGAATTTGCGT-3′. The reaction conditions were as follows:
95°C for 2 min and 40 cycles of 95°C for 10 sec, 55°C for 30 sec
and at 72°C for 30 sec. The expression levels of miR-199b-5p in
tissues and cell lines were analyzed by the ΔΔCq method (8).
Cell transfection
According to the manufacturer's protocol, the
expression levels of miR-199b-5p in ACHN and 786-O cells were
transfected with 5 ml miR-199b-5p inhibitor
(5′-GAACAGAUAGUCUAAACACUGGG-3′; Shanghai GenePharma, Co., Ltd.,
Shanghai, China) or inhibitor negative control
(5′-CAGUACUUUUGUGUAGUACAA-3′; Shanghai GenePharma, Co., Ltd.) by
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) and Opti-MEM® I Reduced Serum Medium
(Gibco; Thermo Fisher Scientific, Inc.). The efficiency of
transfection was detected using RT-qPCR after 24 h.
Cell Counting Kit-8 (CCK-8) assay. The
proliferation ability of ACHN and 786-O cells was assessed using a
CCK-8 assay (Beyotime Institute of Biotechnology, Haimen, China)
according to the manufacturer's protocol. ACHN or 786-O cells were
respectively seeded in each well of a 96-well plate at
5×103 cells/well, cultured for 24 h and then transfected
with miR-199b-5p inhibitor or inhibitor negative control (NCin). At
0, 24, 48 and 72, 10 µl CCK-8 stain was added to each well,
followed by further culture for 30 min in the dark at 37°C in a
humidified atmosphere with 5% CO2 prior to measurement
of the optical density (OD) value with an ELISA microplate reader
at a wavelength of 450 nm (with 620 nm as the reference wave
length).
MTT assay
The number of viable ACHN and 786-O cells was
detected using an MTT assay. ACHN or 786-O cells were respectively
seeded in each well of a 96-well plate at 5×103
cells/well, cultured for 24 h and then transfected with miR-199b-5p
inhibitor or NCin by using Lipofectamine® 2000. After 4
days of incubation, 20 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was added to each well of 96-well plate. After
culture for 4 h, the medium was discarded and 100 µl
dimethylsulfoxide (Sigma-Aldrich; Merck KGaA) was added to each
well, followed by incubation under exclusion of light with
agitation at room temperature for 10 min. Subsequently, an ELISA
microplate reader (Bio-Rad Laboratories, Hercules, CA, USA) was
used to measure the OD value of each well at a wavelength of 595 nm
(with 620 nm as the reference wavelength).
Transwell migration and invasion
assay
The migration and invasion ability of the ACHN and
786-O cells in vitro was measured using a Transwell assay.
The Transwell chambers (pore size, 8 µm; cat. no. 3422; BD
Biosciences, Franklin Lakes, NJ, USA) with Matrigel®
were applied to evaluate the invasion ability, while Transwell
chambers without Matrigel® were applied to evaluate the
migration ability. After transfection for 24 h, ~2×104
ACHN or 786-O cells were added into each of the upper chambers with
serum-free medium, while DMEM with 10% FBS was added to the lower
chamber. The chambers were incubated for 48 h at 37°C, and
subsequently, the cells that had transgressed through the
filter/membrane on the lower side were fixed with 4%
paraformaldehyde and then stained with 0.1% crystal violet at room
temperature for 25 min. The cells in the bottom of the chamber were
then counted using a microscope (magnification, ×100).
Scratch wound assay
A scratch wound assay was used to assess the
migration ability of the 786O and ACHN cells in vitro. The
cells were seeded in a 6-well plate at 1×106 cells/well.
After 24 h, they were transfected with miR-199b-5p inhibitor and
NCin by using Lipofectamine® 2000 for 24 h, and a
vertical line was scratched with a sterile 1-ml pipette tip. Images
of the scratches were respectively captured under a microscope
(magnification, ×100; Optical digital microscope; Olympus
Corporation, Tokyo, Japan) at 0 and 12 h.
Flow cytometric assay
The apoptotic rates of ACHN and 786-O cells in
vitro were measured by a flow cytometric assay. Following the
manufacturer's protocols, ACHN or 786-O cells were incubated in
each well of a 6-well plate at a concentration of 1×106
cells/well and transfected with miR-199b-5p inhibitor or NCin.
