Introduction
Contrast-induced nephropathy (CIN) refers to acute
renal dysfunction caused by contrast agents and is the
third-leading in-hospital cause of acute renal injury (1). At present, no effective methods for
treating CIN are available, so that its prevention is essential.
Studies have indicated that stress hyperglycaemia is a stronger
predictor for the prognosis of patients with acute myocardial
infarction than a history of diabetes mellitus and should therefore
be considered during risk assessment (2). In patients with CIN after percutaneous
coronary intervention (PCI), hyperglycaemia tends to increase the
risk of infection and in-hospital mortality (3). Stolker et al (4) reported that hyperglycemia is associated
with contrast-induced kidney injury, while this association is
hardly tenable in diabetic populations. Perkan et al
(5) indicated that hyperglycemia on
admission is linked to an increased risk of CIN, particularly when
it occurs acutely. A study by Barbieri et al (6) indicated that elevated
glycated-haemoglobin but not glucose levels is an independent
factor associated with CIN among patients without diabetes
undergoing coronary angiography or PCI. This issue remains to be
widely debated, and no clear conclusions have been reached.
Therefore, the present study aimed to explore the effects of
hyperglycaemia and elevated glycosylated haemoglobin on CIN after
coronary angiography and their predictive value in patients
undergoing coronary angiography.
Materials and methods
Patients
The present study was a prospective single-centre
cohort study performed at Zhongda Hospital affiliated to Southeast
University (Nanjing, China; trial registration no.
ChiCTR-OOC-17011466). A total of 258 patients, including 172 males
and 86 females, who underwent coronary angiography between May 2017
and November 2017, were enrolled in a prospective, randomized
manner. The inclusion criteria were as follows: Age between 18 and
75 years, and planned coronary angiography due to chest tightness
or chest pain. The exclusion criteria were as follows: Allergy to
contrast agent; chronic renal insufficiency, stage-5 chronic kidney
disease (CKD), haemodialysis and renal transplantation; computed
tomography, magnetic resonance or other angiography within the
previous 2 weeks; acute renal insufficiency or intake of a
nephrotoxic drug within the previous 2 weeks; severe cardiac
insufficiency, severe valvular heart disease or haemodynamic
instability; and severe liver dysfunction, malignancy or severe
infectious disease.
The present study was approved by the Ethics
Committee of Zhongda Hospital (Nanjing, China) and all patients
provided written informed consent. The subjects were divided into a
CIN and a non-CIN group. The baseline information of the patients
is presented in Table I.
 | Table I.Baseline data and medical history of
258 patients undergoing coronary angiography stratified by the
presence of contrast-induced nephropathy. |
Table I.
Baseline data and medical history of
258 patients undergoing coronary angiography stratified by the
presence of contrast-induced nephropathy.
| Variables | Non-CIN group
(n=213) | CIN group (n=45) | P-value |
|---|
| Male gender | 145.000 (68.100) | 27.000 (60.000) | 0.296 |
| Age (years) |
66.670±10.490 |
69.330±11.080 | 0.045 |
| Height (cm) | 164.140±13.570 | 164.460±8.140 | 0.882 |
| Weight (kg) |
69.170±12.040 |
66.330±12.700 | 0.170 |
| SBP (mmHg) | 134.170±18.040 |
137.290±19.400 | 0.299 |
| DBP (mmHg) |
77.290±11.789 |
80.330±15.190 | 0.137 |
| BMI
(kg/m2) | 25.450±3.690 |
24.470±3.920 | 0.123 |
| Hypertension | 150.000 (70.400) | 31.000 (68.900) | 0.838 |
| Diabetes | 86.000
(53.300) | 24.000 (40.400) | 0.110 |
| Anemia | 0.000
(0.000) | 1.000 (2.200) | 0.174 |
| Hypotension | 0.000
(0.000) | 0.000 (0.000) | 1.000 |
| Chronic kidney
dysfunction | 9.000
(4.200) | 3.000 (6.700) | 0.444 |
| NYHA class | 1.850 | 1.820 | 0.844 |
| Previous myocardial
infarction | 43.000
(18.000) | 2.000
(10.500) | 0.409 |
| ACS | 34.000
(22.000) | 11.000 (16.300) | 0.344 |
| AMI | 25.000
(11.700) | 7.000
(15.600) | 0.480 |
| Emergency PCI | 4.000
(1.900) | 2.000 (4.400) | 0.281 |
| Atrial
fibrillation | 42.000
(17.900) | 3.000
(13.000) | 0.775 |
Evaluation of CIN and
hyperglycaemia
The pre- and post-operative serum creatinine levels
within 48–72 h after coronary angiography were assessed to evaluate
whether CIN occurred. On admission, blood glucose and glycosylated
haemoglobin (HbA1c) were determined, and the differences in
hyperglycaemia and elevated HbA1c were compared between the CIN and
non-CIN groups. Binary logistic regression analysis was performed
and the receiver operating characteristic (ROC) curve was drawn to
assess whether hyperglycaemia and elevated HbA1c are independent
risk factors for CIN, and thereby determine their predictive
value.
Observational indexes and evaluation
methods
Hyperglycaemia, elevated HbA1C, baseline data,
haematology indexes, medication history and the intraoperative
condition were compared between the CIN and non-CIN groups in order
to identify independent risk factors for CIN.
