Introduction
Lumbar degenerative disease is one of the common
orthopedic diseases, mainly including protrusion of lumbar
intervertebral disc, lumbar spinal stenosis, scoliosis and lumbar
spondylolisthesis, which is complicated with or without low back
pain and intermittent claudication (1,2).
Patients are usually treated due to unbearable low back pain
(3). Lumbar degenerative disease is
one of the major factors leading to disability in the working
population. After the conservative treatment failes, patients can
be treated via surgery, which will shorten the recovery time and
alleviate the symptoms (4).
Posterior lumbar interbody fusion (PLIF) is a surgical method that
started to be used and promoted clinically in the 1950s, which can
effectively stabilize the patient's spine, maintain the
intervertebral height, enhance the anterior spinal bearing and
relieve the nerve compression (5).
In this study, patients with single-segment lumbar degenerative
disease were treated with different surgical methods, and the
curative effects were compared, so as to provide a basis for the
development and implementation of reasonable treatment plan.
Patients and methods
Basic information for the included
patients
A total of 86 patients with single-segment lumbar
degenerative disease treated in Jiangyin Hospital (Jiangyin, Wuxi,
China), from January 2013 to October 2016 were randomly selected.
Inclusion criteria: i) patients diagnosed with single-segment
lumbar degenerative disease via imaging examination; ii) patients
with intermittent claudication complicated with or without low back
pain or lower limb radiation pain and who received conservative
treatment that failed for 6 months; iii) patients receiving
single-segment surgery; iv) patients who signed the informed
consent. Exclusion criteria: i) patients with multi-segment lumbar
spinal stenosis or protrusion of intervertebral disc; ii) patients
with severe osteoporosis or intervertebral space infection. This
study was approved by the Ethics Committee of the Affiliated
Jiangyin Hospital of Southeast University Medical School (Jiangyin,
Wuxi, China). Signed informed consents were obtained from all
participants before the study. The patients were divided into
control group (n=43) and observation group (n=43) using a random
number table. The control group was treated with posterolateral
lumbar fusion (PLF), while the observation group was treated with
PLIF. There were no statistically significant differences in the
general data of patients between the two groups (P>0.05)
(Table I).
 | Table I.General data of patients. |
Table I.
General data of patients.
| Items | Control group
(n=43) | Observation group
(n=43) | t/χ2 | P-value |
|---|
| Sex
(male/female) | 23/20 | 21/22 | 0.046 | 0.829 |
| Age (years) | 45–75 | 45–80 |
|
|
| Average age
(years) | 58.74±8.57 | 58.89±8.38 | 0.082 | 0.934 |
| Course of disease
(month) | 23.83±3.54 | 24.16±3.27 | 0.449 | 0.654 |
| Surgical segment (n,
%) |
|
|
|
|
|
L4/L5 | 29 (67.44) | 27 (62.79) | 0.051 | 0.821 |
|
L5/S1 | 14 (32.56) | 16 (37.21) |
|
|
| Symptom (n, %) |
|
|
|
|
| Lumbar
spinal stenosis | 18 (41.86) | 19 (44.19) | 0.262 | 0.877 |
|
Protrusion of lumbar
intervertebral disc | 14 (32.55) | 15 (34.88) |
|
|
| Lumbar
spondylolisthesis | 11 (25.58) | 9 (20.93) |
|
|
Preoperative preparation
Before operation, patients were comprehensively
evaluated to exclude surgical contraindications, and the square
titanium alloy cage was chosen. The appropriate operation time of
patients was selected; both groups of patients received the
operation under combined spinal-epidural anesthesia.
Surgical procedures
Under the prone position, a posterior median
incision (8–10 cm) was made on the back of patients in both groups,
and the deep fascia and paravertebral muscle was peeled off to
expose the vertebral plate lesion and one upper and one lower
normal vertebral plate, and they were fixed firmly using the fix
screws. The control group was treated with PLF for total or
semi-laminectomy; the vertebral spinous process was removed using
rongeur forceps and the proliferative ligamentum flavum was
cleared; the vertebral plates causing stenosis was expanded and
removed for effective decompression. After the reduction fixation
via pedicle screw system, the vertebral plate was washed with
normal saline; the spinous process and vertebral plate was removed
and trimmed into the bone block in appropriate size and implanted
into the intervertebral space, followed by drainage tube indwelling
and incision suture layer by layer.