After 24 h of incubation, the ACHN or 786-O cells were collected,
washed twice with cold PBS and then resuspended in a flow cytometry
tube in 100 µl 1X binding buffer. Next, 5 µl Annexin V-fluorescein
isothiocyanate (Invitrogen; Thermo Fisher Scientific, Inc.) and 5
µl propidium iodide (Invitrogen; Thermo Fisher Scientific, Inc.)
were added to each sample, followed by incubation under the
exclusion of light at room temperature for 15 min. Finally, after
addition of 400 µl 1X binding buffer to each tube, the apoptotic
rate of the ACHN or 786-O cells was analyzed by flow cytometry
(EPICS Xl-4; Beckman-Coulter, Brea, CA, USA). All assays were
repeated at least 3 times.
Statistical analysis
Values are expressed as the mean ± standard error.
Differences between pairs of groups were analyzed by using
Student's t-test, while the paired t-test was used to compare the
expression levels of miR-181a-5p in corresponding tumor/normal
tissues. Prior to the t-test, a normality test had been performed
to confirm the normal distribution of the data. Comparisons between
cell lines were performed using one way analysis of variance
followed by a Tukey's post-hoc test. The SPSS 23.0 statistical
software package (IBM Corp., Armonk, NY, USA) was applied for
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-199b-5p is downregulated in RCC
tissues and cell lines
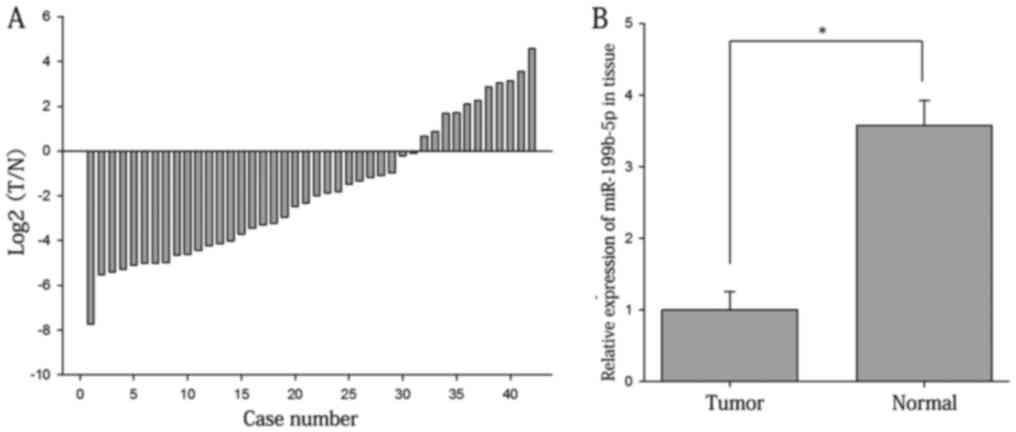
The ratios of the expression of miR-199b-5p in RCC
tissues vs. adjacent normal tissues are presented in Fig. 1A. As displayed in Fig. 1B the expression levels of miR-199b-5p
in RCC tissues were significantly lower than those in adjacent
normal tissues (1.000±0.257 vs. 3.572±0.729; P<0.05). The
results on renal cell lines suggested that the relative expression
levels of miR-199b-5p were higher in 293T (1.000±0.025) than those
in 786-O (0.771±0.064; P<0.05) and ACHN cells (0.667±0.063;
P<0.01; Fig. 2).
Cell transfection efficiency
RT-qPCR was used to measure the knockdown efficiency
of miR-199b-5p inhibitor compared with NCin. The results indicated
that after transfection with miR-199b-5p inhibitor for 24 h, the
expression levels of miR-199b-5p in 786-O and ACHN cells were
decreased to 60 and 21%, respectively, of those in the
NCin-transfected group (P<0.01; Fig.
3).
Inhibition of miR-199b-5p promotes
ACHN- and 786-O-cell proliferation
The proliferation ability of the ACHN and 786-O
cells was assessed with a CCK-8 assay. The results suggested that
the inhibition of miR-199b-5p enhanced the proliferation of the
ACHN and 786-O cells. At 24, 48 and 72 h of incubation,
respectively, the proliferation rate of the ACHN cells transfected
with miR-199b-5p inhibitor was increased by 15.42, 11.80 and 13.47%
of that of the control (Fig. 4A),
and that of 786-O cells was increased by 17.21, 54.95 and 69.49%
(Fig. 4B; P<0.05 or <0.01 for
all).