Definitions of hyperglycaemia,
elevated HbA1C and CIN
No exact criteria have been established to define
stress hyperglycaemia in China or other countries. The
international literature generally defines hyperglycaemia as blood
glucose >11.1 mmol/l on admission (7). Therefore, the patients of the present
study were divided into a hyperglycaemia group (blood glucose on
admission, >11.1 mmol/l; 84 cases) and a control group (blood
glucose on admission, ≤11.1 mmol/l). A reasonable HbA1c control
target is <7%. Therefore, HbA1c ≥7% may be used as a criterion
for type 2 diabetes patients who require clinical treatment or a
treatment adjustment. Patients with HbA1c ≥7% on admission were
considered as the elevated HbA1c group. CIN was diagnosed based on
an increase in serum creatinine by >25% or by 44.2 µmol/l within
48–72 h of coronary angiography after excluding other factors that
cause acute kidney injury. Accordingly, the patients were divided
into a non-CIN group (213 cases) and a CIN group (45 cases). The
CKD-Epidemiology Collaboration formula was used to calculate the
estimated glomerular filtration rate (eGFR) (8).
PCI and clinical medication
Using the Seldinger puncture method, the right
radial or right femoral artery was selected. If necessary, PCI was
performed. One of two types of contrast agent, iodopuron (a
non-ionic hypotonic contrast agent; Bayer AG, Leverkusen, Germany)
or iodixanol (a non-ionic isotonic contrast agent; GE Healthcare,
Little Chalfont, UK), was selected. Based on the individual
clinical condition, the use of drugs including aspirin,
renin-angiotensin converting enzyme inhibitors, angiotensin II
receptor antagonists, beta-blockers, calcium channel antagonists,
statins, diuretics, alprostadil and cardiac drugs, as well as
measures including hydration and intensive statin treatment were
adopted. For patients with chronic renal insufficiency or elderly
patients, the reduction of contrast agent use, hydration, the close
monitoring of renal function, and temporary blood purification
treatment was performed depending on the clinicians' decision based
on the patient's individual condition.
Statistical analysis
SPSS 19.0 statistical software (IBM Corp., Armonk,
NY, USA) was used for data analysis. The normally distributed
numerical data were expressed as the mean ± standard deviation and
compared using the independent-samples t-test. Non-normally
distributed numerical data were expressed as the median and 25–75
interquartile range and compared using the rank-sum test.
Classification data were expressed as numbers and percentages and
compared using the χ2 test. Binary logistic regression
analysis was used to assess the risk factors for CIN. The
predictive value of blood glucose for CIN was evaluated by
generating a ROC curve. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics in the CIN
and non-CIN groups
Table I compares the
baseline data and past history of the CIN and non-CIN patients.
Results demonstrated that CIN occurred in 45 patients (Table I). No significant differences in sex,
height, weight, body mass index, systolic or diastolic blood
pressure, diabetes, hypertension, acute myocardial infarction, New
York Heart Association class, emergency PCI, anemia, hypotension,
CKD, past myocardial infarction, atrial fibrillation or acute
coronary syndrome (ACS) were present between the CIN and non-CIN
groups (P>0.05). However, the age in the CIN group was
significantly higher than that in the non-CIN group (P=0.045).
Comparison of the haematological parameters
(Table II), drug use and
intraoperative conditions (Table
III) between the CIN and non-CIN groups revealed no significant
differences in white or red blood cell counts, Hb, platelet count,
alanine aminotransferase, aspartate transaminase, lactate
dehydrogenase, blood glucose, triglycerides, cholesterol
low-density lipoprotein, pre-operative serum urea nitrogen, as well
as the intake of aspirin, beta blockers, renin-angiotensin
converting enzyme inhibitor, angiotensin II receptor antagonist,
calcium channel blocker or renal protective drugs, and the dosage
of contrast agent, type of contrast agent, multiple vasculopathy,
multi-stent implantation, hydration and hydration dosage
(P>0.05). Pre-operative serum creatinine, total cholesterol,
pre-operative eGFR, hyperglycemia and elevated HbA1c in the CIN
group were significantly higher than those in the non-CIN group
(P=0.048, 0.026, 0.028, 0.028 and 0.022, respectively).
Hyperglycaemia was present in 84 of 258 (32.6%) patients upon
hospital admission and 69 (26.7%) patients exhibited elevated
HbA1c.
 | Table II.Comparison of the haematological
indexes between CIN and non-CIN groups. |
Table II.
Comparison of the haematological
indexes between CIN and non-CIN groups.
| Variables | Non-CIN group
(n=213) | CIN group (n=45) | P-values |
|---|
| WBC P50
(P25-P75) (109/l) | 6.440
(5.250–8.140) | 6.700
(5.400–8.900) | 0.425 |
| RBC
(1012/l) |
4.510±0.530 |
4.480±0.650 | 0.733 |
| Hb (g/l) | 137.340±15.540 | 135.280±19.350 | 0.450 |
| PLT P50
(P25-P75) (109/l) | 189.000
(154.000–231.000) | 184.000
(154.000–273.000) | 0.687 |
| ALB (g/l) | 40.350±4.560 | 39.380±4.670 | 0.197 |
| ALT P50
(P25-P75) (IU/l) | 19.000
(14.000–30.000) | 18.500
(13.750–31.000) | 0.506 |
| AST 18.5 (13.75–31)
(IU/l) | 20.000
(16.000–27.000) | 20.000
(16.000–27.750) | 0.645 |
| LDH P50
(P25-P75) (IU/l) | 182.000
(162.000–214.000) | 184.000
(152.250–236.000) | 0.641 |
| Glu P50
(P25-P75) (mmol/l) | 6.160
(5.130–8.530) | 7.760
(5.080–12.050) | 0.089 |
| TG P50
(P25-P75) (mmol/l) | 6.160
(8.530–12.610) | 1.330
(0.960–1.980) | 0.970 |
| TC P50
(P25-P75) (mmol/l) | 4.130
(3.390–4.990) | 4.470
(3.670–5.470) | 0.026 |
| LDL-C
P50 (P25-P75) (mmol/l) | 2.370
(1.830–2.950) | 2.5900
(2.140–3.160) | 0.090 |
| Pre-operative Scr
P50 (P25-P75) (µmol/l) | 80.000
(70.000–94.000) | 65.000
(55.000–83.500) | 0.048 |
| Pre-operative Bun
P50 (P25-P75) (mmol/l) | 5.800
(4.900–7.000) | 5.500
(4.500–6.350) | 0.167 |
| Pre-operative eGFR
P50 (P25-P75) (ml/min/1.73
m2) | 80.300
(65.600–92.700) | 91.700
(74.500–100.500) | 0.028 |
| Elevated HbA1c | 51.000
(23.944) | 18.000
(40.000) | 0.022 |
| Hyperglycaemia | 63.000
(29.577) | 21.000
(46.667) | 0.028 |
| Elevated HbA1c | 69 |
|
|
| Hyperglycaemia | 84 |
|
|
 | Table III.Comparison of drug use and
intraoperative situation between the CIN and non-CIN groups. |
Table III.
