Introduction
Although kidney transplantation is currently
considered as the preferred therapy for end-stage renal disease,
morbidity induced by the use of immunosuppressive regimens and
recipient-mediated immunological rejection remain the primary
challenge following transplantation (1). It has previously been demonstrated that
graft function may be affected by immunologic and non-immunologic
factors, including race, height, age, sex and weight (2). Although the application of
immunosuppressive agents is able to reduce pathological autoimmune
reactions, it may also interfere with normal immune response, thus
increasing the incidence of side effects, such as cardiovascular
diseases, infection, malignancies, diabetes and other metabolic
disorders (3,4). Therefore, the development of novel
immune tolerance protocols is required for transplantation, so that
the long-term immunosuppression induced by currently used agents
does not occur. However, the mechanisms associated with the
induction and maintenance of immune tolerance remain elusive. The
present case report presents one case of potential immunological
tolerance following kidney transplantation from a living donor and
transient immunosuppressive therapy.
Case report
A 58-year-old female patient with end-stage renal
disease secondary to chronic nephritis underwent living-donor
kidney transplantation at the 251st Hospital of People's Liberation
Army of China (Hebei, China) in February 2005. The kidney was
donated by the patient's sister with ABO compatibility (blood type
of donor and recipient were Type B) and human leukocyte antigen
(HLA) incompatibilities (2/6 mismatch of HLA typing). De novo
donor-specific antibodies were negative. Immunosuppression was
performed following transplantation via a standard method including
FK506 (0.1 mg/kg/day), mycophenolate mofetil (1,000 mg/kg/day) and
prednisone for five days (500 mg/kg/day), and normal graft function
was observed 3 days following transplantation. However, due to the
side effects of the immunosuppressive therapy, including
thoracalgia and diarrhea, 7 days following transplantation, the
patient refused to take mycophenolate mofetil capsules on the 8th
day of treatment and the dosage of FK506 was decreased to 2 mg per
day, which resulted in hypouresis, edema and ascites. Therefore,
the patient was obliged to start and maintain hemodialysis 40 days
following kidney transplantation.
Following hemodialysis for two months, the patient's
urine output increased to 500 ml/day; therefore, she stopped using
all immunosuppressive agents and subsequently stopped hemodialysis
five months later. Notably, graft function subsequently normalized
with a baseline serum creatinine level of 170–180 µmol/l (normal
range, 57–97 µmol/l), accompanied by urine output increasing to
1,200 ml/day (normal range, 1,000–3,000 ml/day) (5,6). In the
last decade, the patient's graft function has been relatively
stable, except for occasional body chills, joint pain (suspected
rheumatoid arthritis) and renal pain.
The patient first attended Beijing Chao-Yang
hospital (Beijing, China) for consultation as she often experienced
pain around the area of transplanted kidney in the last four years.
The medical history showed that her blood pressure was stable, at
~130/90 mmHg. She seldom had a fever, although laboratory tests
indicated the presence of urinary tract infection [white blood cell
(WBC) count, 287.7/µl; bacteria count, 18,996.1/µl]. The serum
creatinine level was 119.2 µmol/l and urea nitrogen was 8.66
µmol/l, whereas a high cystatin C level was observed (2.24 ng/l).
Negative results were obtained for panel reactive antibodies class
I and II screening, and 24-h urinary protein quantity was 88 mg.
The level of fasting blood-glucose was 5.46 mmol/l, whereas
glycated albumin and glycosylated hemoglobin were 16.7 and 7.2%,
respectively. Other laboratory findings, including cholesterol,
glutamic-oxalacetic transaminease, glutamic-pyruvic transaminase
and total bilirubin were normal.
T cells were isolated from peripheral blood
mononuclear cells and determined by flow cytometry. A total of
5×107/ml peripheral blood mononuclear cells (PBMCs) were
isolated from freshly heparinized whole blood by standard Ficoll
density gradient centrifugation (room temperature; 800 × g for 30
min; GE Healthcare, Chicago, IL, USA). To determine the frequency
and phenotype of myeloid derived suppressor cell; cluster of
differentiation (CD)4 cells, CD8 cells, T regulatory and nature
killer cells in PBMCs, multicolor fluorescence-activated cell
staining analysis was performed using the following antibodies
sourced from BD Pharmingen; BD Biosciences (Franklin Lakes, NJ,
USA): anti-CD3 (cat. no. 557832; 1:10), anti-CD4 (cat. no. 562281;
1:10), anti-CD8 (cat. no. 555366; 1:10), anti-CD14 (cat. no.