The number of viable cells was also determined with
an MTT assay. The results suggested that after 4 days of
incubation, the viability of ACHN and 786-O cells transfected with
miR-199b-5p inhibitor were 1.3 and 1.2 times increased compared
with that in the NCin-transfected group (P<0.001 and P<0.05;
Fig. 4C and D, respectively).
Inhibition of miR-199b-5p promotes
ACHN and 786-O cell motility
In order to assess the effect of miR-199b-5p in the
motility of ACHN and 786-O cells, a Transwell assay and a scratch
wound assay were performed. As presented in Fig. 5A and B, the results of the Transwell
migration assay indicated that the migratory ability of ACHN cells
transfected with miR-199b-5p inhibitor was increased by 101.39%
compared with that in the NCin-transfected group (P<0.001).
Furthermore, the migratory ability of 786-O cells transfected with
miR-199b-5p inhibitor was increased by 73.60% compared with that in
the NCin-transfected group (P<0.01; Fig. 5C and D). The Transwell invasion assay
indicated that the invasive ability of 786-O cells was increased by
78.45% following transfection with miR-199b-5p inhibitor
(P<0.01; Fig. 5C and D); however,
the effect of miR-199b-5p inhibitor on the invasion of ACHN cells
was not significant (Fig. 5A and
B).
The results of the scratch wound assay demonstrated
that the migratory ability of ACHN cells transfected with
miR-199b-5p inhibitor was increased by 65.00% compared with that in
the NCin-transfected group (P<0.001; Fig. 6A and B). Furthermore, 786-O cells
exhibited a 213.30% increase in cell migration after transfection
with the inhibitor (P<0.001; Fig. 6C
and D).
Inhibition of miR-199b-5p reduces the
apoptotic rate of ACHN and 786-O cells
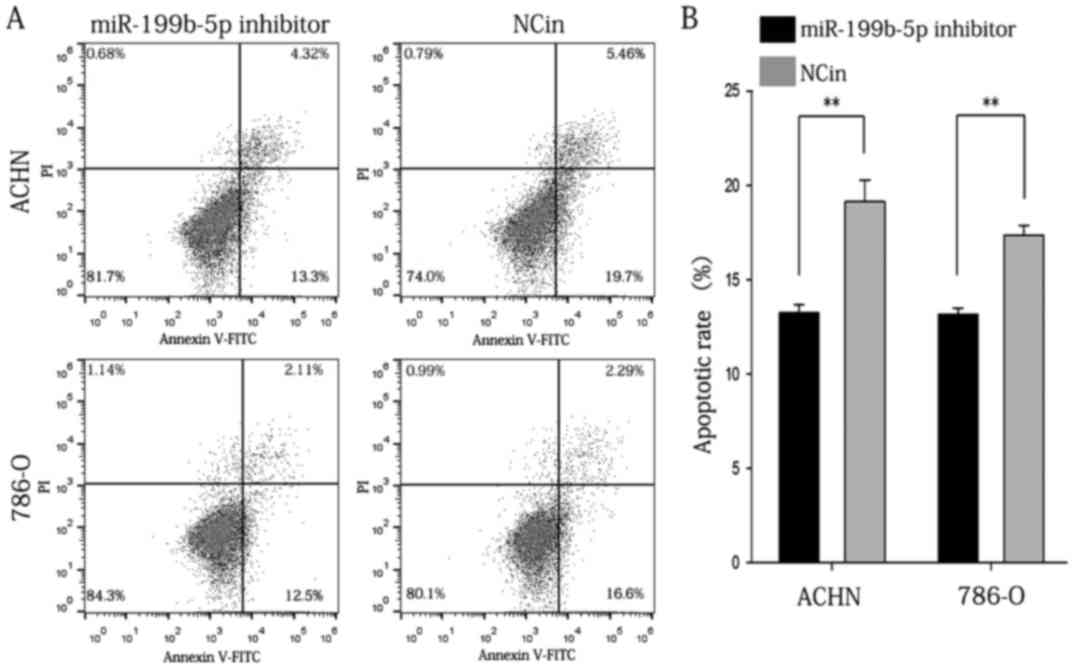
A flow cytometric assay was applied to analyze the
effect of miR-199b-5p inhibitor on the apoptotic rate of RCC cells.