Comparison of drug use and
intraoperative situation between the CIN and non-CIN groups.
| Parameter | Non-CIN group
(n=213) | CIN group
(n=45) | P-values |
|---|
| Aspirin | 193.000
(90.600) | 41.000
(91.100) | 0.916 |
| Beta-blocker | 170.000
(79.800) | 36.000
(80.000) | 0.977 |
| ACEI | 115.000
(54.000) | 24.000
(53.300) | 0.936 |
| CCB | 86.000
(40.400) | 12.000
(26.700) | 0.085 |
| Statin | 205.000
(96.200) | 44.000
(97.800) | 0.610 |
| Renoprotective
drug | 7.000
(3.300) | 2.000 (4.400) | 0.658 |
| Digoxin | 4.000
(1.900) | 3 (6.700) | 0.072 |
| Spironolactone | 33.000
(15.500) | 11.000
(24.400) | 0.147 |
| Hydration | 163.000
(76.500) | 35.000
(77.800) | 0.857 |
| Hydration dosage
(ml) | 500.000 | 500.000 | 1.000 |
| Dosage of contrast
agent P50 (P25-P75) (ml) | 146.000
(135.000–163.000) | 158.000
(142.000–175.000) | 0.403 |
| Iodixanol | 153.000
(71.800) | 36.000
(80.000) | 0.261 |
| Multiple
vasculopathy | 107.000
(50.200) | 24.000
(53.300) | 0.706 |
| Multi-stent
implantation | 44.000
(20.700) | 6.000
(13.300) | 0.259 |
Binary logistic regression analysis of potential
risk factors for CIN revealed that age, pre-operative eGFR, total
cholesterol and hyperglycaemia are independent risk factors
[P=0.037, 0.024, 0.009 and 0.039, respectively; odds ratio
(OR)=1.075, 0.779, 1.500 and 2.815; 95% confidence interval (CI)
1.005–1.151, 0.628–0.869, 1.105–2.030 and 1.042–4.581; Table IV].
 | Table IV.Binary logistic regression analysis
of the risk factors for contrast-induced nephropathy after coronary