The observation group was treated with
PLIF, and the total laminectomy was performed for the affected
vertebrae
The nerve root was fully decompressed and the upper
and lower adjacent segment stenosis received the potential
decompression to fully expose the spinous process and the bilateral
vertebral plates. The articular process was retained as far as
possible, the connecting rods were connected, and the
intervertebral space was expanded moderately using the distracter;
the posterior vertebral osteophyte and intervertebral disc tissues
were completely removed, and the upper and lower cartilage
endplates and residual disc tissues were remove; the cage was
chosen according to the height of intervertebral space; the spinous
process and vertebral plate removed were cut into pieces of bone
and implanted into the front section of intervertebral space; the
single cage was implanted into the second half of intervertebral
space (~3 mm away from the posterior margin of vertebral body);
then the horizontal connection was installed to ensure the spinal
stability. After the spinal dura mater was covered with gelatin
sponge, the drainage tube was placed and the incision was
sutured.
Postoperative care. After operation, patients rested
under supine position and were treated with conventional
dehydration, infection prevention and neurotrophic drugs. At 36 h
after operation, the drainage tube was removed and patients
received training for straight-leg-raising and waist function at 72
h after operation. After 1 week, they exercised off the bed wearing
waist belt for no less than 3 months; after the drainage tube was
removed, the internal fixation was observed via the lumbar
anteroposterior and lateral film and flexion-extension film.
Magnetic resonance imaging (MRI) was performed at 3 months after
operation. The MRI-T2 relaxation time was measured for the
multifidus muscle in the central plane of fusion segment (~1.5×1.5
cm). The patients were followed up for 12 months after operation
and the rehabilitation was evaluated.
Evaluation indexes
The clinical surgical effect of patients was
compared, including operation time, bleeding amount (intraoperative
bleeding amount + postoperative drainage amount) and
hospitalization time. Patients were followed up for 1 year, and the
function was evaluated according to MacNab score: i) excellent, the
patient can raise the leg straight for >70°, the muscle strength
and exercise of lower limb are normal, and the low back pain has
disappeared; ii) good, the patient can raise the leg straight for
30° more than that before operation, but <70°, the muscle
strength is level 4, and they can work and live normally
accompanied occasionally with slight waist-leg pain; iii) general,
the patients can raise the leg straight for 15° more than that
before operation, but <30°, the muscle strength is level 3, and
the low back pain is alleviated; but they still take drugs
occasionally; iv) poor, there is no change before and after
operation or even exacerbation, and analgesics are still needed;
excellent-good rate = (excellent + good)/total cases.
Oswestry disability index (ODI)
The patient's dysfunction was scored according to
the ODI (0–5 points: 0, no dysfunction; 5 points, the most obvious
dysfunction) from a total of 9 items in 3 dimensions: individual
capacity, pain and personal comprehensive ability. ODI is
positively correlated with the degree of dysfunction. The levels of
creatine phosphokinase (CPK) in patients were measured at 1, 3, 5
and 7 days after operation to evaluate the muscle injury.
Bridwell fusion grading
At 12 months after operation, the fusion degree was
evaluated via anterioposterior and lateral film and over
flexion-extension X-ray according to Bridwell fusion grading
criteria (6): i) grade I, bone graft
reconstruction and fusion, and ingrowth of bone trabecula; ii)
grade II, complete bone graft, incomplete reconstruction fusion,
but no translucent area; iii) grade III, complete bone graft, and
potential translucent area below and above the bone block; iv)
grade IV, collapsed and absorbed bone block, and no bone fusion.
The MRI was performed at 3 months for the multifidus muscle after
operation to detect the MRI-T2 relaxation time.