The results indicated that the apoptotic rate of ACHN cells
transfected with miR-199b-5p inhibitor or NC in was 13.267±0.433
vs. 19.133±1.161% (P<0.01), while that of 786-O cells was
13.167±0.353 vs. 17.367±0.536% in the miR-199b-5p inhibitor and
NCin group, respectively (P<0.01; Fig. 7). Propidium iodide was used to
distinguish between viable early cells and necrotic or late
apoptotic cells. Therefore, the cells in the upper right quadrant
contains necrotic cells and the population in the lower right
quadrant of the flow cytometry dot plots represents the early
apoptotic cells. The aforementioned data were the average result of
three replicates of experimental data. The graph that was selected
was typically represented. The above results indicated that
inhibition of miR-199b-5p reduced the apoptotic rate of ACHN and
786-O cells in vitro.
Discussion
The molecular mechanisms of tumorigenesis and tumor
development have remained to be fully elucidated. However, numerous
tumor suppressor genes and oncogenes are known to be involved in
biological processes of tumorigenesis and tumor development,
including the dysregulation of cell proliferation and apoptosis.
According to a growing number of studies, certain miRNAs have
important functions in numerous types of malignant tumor by
regulating the expression of their target genes (9). In malignant tumor cells, the expression
levels of certain miRNAs are altered (10). miRNAs are a class of small non-coding
and evolutionarily conserved RNAs of 19–25 nucleotides in length,
which regulate mRNA expression at the post-transcriptional level
(11). According to statistics,
~1,500 miRNA sequences have been identified, and >50% of the
genes in the human genome were revealed to be targets of miRNAs
(12). This provides a theoretical
basis for the potential of miRNAs for use in tumor diagnosis,
treatment and prognosis.
A number of studies have suggested that miRNAs have
a crucial role in the occurrence and development of malignances
(13). For instance, Won et
al (14) suggested that
miR-199b-5p is involved in Notch signaling pathway in osteosarcoma.
Joshi et al (15) reported
that low expression levels of miR-199b were associated with
imatinib drug resistance in 9q34.1-deleted breakpoint cluster
region protein/Abelson murine leukemia viral oncogene homolog
1-positive chronic myeloid leukemia patients. In addition, a study
by Fang et al (16)
demonstrated that downregulation of miR-199b-5p is correlated with
aggressive clinical characteristics of breast cancer. Furthermore,
growing evidence has indicated that miR-199b-5p acts as a biomarker
in various cancer types, including acute myeloid leukemia (AML) and
hepatocellular carcinoma (17,18).
Furthermore, a study by Favreau et al (17) indicated that downregulation of
miR-199b is correlated with a worse overall survival of AML
patients and appears to be a promising prognostic marker for the
French-American-British M5 subtype. Apart from the above studies,
abnormal expression levels of miR-199b has been reported in other
tumor types, including ovarian cancer (19), prostate cancer (20), human osteosarcoma (21) and medulloblastoma (22), while it has largely remained to be
elucidated in RCC.
In the present study, the expression levels of
miR-199b-5p in RCC tissues and cell lines were quantified by
RT-qPCR, revealing that miR-199b-5p was downregulated in RCC. As
presented in Fig. 1A, the expression
of miR-199b was still upregulated in certain patients; however,
this may be due to instrumental measurement errors and the small
quantity of specimens used. The present study assessed the function
of miR-199b-5p in RCC in vitro by performing a CCK-8, MTT
assay, scratch wound, Transwell and flow cytometric assays, which
indicated that after transfection with miR-199b-5p inhibitor, RCC
cells present with increased cellular proliferation, migration and
invasion, but less cellular apoptosis compared with that in the
negative control group. The present results demonstrate that
miR-199b-5p may serve as a tumor suppressor in RCC by regulating
various biological processes, including cell proliferation,
migration, invasion and cell apoptosis. It is undeniable that more
in-depth experiments, including those aiming to identify cell
migration markers as well as specific analysis of cellular key
proteins, are lacking in the present study. For example, the
relationship between tumor stage and the down-regulation of
miRNA-199b-5p may be better assessed by increasing the sample size
and analyzing the samples in greater detail. The specific molecular
mechanisms of the regulatory effects of miR-199b-5p still require
further study. For instance, a subcutaneous tumorigenesis test
(23,24) in animals may further verify the tumor
biological function of miRNA in vivo in the future. However,
Brodaczewska et al (25)
demonstrated that in vitro RCC cell lines may be unable to
represent the full pathological features of RCC, which is a
limitation that requires further study. In addition, 293T cells are
only used as a representation of kidney physiology, and whilst they
may be used as controls in RCC in vitro studies, caution is
required for interpretation (26,27).