angiography. |
Table IV.
Binary logistic regression analysis
of the risk factors for contrast-induced nephropathy after coronary
angiography.
|
| Single-factor
analysis | Multiple logistic
regression |
|---|
|
|
|
|
|---|
| Variable | OR | 95% CI | P-values | OR | 95% CI | P-values |
|---|
| Age | 1.025 | 1.013–1.058 | 0.045 | 1.075 | 1.005–1.151 | 0.037 |
| Preoperative
Scr | 1.021 | 1.002–1.041 | 0.048 | 0.990 | 0.968–1.020 | 0.590 |
| Preoperative
eGFR | 0.984 | 0.969–1.000 | 0.028 | 0.779 | 0.628–0.869 | 0.024 |
| Elevated HbA1C | 2.206 | 1.108–4.393 | 0.022 | 1.240 | 0.843–1.450 | 0.630 |
| Cholesterol | 1.365 | 1.034–1.802 | 0.026 | 1.500 | 1.105–2.030 | 0.009 |
| Hyperglycaemia | 2.083 | 1.082–4.012 | 0.028 | 2.815 | 1.042–4.581 | 0.039 |
| ACS | 1.443 | 0.673–3.097 | 0.346 | 1.389 | 0.553–3.493 | 0.484 |
| Diabetes | 1.688 | 0.884–3.221 | 0.110 | 1.474 | 0.585–3.713 | 0.410 |
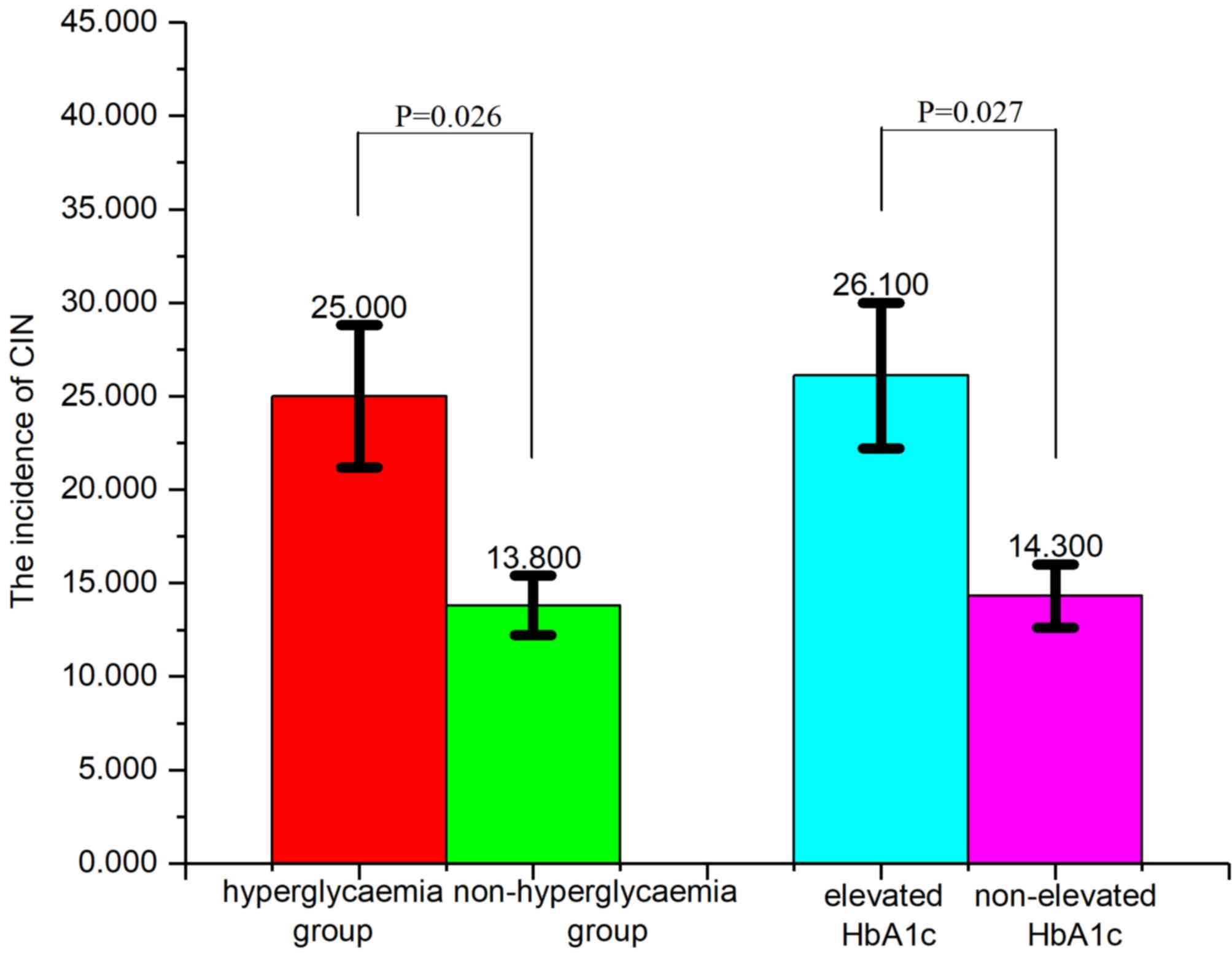
CIN incidence in different groups
The incidence of CIN in the hyperglycaemia group
(25.0%; blood glucose on admission, >11.1 mmol/l) was
significantly higher (P=0.026; Fig.
1) compared with the non-hyperglycemia group (13.8%; blood
glucose on admission, >11.1 mmol/l). Furthermore, the incidence
of CIN in the elevated HbA1c group (26.1%; HbA1c on admission,
upper limit of normal) was significantly higher (P=0.027; Fig. 1) compared with the group without
elevated HbA1c (14.3%).
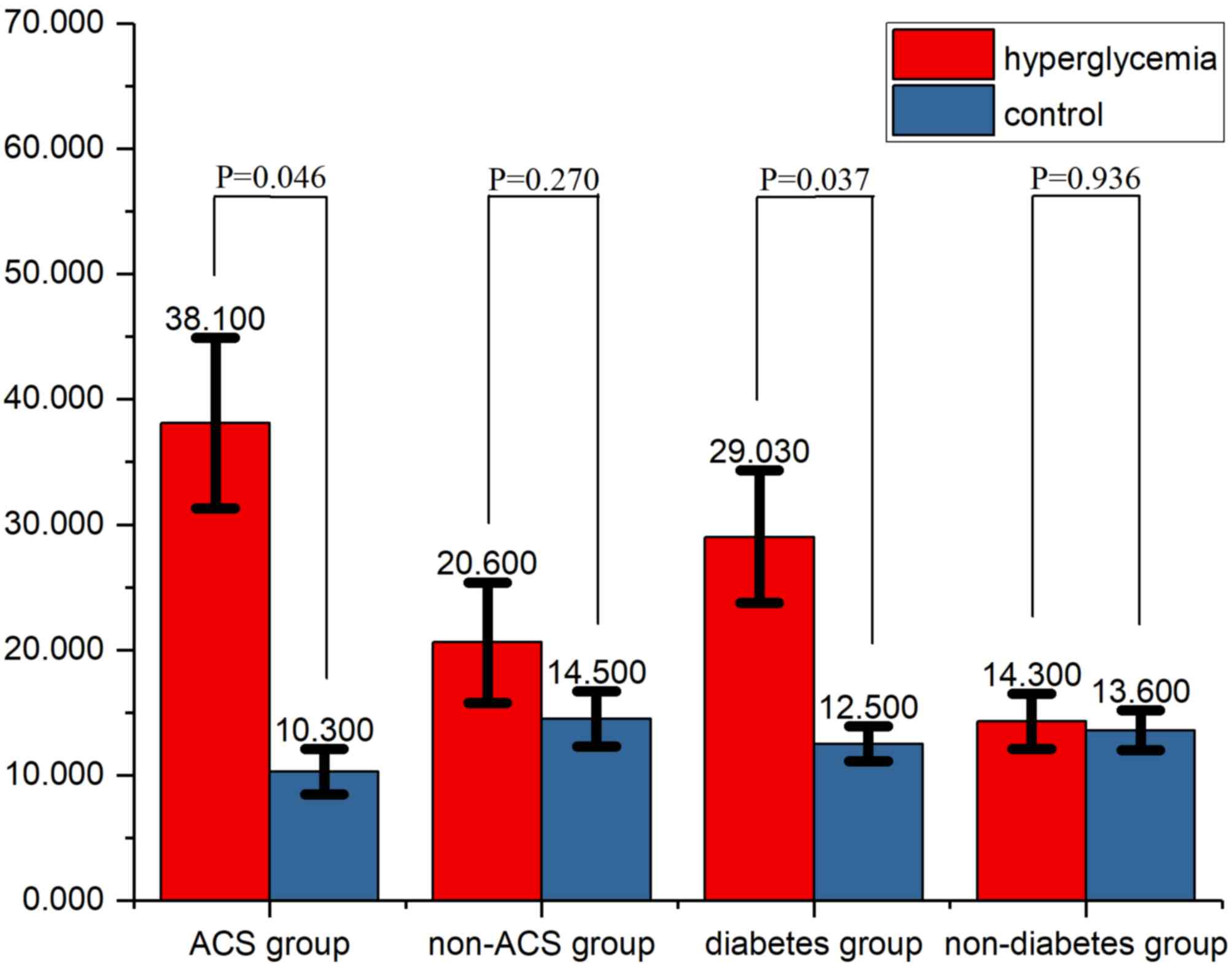
Subgroup analyses
In the ACS subgroup, the incidence of CIN was 38.1%.