Pfirrmann grading
The adjacent segment was scored (0–10 points) using
Pfirrmann grading method (7)
combined with the patient's clinical data. Scoring method: i)
Pfirrmann grading: grade I, 4 points; grade II, 3 points; grade
III, 2 points; grade IV, 1 point; grade V, 0 point; ii) imaging
findings: adjacent segment sagittal plane angle ≥10°, 1 point;
lateral dislocation ≤3 mm, 1 point; cone shaped deformation of
intercalated disc ≤5°, 1 point; sagittal dislocation ≤4 mm, 1
point; iii) clinical data: patients aged ≤60 years, 1 point; body
mass index (BMI) ≤25, 1 point. The average spinal canal area was
calculated via MRI imaging.
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) software was
used for data processing. Comparison between groups was done using
One-way ANOVA test followed by Post Hoc Test (Least Significant
Difference). Enumeration data were presented as ratio, and
Chi-square test was used. Rank sum test was used for ranked data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of clinical operation
effects between the two groups
There were no significant differences in the
intraoperative bleeding amount and postoperative drainage amount
between the two groups (P>0.05); the operation time in the
observation group was significantly longer than that in the control
group, but the hospitalization time was significantly shorter than
that in the control group (P<0.05) (Table II).
 | Table II.Comparison of clinical operation
effects between the two groups. |
Table II.
Comparison of clinical operation
effects between the two groups.
| Groups | n | Operation time
(min) | Intraoperative
bleeding amount (ml) | Postoperative
drainage amount (ml) | Hospitalization time
(days) |
|---|
| Observation
group | 43 | 196.83±23.62 | 371.47±26.63 | 237.86±17.45 | 7.23±1.53 |
| Control group | 43 | 148.14±23.57 | 367.57±29.38 | 231.97±18.37 | 12.56±1.47 |
| t value |
| 9.568 | 0.766 | 1.524 | 16.437 |
| P-value |
| <0.001 | 0.446 | 0.131 | <0.001 |
Evaluation of therapeutic effects on
patients in the two groups via MacNab score
The excellent-good rate of treatment in the
observation group (90.69%) was significantly higher than that in
the control group (62.79%) (P<0.05) (Table III).
 | Table III.Comparison of therapeutic effects on
patients between the two groups (n, %). |
Table III.
Comparison of therapeutic effects on
patients between the two groups (n, %).
| Groups | n | Excellent | Good | General | Poor |
|---|
| Observation
group | 43 | 23 (53.48) | 16 (37.21) | 2 (4.65) | 1 (2.32) |
| Control group | 43 | 17 (39.53) | 11 (25.58) | 7 (16.28) | 8 (18.61) |
Comparison of CPK levels in the two
groups before and after operation
Before operation, the CPK level was 71.83±3.14 U/l
in the observation group and 71.06±3.23 U/l in the control group,
respectively, and the difference was statistically significant
(P>0.05); at 1, 3 and 5 days after operation, the CPK levels
were 523.16±9.13, 242.35±7.14 and 161.03±6.12 U/l in the
observation group and 534.36±9.25, 247.08±7.26 and 162.76±6.17 U/l
in the control group, which were increased compared with those
before operation (P<0.05). The CPK level reached the peak at 1
day after operation, and it was 78.61±3.14 U/l in the observation
group and 79.15±3.48 U/l in the control group at 7 days after
operation, respectively. There were no significant differences in
the CPK levels at different time-points after operation between the
two groups (P>0.05) (Fig. 1).
Comparison of ODIs between the two
groups
ODIs in observation group at 1, 6 and 12 months
after operation were significantly lower than those in the control
group, and the differences were statistically significant
(P<0.05) (Table IV).
 | Table IV.Comparison of ODIs between the two
groups. |
Table IV.
Comparison of ODIs between the two
groups.
| Groups | n | Before operation | 1 month after
operation | 6 months after
operation | 12 months after
operation |
|---|
| Observation
group | 43 | 30.81±3.24 | 11.62±3.63 | 5.78±2.24 | 4.13±2.23 |
| Control group | 43 | 30.29±2.34 | 15.53±3.37 | 7.93±2.36 | 6.76±2.35 |
| t value |
| 0.853 | 5.176 | 4.333 | 5.323 |
| P-value |
| 0.396 | <0.001 | <0.001 | <0.001 |
Comparison of fusion rates between the
two groups
Patients were followed up for 1 year; the grade I
and II interbody fusion rates in the observation group (93.02%)
were significantly higher than those in the control group (74.41%);
the differences were statistically significant (P<0.05)
(Table V).
 | Table V.Comparison of postoperative fusion
rates between the two groups. |
Table V.