In summary, the results of the present study
revealed that miR-199b-5p was downregulated in RCC and functioned
as a tumor suppressor. Inhibition of miR-199b-5p in RCC promoted
cellular proliferation, migration and invasion, while inhibiting
cellular apoptosis; however, the specific molecular mechanism
remains elusive. In the present study, the expression levels of
miR-199b-5p were downregulated in RCC lines, as well as in RCC vs.
paired adjacent normal tissues. RCC is not sensitive to traditional
radiotherapy and chemotherapy, and the prognosis remains poor;
therefore, easily detectable and specific biomarkers for early
detection and treatment guidance are required. To further reveal
the mechanisms associated with RCC and to facilitate early
diagnosis, treatment and prognostication of RCC patients, the
search of new biomarkers for renal cell carcinoma is imminent.
However, the focus of further research will be on the roles of
miR-199b-5p in RCC with regard to its diagnostic and prognostic
value for RCC. In addition, further study is essential to identify
the molecular mechanisms of miR-199b-5p in the genesis of RCC and
its potential in early detection, prognosis prediction and targeted
therapy for RCC. Perhaps in the future, circulating miR-199b-5p may
be able to reflect the disease status of RCC patients and be of
great early diagnostic and prognostic value.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81101922), the Science and
Technology Development Fund Project of Shenzhen (grant nos.
JCYJ20150403091443329 and JCYJ20170307111334308), the fund of the
‘San-ming’ project of medicine in Shenzhen and the fund of the
Guangdong Key medical subject.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LCN and YQL conceived and refined experimental
designs; YLL, JQ, JH, PJC collected data; JLX, XG and WJX performed
the experiments; YLL and JQ evaluated the data; YLL drafted the
manuscript; and YLL and JQ edited the manuscript. All authors read
and approved the final manuscript.
Ethical approval and consent to
participate
All patients provided written informed consent. The
present study was approved by the Ethical Review Committee of
Peking University Shenzhen Hospital (Shenzhen, China) and abided by
the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y, Han X, Yu Y, Ding Y, Ni C, Liu W,
Hou X, Li Z, Hou J, Shen D, et al: A genetic polymorphism affects
the risk and prognosis of renal cell carcinoma: Association with
follistatin-like protein 1 expression. Sci Rep. 6:266892016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Y, Zhang N, Li K, Chen H, Lin X, Yu Y,
Gou Y, Hou J, Jiang D, Na R, et al: Genetic scores based on
risk-associated single nucleotide polymorphisms (SNPs) can reveal
inherited risk of renal cell carcinoma. Oncotarget. 7:18631–18637.
2016.PubMed/NCBI
|
|
3
|
Lin YW, Lee LM, Lee WJ, Chu CY, Tan P,
Yang YC, Chen WY, Yang SF, Hsiao M and Chien MH: Melatonin inhibits
MMP-9 transactivation and renal cell carcinoma metastasis by
suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J
Pineal Res. 60:277–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou J, Yun EJ, Chen W, Ding Y, Wu K, Wang
B, Ding C, Hernandez E, Santoyo J, Pong RC, et al: Targeting
3-phosphoinositide-dependent protein kinase 1 associated with
drug-resistant renal cell carcinoma using new oridonin analogs.