The difference in the incidence of CIN between the hyperglycaemia
and control groups was significant in the ACS (P=0.046) and
diabetes (P=0.037) subgroups (Fig.
2), but not in the non-ACS or non-diabetic subgroups (P=0.270
and 0.936, respectively).
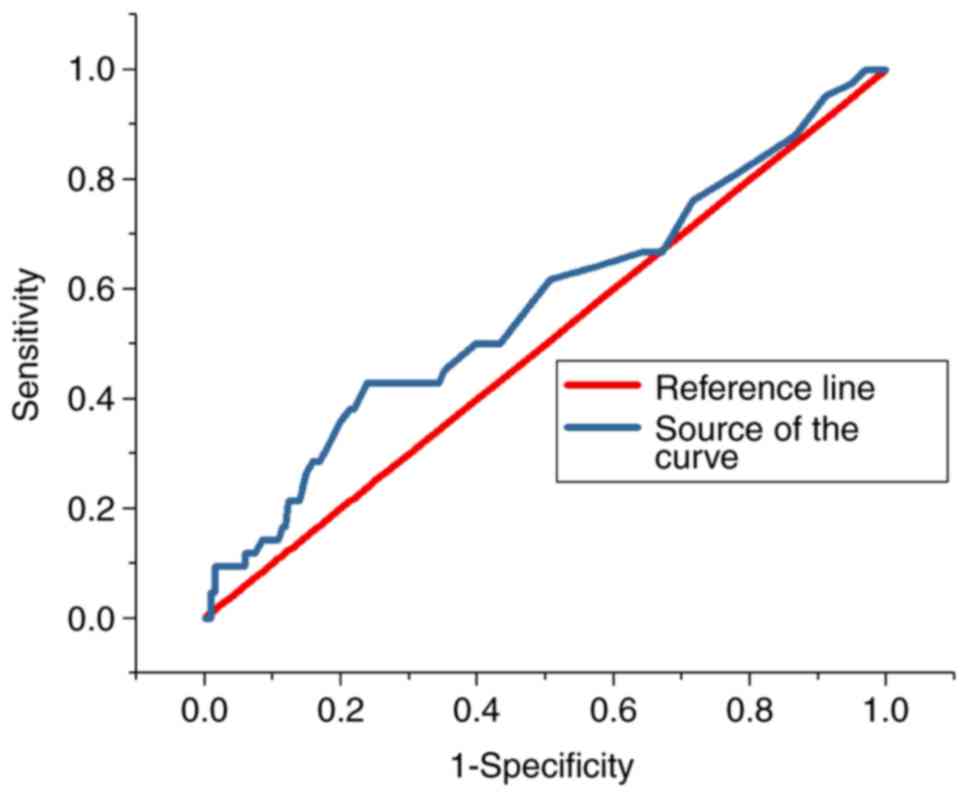
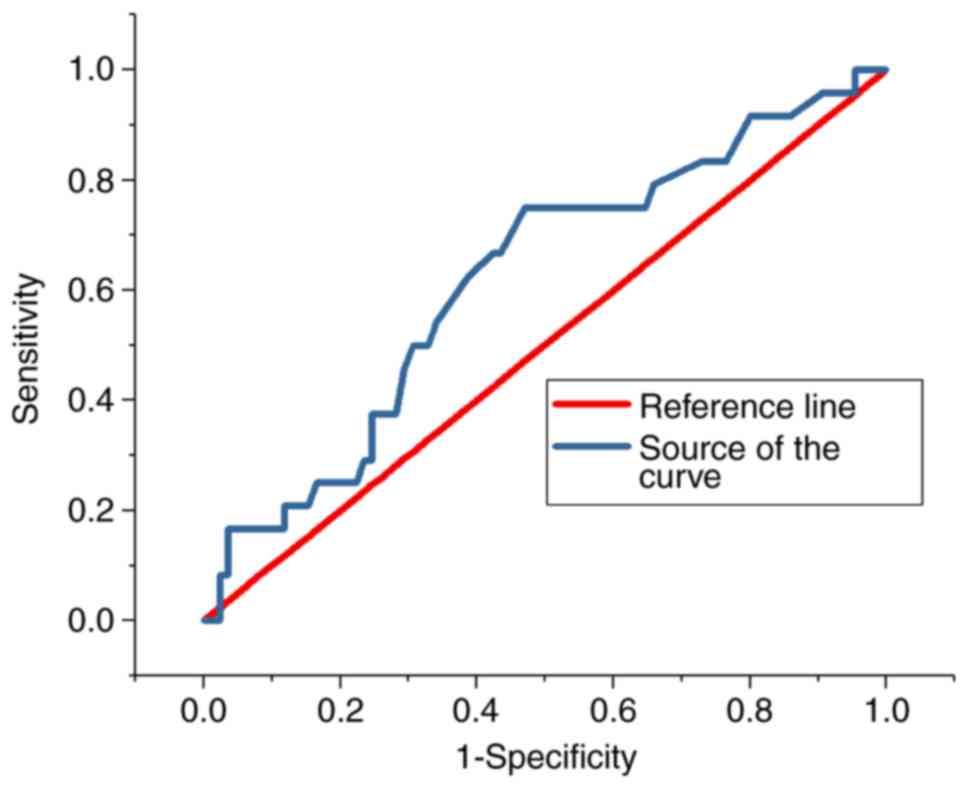
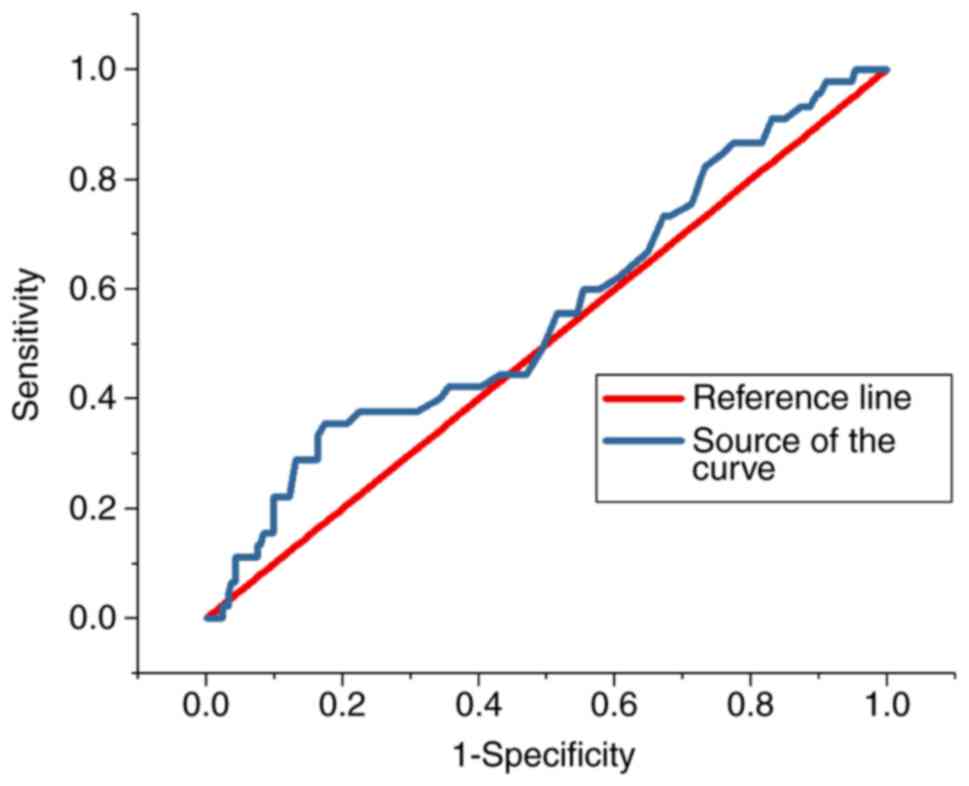
ROC analysis
The ROC curves for the predictive value of the blood
glucose levels for CIN for all patients and diabetes patients are
presented in Figs. 3 and 4, respectively; the areas under the curve
with 95% CIs were 0.569 (0.469–0.669) and 0.652 (0.427–0.876),
respectively. The ROC curves for the predictive value of
post-operative serum creatinine for CIN is presented in Fig. 5, and the area under the curve with
95% CI was 0.559 (0.427–0.618). The area under the curve value was
relatively poor, indicating that blood glucose levels do not
exhibit a strong predictive value for CIN; however, the area under
the curve value was stronger in patients with diabetes (Fig. 4).
Discussion
CIN is an acute renal injury caused by contrast
agents. It is the third major cause of acute in-hospital renal
injury and increases the burden on patients and society in terms of
hospitalisation time, all-cause mortality and kidney disease
morbidity (9). Therefore, CIN has
drawn the attention of radiologists, interventionists,
nephrologists and cardiovascular specialists.