Comparison of postoperative fusion
rates between the two groups.
| Groups | n | Grade I | Grade II | Grade III | Grade IV |
|---|
| Observation
group | 43 | 26 (60.47) | 14 (32.56) | 3 (6.98) | 0 (0.00) |
| Control group | 43 | 20 (46.51) | 12 (27.91) | 10 (23.26) | 1 (2.33) |
| χ2 |
|
| 4.181 |
|
| P-value |
|
| 0.041 |
|
Comparison of MRI-T2 relaxation time
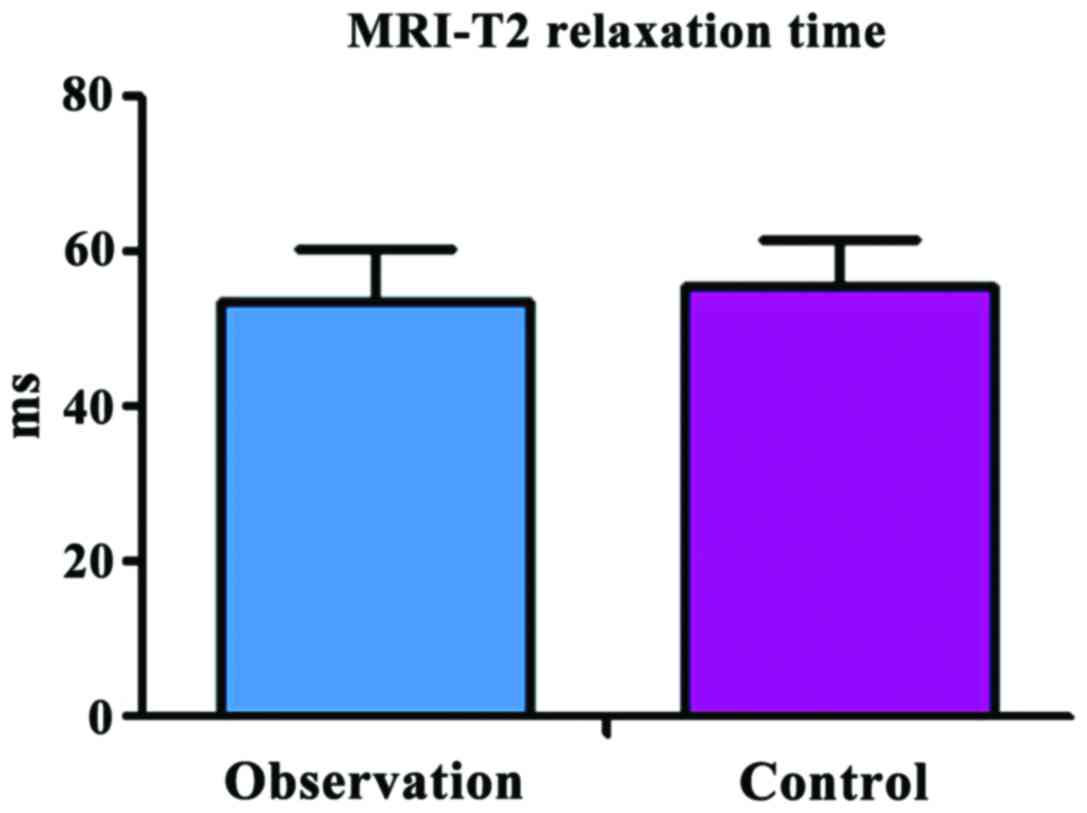
at 3 months after operation between the two groups
The MRI-T2 relaxation time was 53.83±5.24 msec in
the observation group and 55.64±6.47 msec in the control group, and
there was no significant difference between the two groups
(t=1.426, P=0.157) (Fig. 2).