Cell Death Dis. 8:e27012017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshino H, Seki N, Itesako T, Chiyomaru T,
Nakagawa M and Enokida H: Aberrant expression of microRNAs in
bladder cancer. Nat Rev Urol. 10:396–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ling H, Girnita L, Buda O and Calin GA:
Non-coding RNAs: The cancer genome dark matter that matters! Clin
Chem Lab Med. 55:705–714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cortés-Sempere M and de Cáceres Ibáñez I:
microRNAs as novel epigenetic biomarkers for human cancer. Clin
Transl Oncol. 13:357–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rotomskis A, Margevičiūtė R, Germanavičius
A, Kaubrys G, Budrys V and Bagdonas A: Differential diagnosis of
depression and Alzheimer's disease with the Addenbrooke's cognitive
Examination-revised (ACE-R). BMC Neurol. 15:572015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roomi MW, Kalinovsky T, Rath M and
Niedzwiecki A: In vitro modulation of MMP-2 and MMP-9 in pediatric
human sarcoma cell lines by cytokines, inducers and inhibitors. Int
J Oncol. 44:27–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lawless N, Vegh P, O'Farrelly C and Lynn
DJ: The role of microRNAs in bovine infection and immunity. Front
Immunol. 5:6112014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kefas B, Godlewski J, Comeau L, Li Y,
Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, Lawler S and
Purow B: microRNA-7 inhibits the epidermal growth factor receptor
and the Akt pathway and is down-regulated in glioblastoma. Cancer
Res. 68:3566–3572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Won KY, Kim YW, Kim HS, Lee SK, Jung WW
and Park YK: MicroRNA-199b-5p is involved in the Notch signaling
pathway in osteosarcoma. Hum Pathol. 44:1648–1655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Joshi D, Chandrakala S, Korgaonkar S,
Ghosh K and Vundinti BR: Down-regulation of miR-199b associated
with imatinib drug resistance in 9q34.1 deleted BCR/ABL positive
CML patients. Gene. 542:109–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang C, Wang FB, Li Y and Zeng XT:
Down-regulation of miR-199b-5p is correlated with poor prognosis
for breast cancer patients. Biomed Pharmacother. 84:1189–1193.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Favreau AJ, McGlauflin RE, Duarte CW and
Sathyanarayana P: miR-199b, a novel tumor suppressor miRNA in acute
myeloid leukemia with prognostic implications. Exp Hematol Oncol.
5:42016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang C, Song B, Song W, Liu J, Sun A, Wu
D, Yu H, Lian J, Chen L and Han J: Underexpressed microRNA-199b-5p
targets hypoxia-inducible factor-1α in hepatocellular carcinoma and
predicts prognosis of hepatocellular carcinoma patients. J
Gastroenterol Hepatol. 26:1630–1637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu MX, Siu MK, Liu SS, Yam JW, Ngan HY
and Chan DW: Epigenetic silencing of microRNA-199b-5p is associated
with acquired chemoresistance via activation of JAG1-Notch1
signaling in ovarian cancer. Oncotarget. 5:944–958. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shang W, Chen X, Nie L, Xu M, Chen N, Zeng
H and Zhou Q: MiR199b suppresses expression of hypoxia-inducible
factor 1alpha (HIF-1α) in prostate cancer cells. Int J Mol Sci.
14:8422–8436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng H, Zhang Z, Dai X, Chen Y, Ye J and
Jin Z: Increased expression of microRNA-199b-5p associates with
poor prognosis through promoting cell proliferation, invasion and
migration abilities of human osteosarcoma. Pathol Oncol Res.
22:253–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andolfo I, Liguori L, De Antonellis P,
Cusanelli E, Marinaro F, Pistollato F, Garzia L, De Vita G,
Petrosino G, Accordi B, et al: The micro-RNA 199b-5p regulatory
circuit involves Hes1, CD15, and epigenetic modifications in
medulloblastoma. Neuro Oncol. 14:596–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang J, Wang F, Cheng G, Si S, Sun X, Han
J, Yu H, Zhang W, Lv Q, Wei JF and Yang H: Wilms' tumor
1-associating protein promotes renal cell carcinoma proliferation
by regulating CDK2 mRNA stability. J Exp Clin Cancer Res.
37:402018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang M, Li C, Yu B, Su L, Li J, Ju J, Yu
Y, Gu Q, Zhu Z and Liu B: Overexpressed miR-301a promotes cell
proliferation and invasion by targeting RUNX3 in gastric cancer. J
Gastroenterol. 48:1023–1033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brodaczewska K, Szczylik C, Fiedorowicz M,
Porta C and Czarnecka AM: Choosing the right cell line for renal
cell cancer research. Mol Cancer. 15:832016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zaravinos A, Pieri M, Mourmouras N,
Anastasiadou N, Zouvani I, Delakas D and Deltas C: Altered
metabolic pathways in clear cell renal cell carcinoma: A
meta-analysis and validation study focused on the deregulated genes
and their associated networks. Oncoscience. 1:117–131. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Araújo Júnior RF, Oliveira Leitão AL,
de Melo Silveira RF, de Oliveira Rocha HA, de França Cavalcanti P
and de Araújo AA: Telmisartan induces apoptosis and regulates Bcl-2
in human renal cancer cells. Exp Biol Med (Maywood). 240:34–44.
2015. View Article : Google Scholar : PubMed/NCBI
|