557832; 1:10), anti-HLA DR (cat. no. 555812; 1:10), anti-CD11b
(cat. no. 340937; 1:10), anti-CD56 (cat. no. 556647; 1:10),
anti-CD33 (cat. no. 555450; 1:10), anti-CD34 (cat. no. 550761;
1:10), anti-CD25 (cat. no. 560990; 1:10), anti-CD127 (cat. no.
557938; 1:10), anti-CD19 (cat. no. 555415; 1:10), anti-CD20 (cat.
no. 563780; 1:10) and anti-forkhead box P3 (FOXP3 1:10; cat. no.
77-5776-40; eBioscience; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). For surface marker staining, PBMCs were incubated with
the appropriate antibodies following three gentle washes with PBS.
For intracellular marker staining, PBMCs were washed with PBS, then
fixed and permeabilized with Cytofix/Cytoperm solution on ice for 1
h (BD Pharmingen; BD Biosciences), anti-FOXP3 antibodies were
utilized for intracellular staining by incubating with PBMCs at 4°C
for 30 min in the dark. Flow cytometry was performed using a
Beckman Coulter FC500 MPL (Beckman Coulter, Inc., Brea, CA, USA).
Analysis of FACS-data was completed using FlowJo software 7.6.3
(Tree Star, Inc., Ashland, OR, USA). Isotype-matched antibodies
were used with all the samples as controls. The CD3+ T cell count
was 1,054/µl (59.4%), CD3+CD4+ was 643/µl (33%) and CD3+CD8+ was
391/µl (22.1%). The proportions of regulatory B cells, T cells,
suppressor T cells and nature killer cells were 12.0, 3.0, 13.4 and
12.9%, respectively. The proportion of myeloid-derived suppressor
cells was 1%, which was within the normal range (1–2%) (7).
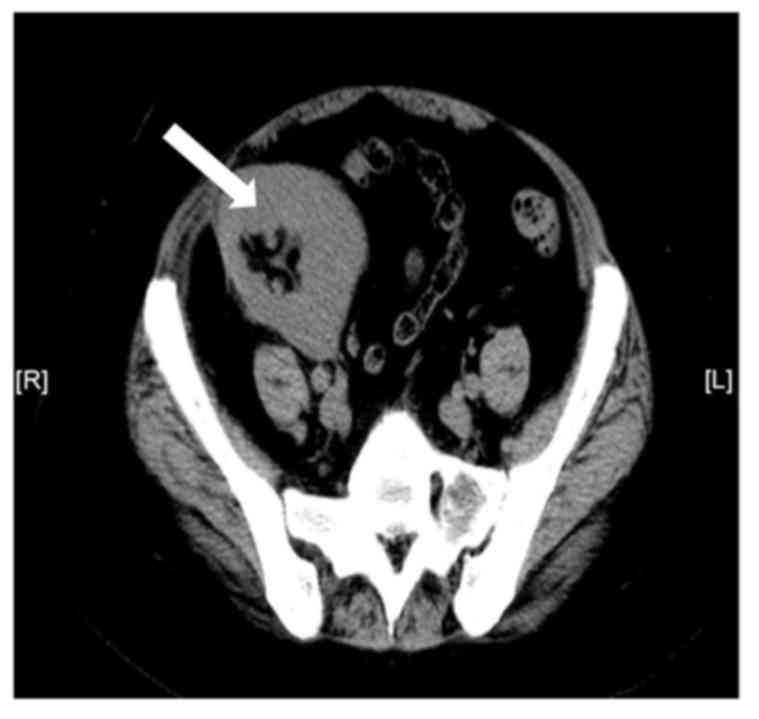
Spiral abdominal computed tomography revealed that
two native kidneys were atrophied and that the size, shape and
density of the transplanted kidney, which was located on the right
iliac fossa, showed no marked abnormalities (Fig. 1).