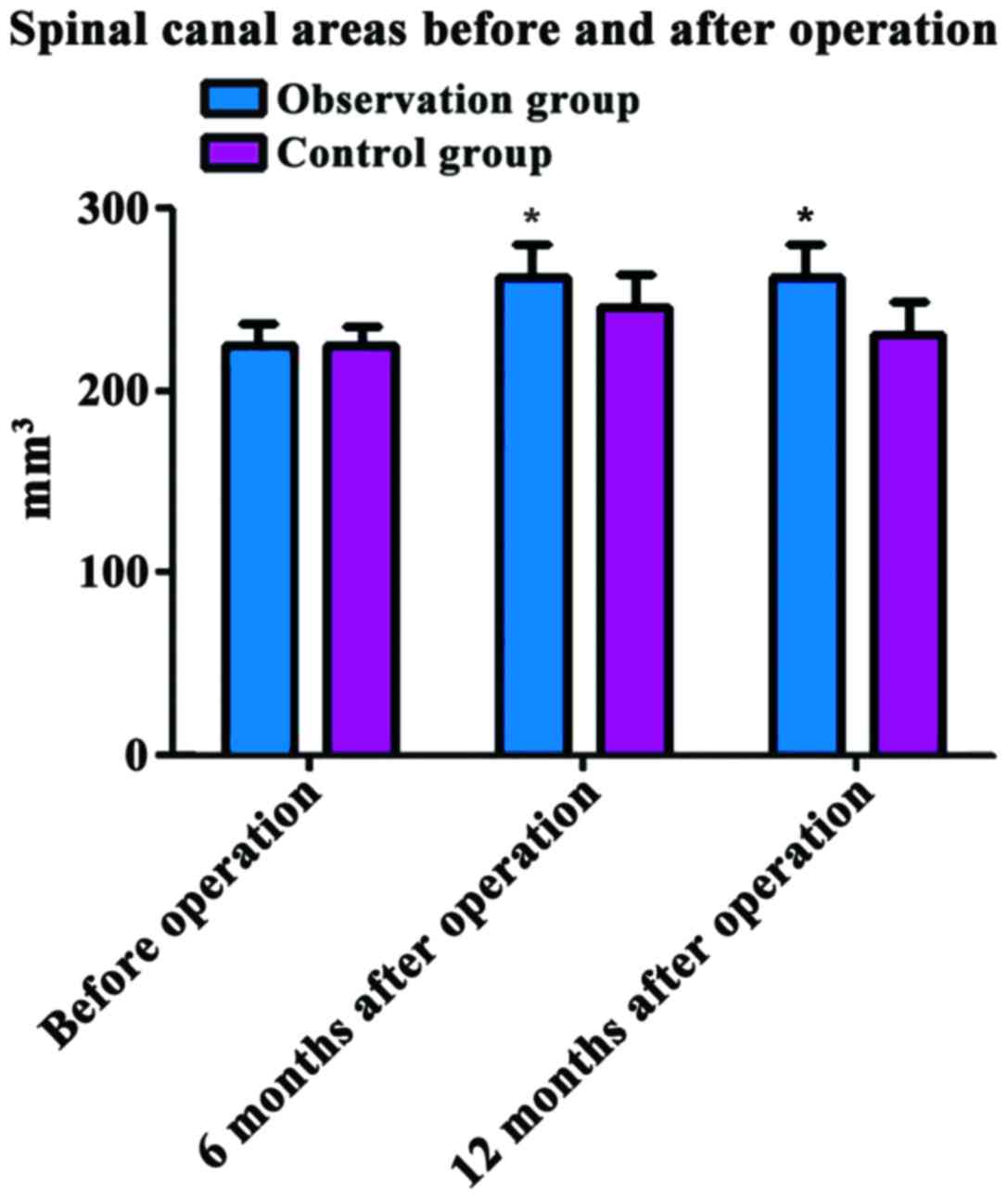
Comparison of spinal canal areas
before and after operation between the two groups
Before operation, the spinal canal area was
224.83±13.14 mm3 in the observation group and
225.06±13.23 mm3 in the control group, and the
difference was not statistically significant (P>0.05). At 6 and
12 months after operation, the spinal canal areas in the
observation group (263.16±9.13 and 262.35±7.14 mm3) were
significantly larger than those in the control group (246.36±9.25
and 231.08±7.26 mm3); the differences were statistically
significant (P<0.05) (Fig.