Diabetic patients have twice the risk of
cardiovascular events of that of healthy individuals.
Cardiovascular disease is the leading cause of mortality in
diabetic patients, accounting for 75% of mortalities (10). A 20-year follow-up of the Whitehall
study demonstrated an association between hyperglycaemia and
cardiovascular events in non-diabetic populations (11). In 2010, a Chinese chronic disease
surveillance and diabetes investigation revealed that the
prevalence of diabetes in China was 11.6% (12). In diabetes patients with
cardiovascular disease who required coronary angiography or
percutaneous coronary intervention, diabetes is a risk factor for
acute renal injury after coronary angiography (13). Therefore, it is important to study
the correlation between blood glucose and CIN.
Stress hyperglycaemia usually refers to the
transient hyperglycaemia that occurs during a disease. The American
Diabetes Association consensus defined the concept of
hyperglycaemia as fasting glucose >6.9 mmol/l or random glucose
>11.1 mmol/l without any evidence of previous diabetes (7). This concept was used to define stress
hyperglycemia: Random glucose on admission, >11.1 mmol/l,
regardless of the patient's diabetes status.
Stress hyperglycaemia is widely recognised as a
predictive factor of poor prognosis in myocardial infarction
patients (14). It is also closely
associated with mortality and in-hospital complications (15,16).