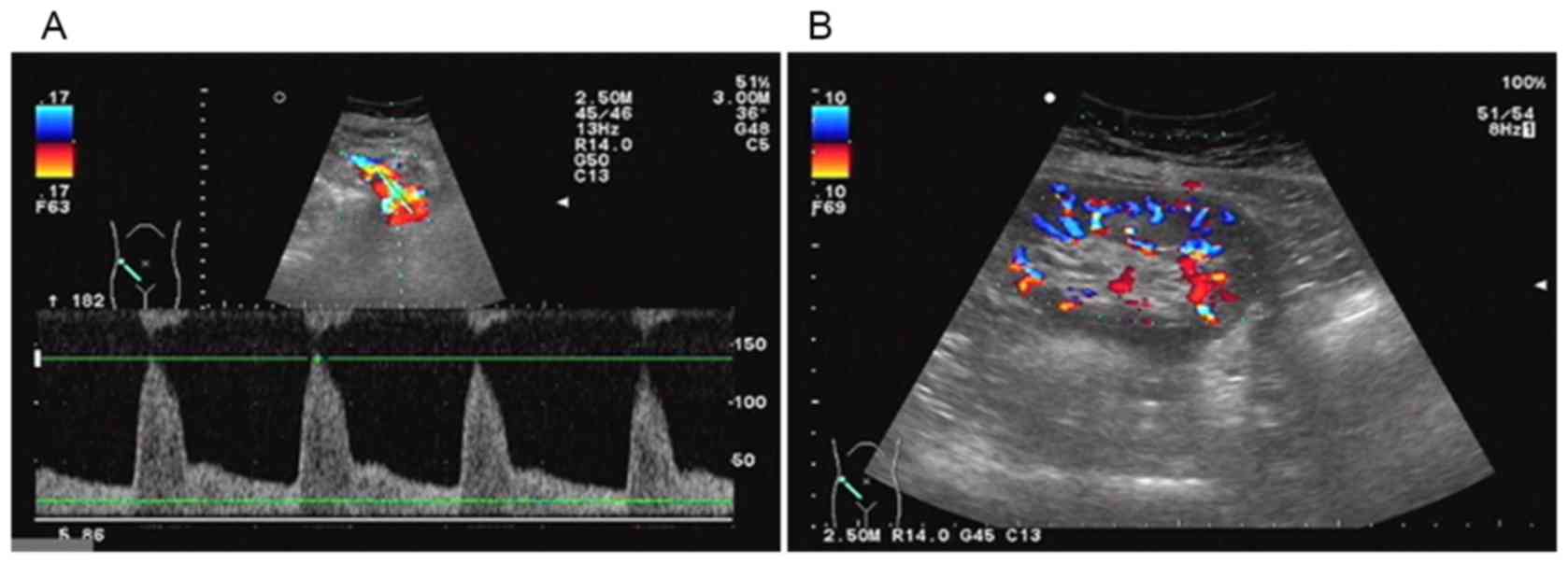
Color Doppler ultrasonography examination revealed
that the size of the transplanted kidney was 9.9×4.8 cm, the
thickness of kidney parenchyma was 1.6 cm, the blood supply of
transplanted kidney was good and there was no pelviectasis;
however, kidney artery blood flow resistance index had increased to
0.74–0.78 (Fig. 2).
Graft biopsy for this patient was performed twice by
nephrocentesis under guidance of ultrasound, as the initial
pathological findings were contrary to the patient's clinical
manifestation. The result revealed ischemic sclerosis in the
glomerulus, the kidney tubular epithelial cells were flat and a
number of kidney tubules were atrophied. Protein cast, cell cast
and inflammatory cell infiltration were also observed in some
kidney tubules. Kidney interstitial hyperplasia was also observed
(Fig. 3A). To further confirm the
pathological conditions of the transplanted kidney, graft biopsy
was conducted for the second time. The results demonstrated few
glomerulus ischemic sclerosis, partial renal tubular dilatation and
infiltration of inflammatory cells (Fig.
3B). The patient was in stable condition without other dominant
diseases.
The present study was approved by the Ethics
Committee of Beijing Chao-Yang Hospital (Capital Medical
University, Beijing, China). Written informed consent form was
obtained from the patient. All procedures performed were in
accordance with the ethical standards of the institutional and/or
national research committee and with the 1964 Helsinki declaration
and its later amendments or comparable ethical standards.
Discussion
Following the discovery of immunologic tolerance by
Ray Owen in 1945, who observed that placental interchange resulted
in red cell chimerism between dizygotic bovine twins (8), there has been a great effort to
establish tolerance through various different strategies. The
original definition of tolerance was provided by Billingham et
al in the 1950s (9), which
referred to non-responsiveness to antigens. At present, tolerance
is categorized in three subtypes: Central tolerance, which is
mediated by transplanted donor hematopoietic cells and concerns the
recognition of donor antigens as self-antigens; peripheral
tolerance, which is not inducted by hematopoetic cell
transplantation, but by either pharmacological immunosuppressive
agents or biological agent-led deletion or suppression of
self-reactive T cells; and operational tolerance, which is
typically observed in patients who stopped immunosuppressive
therapy for at least one year and in whom no destructive alloimmune
response was observed (10).