3).
Comparison of adjacent segment
quantitative scores before and after operation between the two
groups
At 6 months and 12 months after operation, the
scores were 4.54±0.34 and 6.12±0.53 points in the observation
group, and 3.58±0.46 and 4.87±0.57 points in the control group; the
scores in the observation group were significantly superior to
those in the control group, and the differences were statistically
significant (P<0.05) (Table
VI).
 | Table VI.Comparison of adjacent segment
quantitative scores before and after operation between the two
groups. |
Table VI.
Comparison of adjacent segment
quantitative scores before and after operation between the two
groups.
| Groups | n | Before
operation | 6 months after
operation | 12 months after
operation |
|---|
| Observation
group | 43 | 2.82±0.34 | 4.56±0.35 | 6.24±0.36 |
| Control group | 43 | 2.78±0.32 | 3.34±0.33 | 4.89±0.34 |
| t value |
| 0.562 | 16.631 | 17.878 |
| P-value |
| 0.576 | <0.001 | <0.001 |
Discussion
Degeneration is the normal aging process of human
body. Walking upright exerts large pressure to the lumbar vertebra,
and it begins to degenerate after the age of 20 years. According to
statistics, ~90% of people aged >50 years suffer from varying
degrees of spinal structure or lumbar intervertebral disc
degeneration (8). Lumbar
degeneration usually begins from the intervertebral disc, and with
the increase of age and long-term repeated abrasion, intervertebral
disc nucleus suffers from continuous dehydration and calcification,
leading to uneven pressure dispersion and resulting in uneven
stress to the peripheral fibrous rings; and it leads to
intervertebral height loss and spinal instability, thereby
increasing the oppression against the intervertebral disc and
forming a vicious cycle (9). The
combined action among protrusion of intervertebral disc, ligamentum
flavum relaxation thickening, superior articular process
hyperplasia and vertebral posterior margin hyperostosis leads to
degenerative lumbar spinal stenosis. The initial symptom of lumbar
degeneration is low back pain, and neurological dysfunction and
intermittent claudication will occur with the progression of
disease, causing inconvenience to the daily life of patients
(10,11).
The treatment of lumbar degenerative diseases can be
divided into conservative treatment and surgical treatment. The
conservative treatment usually includes general treatment (waist
protection, back muscle exercise, pelvic traction and bed rest),
physical therapy (massage, cupping, acupuncture and magnetic
therapy), and drug therapy (steroids, analgesics and antispasmodic
drugs) (12). The conservative
treatment is the preferred method for most patients, which can
effectively delay and prevent the disease. However, there are often
some problems in conservative treatment: patients have poor
tolerance to the pain in conservative treatment, the nervous system
injury progresses rapidly, and cauda equina symptoms occur;
conservative treatment is invalid for some patients, so the spinal
decompression and spinal fusion should be considered via surgery at
this time, thus effectively solving the spinal instability and pain
relief (13). Spinal fusion may
include transforaminal lumbar interbody fusion (TLIF), anterior
lumbar interbody fusion (ALIF), PLF and PLIF (14). PLIF can not only relieve pain
effectively through the nerve decompression, but also effectively
maintain the intervertebral height and stability of surgical
segment through cage placing, so that the biomechanical properties
are superior, the bone graft fusion area is larger and the fusion
effect is better (15).
Many studies have shown that the bone graft fusion
internal fixation effect of PLIF is more significant than that of
PLF, the fusion rate is higher, and the postoperative long-term
effective rate is ~95%. The results of this study showed that ODIs
at 1, 6 and 12 months after operation in the observation group were
significantly lower than those in the control group (P<0.05).