Stress hyperglycaemia increases the risk of acute kidney injury and
is an independent risk factor for acute renal injury in acute
myocardial infarction patients. Furthermore, acute kidney injury is
correlated with in-hospital mortality (17). A study by Stolker et al
(4) comprising 6,358 non-diabetic
patients with acute myocardial infarction indicated that
hyperglycemia is associated with contrast-induced kidney injury,
the risk of CIN is dependent on hyperglycemia. In a presentation at
the European Society of Cardiology congress in 2013, Perkan et
al (5) reported that
hyperglycemia on admission is associated with an increased risk of
CIN, particularly when hyperglycemia occurs acutely. A study
including high-risk elderly patients with impaired renal function
indicated that the incidence of CIN is associated with diabetes
mellitus (18).
Due to hyperglycaemia leading to CIN, hyperglycaemia
may be a stress response phenomenon due to sympathetic nervous
system activation, which promotes cortisol and catecholamine
release, leading to ischemic injury of the renal outer medulla.
Hyperglycaemia increases the production of oxygen free radicals and
inhibits blood flow-mediated vascular dilation, leading to hypoxia
and ischemia. Acute hyperglycaemia may also act as an osmotic
diuretic, resulting in insufficient renal perfusion, which
increases the risk of CIN. Hyperglycaemic patients also develop
more metabolic abnormalities in comparison with non-hyperglycaemic
patients, resulting in elevated levels of inflammatory biomarkers.
These increase the systemic inflammatory response and the risk of
nephrotoxicity. Particularly in patients with acute myocardial
infarction, a variety of neurohormones, as well as immune and
inflammatory pathways are activated, leading to kidney damage
(3,19). Stress hyperglycaemia is also a
response to insulin deficiency, which is associated with increased
circulating free fatty acids, causing kidney injury. In brief,
contrast medium and an elevated glucose levels have a synergistic
effect on CIN (20).
HbA1c is a product of Hb and blood glucose. It more
accurately reflects long-term glycaemic control with blood glucose
and helps to identify undiagnosed diabetic patients (21). A study by Barbieri et al
(6) indicated that among patients
without diabetes undergoing coronary angiography or PCI, elevated
HbA1c but not glucose levels is a factor independently associated
with CIN, and the adjusted OR with 95% CI was 1.69 (1.14–2.51).
In the present study, the subjects were divided into
hyperglycaemia and control groups using a cut-off of 11.1 mmol/l.
The association between hyperglycaemia and the incidence of CIN was
significant. This association was confirmed in the diabetes and ACS
subgroups. However, in patients with stable coronary heart disease
and patients without diabetes, the incidence of CIN was not
significantly associated with hyperglycaemia. The reason may be
that hyperglycaemia was not obvious in the patients without ACS.
The percentage of patients with unstable angina, non-ST-elevation
myocardial infarction or ST-elevation myocardial infarction was
only 19.4% (50 patients). The binary logistic regression analysis
indicated that age, pre-operative eGFR, total cholesterol and
hyperglycemia were independent risk factors for CIN after
regression analysis was performed on patients with ACS and
diabetes. The area under the curve value of pre-operative
creatinine for CIN was 0.559, indicating that blood glucose is
rather poor at predicting CIN, but is more effective when compared
with serum creatinine. This may be due to the following reasons:
When pre-operative serum creatinine levels are low, post-operative
creatinine levels easily rise by >25% of the pre-operative
level, and it is therefore easy to achieve the diagnostic criteria
for CIN. Furthermore, when basal serum creatinine is high,
considering that chronic renal insufficiency may be present, the
probability of CIN is high. Therefore, it is inappropriate to use
pre-operative serum creatinine or eGFR to predict the probability
of CIN. The Chinese type 2 diabetes prevention and treatment
guidelines (2013 version) state that HbA1c is one of the major
indicators of blood glucose control in the long-term (22); for most non-pregnant adults with type
2 diabetes, a reasonable HbA1c control target is <7%. HbA1c ≥7%
may be used as the level at which type 2 diabetes patients need to
start clinical treatment or adjust their treatment. The results of
the present study suggested that the incidence of CIN was
significantly higher in the high HbA1c group than in the control
group, but the binary logistic regression analysis indicated that
HbA1c was not an independent risk factor for CIN. Larger studies
are required to confirm this association.
The results of the present study indicated that the
incidence of CIN is as high as 17%. At present, no specific methods
are available for the prevention or treatment of CIN. Commonly used
measures include minimising the amount of contrast agent,
hydration, using a pre-heated isotonic contrast agent and stopping
the use of nephrotoxic drugs, including non-steroidal
anti-inflammatory drugs, cyclosporine and aminoglycoside
antibiotics prior to angiography (23). In addition, a meta-analysis suggested
that low-dose N-acetylcysteine and statins had a significant effect
on reducing the incidence of CIN (24).
In conclusion, the present study suggested that in
patients undergoing coronary angiography, acute hyperglycaemia is
closely associated with the risk of CIN, particularly in ACS and
diabetes patients. Further research is required to evaluate the
association between CIN and HbA1c.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81600227).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
YQ and CM made substantial contributions to the
conception of the study and acquisition of data. YQ was responsible
for the statistic analysis of data and was a major contributor in
writing the manuscript. GY designed the clinical trial, confirmed
the specific procedure of the trial and gave advice on the specific
parameters for this study. CT and GM helped design the trial,
contributed in the interpretation of data and revised the
manuscript critically for important intellectual content. All
authors read and approved the final manuscript.