Notably, operational tolerance was more often observed following
organ transplantation. Although kidney transplant recipients were
prone to rejection following immunosuppressive withdrawal, >200
cases of operational tolerance persisting for at least one year
have been reported (11). Therefore,
elucidation of the operational tolerance-related molecular
mechanisms and biomarkers may identify novel targets for kidney
transplantation therapy.
The molecular basis of operational tolerance have
been analyzed by gene-expression profiling in kidney transplant
recipients, in which various genes, including tumor growth factor-β
in the blood of kidney transplant recipients, were demonstrated to
be minimally invasive monitoring tools for guiding operational
tolerance titration (12). In 25
operational tolerant kidney transplant recipients, 30
differentially-expressed genes were identified to be specific for B
cells compared with those patients receiving immunosuppression by
using peripheral blood microarrays (13). Increases of regulatory T cells were
found in the maintenance of immunologic tolerance by suppressing
aberrant or excessive immune responses (14). In addition, patients with operational
tolerance were also characterized by a higher number of B cells in
the blood compared with patients with stable graft function under
immunosuppression and patients with antibody-mediated chronic
rejection (15). Therefore,
strategies for operational tolerance including the pharmacological,
biological and cellular therapies were categorized by T cell
agents, B cell agents and cellular therapies. Krepsova et al
(16) reported that basiliximab
induction may result in an increase of regulatory T cells and
various biomarkers associated with operational tolerance
expression. Additionally, long-term allograft acceptance was
achieved by B cell depletion treatment in kidney transplant
recipients (17). Mixed chimerism,
defined as cells from different donor origins coexisting in the
same organism, is another form of tolerance. In 1995, Kawai et
al (18) developed a
non-myeloablative regimen that induced mixed chimerism and kidney
allograft tolerance in major histocompatibility complex-mismatched
cynomolgus monkeys. Further clinical studies have demonstrated that
operational tolerance may be achieved in HLA-mismatched recipients
by induction of transient chimerism via donor bone marrow
transplantation (19).
In conclusion, the present case report primarily
describes the immunologic tolerance observed in a kidney
transplantation recipient with minimal immunosuppressive agent
induction. In the present case, although the pathological
conditions of the transplanted kidney exhibited a degree of
impaired kidney function, T or B cell biomarkers associated with
immunologic tolerance were not observed; however but the patient
exhibited normal kidney function. This suggests that this patient
exhibited operational tolerance to some extent and that the
underlying mechanism may be mixed chimerism. This condition may
suggest that operational tolerance may be developed into a clinical
reality when appropriate treatment is administered; however, the
underlying mechanisms and biomarkers require further elucidation.
The clinical application of operational tolerance may improve the
prognoses of kidney transplant and other organ transplant
recipients following successful immunosuppression withdrawal, and
improve graft function and host defenses.
Acknowledgements
We would like to thank Dr. Hui Li from the
Department of Radiology (Beijing Chao-Yang Hospital, Capital
Medical University) for providing the CT images, Dr. Zexing Yu from
the Department of Ultrasound (Beijing Chao-Yang Hospital, Capital
Medical University) for providing the Doppler ultrasonography
images, Dr. Lei Jiang from the Department of Pathology (Beijing
Chao-Yang Hospital, Capital Medical University) for providing the
H&E staining images of the renopuncture biopsy.
Funding
The present study was supported by the Beijing
Municipal Administration of Hospitals Clinical Medicine Development
of Special Funding Support (grant no. ZYLX201408) and the China
Postdoctoral Science Foundation (grant no. 2015M581131).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SF contributed to the study design, collection and
interpretation of data, literature search, and drafting of the
manuscript. YZ contributed to the collection and interpretation of
data, and drafting of the manuscript. HL contributed to the study
design, interpretation of data, literature search and manuscript
review. XZ contributed to the interpretation of data, literature
search, manuscript review and collection of funds.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing Chao-Yang Hospital (Capital Medical
University, Beijing, China) and a written informed consent form was
obtained from the patient. All procedures performed were in
accordance with the ethical standards of the institutional and/or
national research committee and with the 1964 Helsinki declaration
and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
All authors have no conflict of interest to
declare.