The grade I and II fusion rates in the observation group at 12
months after operation was 90.69%, which was significantly higher
than that in the control group (P<0.05). In PLIF, intervertebral
disc is removed, and nucleus pulposus and fibrous ring tissues are
completely eliminated, and the intervertebral height can be
increased and the lumbar sagittal axis can be reconstructed through
a cage, thus improving the intervertebral foramen stenosis more
effectively. PLF is usually limited in the intervertebral foramen
and lateral recess decompression, and the incomplete decompression
will still leave the nerve root compression symptoms after
operation, so the postoperative decline in ODI is not as
significant as PLIF (16). Using the
cage can effectively avoid implanted bone displacement and
compression, so as to provide good fusion conditions and share the
burden of spin and increase the fusion area, and its fusion time is
shorter and the effect is better than PLF (17). The CPK levels in the two groups were
significantly increased at 1, 3 and 5 days after operation
(P<0.05), reached the peak at 1 day after operation and returned
to normal at 7 days after operation. There was no statistically
significant difference between the two groups (P>0.05). The
incisions in the two surgeries is longer, and the scope of invasion
is relatively wide, so the rapid increase in CPK level at 1 day
after operation indicates a greater degree of muscle damage; the
wound is healed with time under careful postoperative care for
patients, so the CPK level is gradually decreased. There was no
significant difference in MRI-T2 relaxation time of multifidus
muscle between the two groups at 3 months after operation,
indicating that there is no significant difference in muscle injury
between the two surgeries and the muscle function is gradually
restored with time (18).
In this study, patients were followed up for 12
months; the spinal canal area in the observation group was
significantly larger than that in the control group, and the
maintenance time of improved spinal canal area was also longer in
the observation group, possibly because PLIF can effectively
eliminate the fibrous ring protrusion and ligamentum flavum
folding, so that the improving effect on spinal canal area is more
obvious and more durable (19). The
results of this study showed that the adjacent segment quantitative
scores in observation group at 6 and 12 months after operation were
significantly superior to those in the control group. This is
because in PLIF, the cage is implanted into the intervertebral
space and the lumbar kyphosis can be converted into lordosis via
compression and fixation; the physiological flexion of lumbar spine
can be restored, making its activity similar to normal spine,
effectively reducing the adjacent vertebral slippage, compensatory
activity and stress, so as to avoid the occurrence of degeneration.
The effect of PLIF on the adjacent segment is less than that of PLF
(5).
In conclusion, there are no significant differences
in the effects of PLIF and PLF on soft tissue and muscle damage in
the treatment of single-segment lumbar degenerative disease, but
the cure rate of PLIF is significantly higher than that of PLF, the
former of which can promote the early functional recovery of
patients, increase the lumbar fusion rate, reduce the impact on
adjacent segments and delay the degradation of adjacent
segments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YaZ and ZJ designed the study and performed the
experiments. YaZ, FZ and YuZ collected the data. YaZ and FZ
analyzed the data, YaZ and ZJ prepared the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Affiliated Jiangyin Hospital of Southeast University Medical
School (Jiangyin, Wuxi, China). Signed informed consents were
obtained from all participants before the study.
Consent for publication
Patients or their guardians have provided written
informed consents for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kreiner DS, Shaffer WO, Baisden JL,
Gilbert TJ, Summers JT, Toton JF, Hwang SW, Mendel RC and Reitman
CA: North American Spine Society: An evidence-based clinical
guideline for the diagnosis and treatment of degenerative lumbar
spinal stenosis (update). Spine J. 13:734–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manchikanti L, Benyamin RM, Falco FJ, Kaye
AD and Hirsch JA: Do epidural injections provide short- and
long-term relief for lumbar disc herniation? A systematic review.
Clin Orthop Relat Res. 473:1940–1956. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shamji MF, Mroz T, Hsu W and Chutkan N:
Management of degenerative lumbar spinal stenosis in the elderly.
Neurosurgery. 77 Suppl 4:S68–S74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shin JS, Oh SH and Cho PG: Surgical
outcome of a zero-profile device comparing with stand-alone cage
and anterior cervical plate with iliac bone graft in the anterior
cervical discectomy and fusion. Korean J Spine. 11:169–177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hikata T, Kamata M and Furukawa M: Risk
factors for adjacent segment disease after posterior lumbar
interbody fusion and efficacy of simultaneous decompression surgery
for symptomatic adjacent segment disease. J Spinal Disord Tech.