Ethical approval and consent to
participate
This study was approved by the Ethics Committee of
Zhongda Hospital (Nanjing, China) and all patients provided
informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
James MT, Samuel SM, Manning MA, Tonelli
M, Ghali WA, Faris P, Knudtson ML, Pannu N and Hemmelgarn BR:
Contrast-induced acute kidney injury and risk of adverse clinical
outcomes after coronary angiography: A systematic review and
meta-analysis. Circ Cardiovasc Interv. 6:37–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goyal A, Mehta SR, Gerstein HC, Díaz R,
Afzal R, Xavier D, Zhu J, Pais P, Lisheng L, Kazmi KA, et al:
Glucose levels compared with diabetes history in the risk
assessment of patients with acute myocardial infarction. Am Heart
J. 157:763–770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marenzi G, De Metrio M, Rubino M, Lauri G,
Cavallero A, Assanelli E, Grazi M, Moltrasio M, Marana I,
Campodonico J, et al: Acute hyperglycemia and contrast-induced
nephropathy in primary percutaneous coronary intervention. Am Heart
J. 160:1170–1177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stolker JM, McCullough PA, Rao S, Inzucchi
SE, Spertus JA, Maddox TM, Masoudi FA, Xiao L and Kosiborod M:
Pre-procedural glucose levels and the risk for contrast-induced
acute kidney injury in patients undergoing coronary angiography. J
Am Coll Cardiol. 55:1433–1440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perkan A, Cinquetti M, Giannini F,
Santangelo S, Pirozzi F, Barbati G, Vitrella G, Rakar S, Salvi A
and Sinagra G: Admission hyperglycemia and contrast induced
nephropathy. Eur Heart J. 34:555. 2013. View Article : Google Scholar
|
|
6
|
Barbieri L, Verdoia M, Schaffer A,
Cassetti E, Di Giovine G, Marino P, Suryapranata H and De Luca G:
Pre-diabetes and the risk of contrast induced nephropathy in
patients undergoing coronary angiography or percutaneous
intervention. Diabetes Res Clin Pract. 106:458–464. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dungan KM, Braithwaite SS and Preiser J-C:
Stress hyperglycaemia. Lancet. 373:1798–1807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene
T, et al: A new equation to estimate glomerular filtration rate.
Ann Intern Med. 150:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katsiki N, Athyros VG, Karagiannis A and
Mikhailidis DP: Contrast-induced nephropathy: An ‘All or None’
phenomenon? Angiology. 66:508–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Selvin E, Marinopoulos S, Berkenblit G,
Rami T, Brancati FL, Powe NR and Golden SH: Meta-analysis:
Glycosylated hemoglobin and cardiovascular disease in diabetes
mellitus. Ann Internal Med. 141:421–431. 2004. View Article : Google Scholar
|
|
11
|
Balkau B, Shipley M, Jarrett RJ, Pyorala
K, Pyörälä K, Pyörälä M, Forhan A and Eschwège E: High blood
glucose concentration is a risk factor for mortality in middle-aged
nondiabetic men. 20-year follow-up in the Whitehall Study, the
Paris Prospective Study, and the Helsinki Policemen Study. Diabetes
Care. 21:360–367. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCullough PA, Wolyn R, Rocher LL, Levin
RN and Oneill WW: Acute renal failure after coronary intervention:
Incidence, risk factors, and relationship to mortality. Am J Med.
103:368–375. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Norhammar A, Tenerz Å, Nilsson G, Hamsten
A, Efendíc S, Rydén L and Malmberg K: Glucose metabolism in
patients with acute myocardial infarction and no previous diagnosis
of diabetes mellitus: A prospective study. Lancet. 359:2140–2144.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naruse H, Ishii J, Hashimoto T, Kawai T,
Hattori K, Okumura M, Motoyama S, Matsui S, Tanaka I, Izawa H, et
al: Pre-procedural glucose levels and the risk for contrast-induced
acute kidney injury in patients undergoing emergency coronary
intervention. Circ J. 76:1848–1855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Allison SP, Tomlin PJ and Chamberlain MJ:
Some effects of anaesthesia and surgery on carbohydrate and fat
metabolism. Br J Anaesth. 41:588–593. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shacham Y, Steinvil A and Arbel Y: Acute
kidney injury among ST elevation myocardial infarction patients
treated by primary percutaneous coronary intervention: A
multifactorial entity. J Nephrol. 29:169–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park KW, Koo BK, Kim HL, Kim YS, Jo SH,
Lee HY, Kang HJ, Cho YS, Chung WY, Yeon TJ, et al: The incidence
and predictors of contrast-induced nephropathy in adequately
hydrated elderly patients with impaired renal function. Nephrol
Dial Transplant. 22:1794–1795. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Persson PB and Tepel M: Contrast
medium-induced nephropathy: The pathophysiology. Kidney Int Suppl.
S8–S10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Donnell DH, Moloney MA, Bouchier-Hayes
DJ and Lee MJ: Contrast-induced nephrotoxicity: Possible
synergistic effect of stress hyperglycemia. AJR Am J Roentgenol.
195:W45–W49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldstein DE, Little RR, Lorenz RA, Malone
JI, Nathan DM and Peterson CM: American Diabetes Association: Tests
of glycemia in diabetes. Diabetes Care. 27 Suppl 1:S91–S93. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chinese Diabetes Society, . China
Guideline For Type 2 Diabetes (2013 Edition). Chin J Diabet
Mellitus. 6:447–498. 2014.(In Chinese).
|
|
23
|
Azzalini L, Spagnoli V and Ly HQ:
Contrast-induced nephropathy: From pathophysiology to preventive
strategies. Can J Cardiol. 32:247–255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Subramaniam RM, Suarez-Cuervo C, Wilson
RF, Turban S, Zhang A, Sherrod C, Aboagye J, Eng J, Choi MJ,
Hutfless S and Bass EB: Effectiveness of prevention strategies for
contrast-induced nephropathy: A systematic review and
meta-analysis. Ann Intern Med. 164:406–416. 2016. View Article : Google Scholar : PubMed/NCBI
|