References
|
1
|
Newell KA and Turka LA: Tolerance
signatures in transplant recipients. Curr Opin Organ Transplant.
20:400–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Głyda M, Czapiewski W, Karczewski M, Pięta
R and Oko A: Influence of donor and recipient gender as well as
selected factors on the five-year survival of kidney graft. Pol
Przegl Chir. 83:188–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marcén R: Immunosuppressive drugs in
kidney transplantation: Impact on patient survival, and incidence
of cardiovascular disease, malignancy and infection. Drugs.
69:2227–2243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marchetti P and Navalesi R: The metabolic
effects of cyclosporin and tacrolimus. J Endocrinol Invest.
23:482–490. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okonkwo IN, Ogbu II, Ijoma UN, Ulasi II
and Ijoma CK: Reference intervals for serum cystatin C and
creatinine of an indigenous adult Nigerian population. Niger J Clin
Pract. 18:173–177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luippold G, Benöhr P, Piesch C, Heyne N
and Mühlbauer B: Urinary dopamine excretion in healthy volunteers:
Effect of sodium diet and acute water load. Pflugers Arch.
440:28–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luan Y, Mosheir E, Menon MC, Wilson D,
Woytovich C, Ochando J and Murphy B: Monocytic myeloid-derived
suppressor cells accumulate in renal transplant patients and
mediate CD4(+) Foxp3(+) Treg expansion. Am J Transpl. 13:3123–3131.
2013. View Article : Google Scholar
|
|
8
|
Owen RD: Immunogenetic consequences of
vascular anastomoses between bovine twins. Science. 102:400–401.
1945. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Billingham RE, Brent L and Medawar PB:
Actively acquired tolerance of foreign cells. Nature. 172:603–606.
1953. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Girmanova E, Hruba P and Viklicky O:
Circulating biomarkers of tolerance. Transpl Rev (Orlando).
29:68–72. 2015. View Article : Google Scholar
|
|
11
|
Orlando G, Hematti P, Stratta RJ, Burke GW
III, Di Cocco P, Pisani F, Soker S and Wood K: Clinical operational
tolerance after renal transplantation: Current status and future
challenges. Ann Surg. 252:915–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brouard S, Mansfield E, Braud C, Li L,
Giral M, Hsieh SC, Baeten D, Zhang M, Ashton-Chess J, Braudeau C,
et al: Identification of a peripheral blood transcriptional
biomarker panel associated with operational renal allograft
tolerance. Proc Natl Acad Sci USA. 104:15448–15453. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heidt S and Wood KJ: Biomarkers of
operational tolerance in solid organ transplantation. Exp Opin Med
Diagn. 6:281–293. 2012. View Article : Google Scholar
|
|
14
|
Sakaguchi S: Regulatory T cells: Key
controllers of immunologic self-tolerance. Cell. 101:455–458. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Louis S, Braudeau C, Giral M, Dupont A,
Moizant F, Robillard N, Moreau A, Soulillou JP and Brouard S:
Contrasting CD25hiCD4+T cells/FOXP3 patterns in chronic rejection
and operational drug-free tolerance. Transplantation. 81:398–407.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krepsova E, Tycova I, Sekerkova A,
Wohlfahrt P, Hruba P, Striz I, Sawitzki B and Viklicky O: Effect of
induction therapy on the expression of molecular markers associated
with rejection and tolerance. BMC Nephrol. 16:1462015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kopchaliiska D, Zachary AA, Montgomery RA
and Leffell MS: Reconstitution of peripheral allospecific CD19+
B-cell subsets after B-lymphocyte depletion therapy in renal
transplant patients. Transplantation. 87:1394–1401. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawai T, Cosimi AB, Colvin RB, Powelson J,
Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M and Sachs DH:
Mixed allogeneic chimerism and renal allograft tolerance in
cynomolgus monkeys. Transplantation. 59:256–262. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawai T, Sachs DH, Sprangers B, Spitzer
TR, Saidman SL, Zorn E, Tolkoff-Rubin N, Preffer F, Crisalli K, Gao
B, et al: Long-term results in recipients of combined
HLA-mismatched kidney and bone marrow transplantation without
maintenance immunosuppression. Am J Transpl. 14:1599–1611. 2014.
View Article : Google Scholar
|