27:70–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daubs MD, Lenke LG, Bridwell KH, Kim YJ,
Hung M, Cheh G and Koester LA: Does correction of preoperative
coronal imbalance make a difference in outcomes of adult patients
with deformity? Spine. 38:476–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Urrutia J, Besa P, Campos M, Cikutovic P,
Cabezon M, Molina M and Cruz JP: The Pfirrmann classification of
lumbar intervertebral disc degeneration: An independent inter- and
intra-observer agreement assessment. Eur Spine J. 25:2728–2733.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Williams FM and Sambrook PN: Neck and back
pain and intervertebral disc degeneration: Role of occupational
factors. Best Pract Res Clin Rheumatol. 25:69–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Li F, Yu S, Ma H, Chen Z, Zhang H
and Fu Q: Two-year follow-up results of the Isobar TTL Semi-Rigid
Rod System for the treatment of lumbar degenerative disease. J Clin
Neurosci. 20:394–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui YZ, Yang XH, Liu PF, Wang B and Chen
WJ: Preliminary study on diagnosis of lumbar disc degeneration with
magnetic resonance T1p, T2 mapping and DWI quantitative detection
technologies. Eur Rev Med Pharmacol Sci. 20:3344–3350.
2016.PubMed/NCBI
|
|
11
|
Choudhri TF, Mummaneni PV, Dhall SS, Eck
JC, Groff MW, Ghogawala Z, Watters WC III, Dailey AT, Resnick DK,
Sharan A, et al: Guideline update for the performance of fusion
procedures for degenerative disease of the lumbar spine. Part 4:
Radiographic assessment of fusion status. J Neurosurg Spine.
21:23–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu SW, Yang SC, Ma CH, Wu CH, Yen CY and
Tu YK: Comparison of Dynesys posterior stabilization and posterior
lumbar interbody fusion for spinal stenosis L4L5. Acta Orthop Belg.
78:230–239. 2012.PubMed/NCBI
|
|
13
|
Kong LD, Meng LC, Wang LF, Shen Y, Wang P
and Shang ZK: Evaluation of conservative treatment and timing of
surgical intervention for mild forms of cervical spondylotic
myelopathy. Exp Ther Med. 6:852–856. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berg S and Tullberg T: Letter to the
editor regarding Mannion, Brox, Fairbank. Comparison of spinal
fusion and nonoperative treatment in patients with chronic low back
pain: Long-term follow-up of three randomized controlled trials.
Spine J. 14:10872014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vieweg U and Sola S: Posterior lumbar
interbody fusion with an interbody fusion spacer or cageManual of
Spine Surgery. Vieweg U and Grochulla F: Springer; Berlin,
Heidelberg: pp. 377–384. 2012, https://doi.org/10.1007/978-3-642-22682-3_53
View Article : Google Scholar
|
|
16
|
Lee JC, Kim Y, Soh JW and Shin BJ: Risk
factors of adjacent segment disease requiring surgery after lumbar
spinal fusion: Comparison of posterior lumbar interbody fusion and
posterolateral fusion. Spine. 39:E339–E345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ong KL, Auerbach JD, Lau E, Schmier J and
Ochoa JA: Perioperative outcomes, complications, and costs
associated with lumbar spinal fusion in older patients with spinal
stenosis and spondylolisthesis. Neurosurg Focus. 36:E52014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu ZJ, Fang XQ, Zhou ZJ, Wang JY, Zhao FD
and Fan SW: Effect and possible mechanism of muscle-splitting
approach on multifidus muscle injury and atrophy after posterior
lumbar spine surgery. J Bone Joint Surg Am. 95(e192): 1–9.
2013.PubMed/NCBI
|
|
19
|
Ma C, Wu JB, Zhao M, Dai WX, Wu DH, Wang
ZH, Feng J, Liu C, Zhao QH and Tian JW: Posterior interbody fusion
versus improved transforaminal lumbar interbody fusion in segmental
spinal fixation for aged spondylolisthesis with lumbar spinal canal
stenosis. Zhonghua Yi Xue Za Zhi. 92:620–623. 2012.(In Chinese).
PubMed/NCBI
|