Introduction
Hypothyroidism, also known as underactive thyroid
disease, is a typical endocrine disorder of the thyroid gland that
is caused due to inadequate quantities of thyroid hormones such as
thyroxine (T4) and triiodothyronine (T3). The common symptoms of
hypothyroidism are tiredness, weight gain, constipation, aches, dry
skin, dry hair and feeling cold, accompanied by a low metabolism.
The most common cause of hypothyroidism is Hashimoto's thyroiditis,
an autoimmune disorder. The thyroid gland controls the body's
energy metabolism, which affects the body temperature, heartbeat,
and calorie burning. The thyroid hormones have wide effects on
homeostasis and play an important role in the balance of the
cardiovascular system. Thus, patients with hypothyroidism have an
increased risk of cardiovascular abnormalities such as accelerated
atherosclerosis (1–3). For hypothyroidism treatment, a
synthetic thyroid hormone T4, L-Thyroxine (LT4) has been prescribed
as a first treatment regimen, but thyroid replacement hormones are
usually well tolerated. Symptoms that occur during treatment are
often due to toxic, elevated levels of thyroid hormones and
resulting the symptoms from hyperthyroidism.
Traditional medicines, including traditional Chinese
medicine (TCM) and traditional Korean medicine (TKM), regard the
treatment of both hyperthyroidism and hypothyroidism as concepts of
Yin/Yang imbalance. When treating either condition, acupuncture,
herbal medicine, and dietary therapy are typically employed to
rebalance an individual's imbalance of Yin and Yang. According to
the World Health Organization (WHO), acupuncture can be used to
treat thyroid diseases, and several studies have suggested that
acupuncture can be beneficial in treating hypothyroidism. Although
acupuncture is popularly applied in many countries for the
treatment of various disorders, the scientific evidence of safety
and efficacy is still an important issue that deserves close
attention. Pharmacopuncture therapy, a new form of acupuncture
treatment in TKM, is a stimulating method on acupoints with the
injection of herbal medicines that are frequently used for the
regulation of immune balance in clinical settings (4,5). MOK is
a polyherbal medicine consisting of 10 herbs and is commonly used
for pharmacopuncture treatment of thyroid syndromes such as
hypothyroidism, hyperthyroidism, and heart diseases in Korean
clinics (5,6). MOK has been reported to exhibit
anti-inflammatory activity, antioxidant effects (7,8), and
modulation of Th1/Th2 immune response (9) in in vitro studies and exert
clinical effects on Hwa-Byung (6)
which is known to cause of thyroid syndromes (5,10).
However, it has still little scientific evidence.
Therefore, in this study, we investigated the
effects of acupuncture with MOK (MOK pharmacopuncture) on
Propylthiouracil (PTU)-induced hypothyroidism in rats and studies
the mechanism underlying the anti-hypothyroidism effects of MOK
pharmacopuncture, with a focus on antioxidation and Th1/Th2 immune
regulation.
Materials and methods
Preparation of MOK extract
MOK consists of 10 herbs (Table I). All raw materials of MOK were
purchased from herbal materials company (Jayeondameun, Yangju,
Korea), and authenticated by the Korean Food and Drug
Administration (KFDA). Their voucher specimens (KIPA-MOK01~10) were
deposited at the Korea Immuno-Pharmacopuncture Association (KIPA,
Seoul, Korea). MOK extract was manufactured under a good
manufacturing practice (GMP)-compliant facility (7). Therefore, MOK was extracted with dried
ten herbs (106.2 g) in distilled water (1 L), mixed with alcohol in
a ratio of 1:1 (v/v), filtered through a two-layer mesh, and
adjusted pH 7.2 to 7.6 with NaOH for making a 0.9% isotonic
solution. This solution was concentrated under vacuum pressure, and
freeze-dried (the yield of 53.1 mg/ml). MOK was stored at 4°C until
use, at which time it was dissolved in sterilized water.
 | Table I.Constituents of MOK extract. |
Table I.
Constituents of MOK extract.
| No. of
KIPA-MOK | Herbal name (part
of medicinal use) | Scientific
name | Ratio (g) | Standard
compoundsa |
|---|
| 01 | Hominis Placenta
(placenta) | Hominis
placenta | 4 | Alanine,
luecine |
| 02 | Moschus (bear's
gall) |
Moschusberezovskii | 1 | Muscone |
| 03 | FelUrsi (musk) | Ursusarctos | 0.6 | Ursodeoxycholic
acid |
| 04 | Calculus Bovis Cow
bezoar (cow gallstone) | Bostaurus | 0.6 | Bilirubin |
| 05 | Scutellariae Radix
(root) |
Scutellariabaicalensis | 20 | Baicalein |
| 06 | Phellodendri Cortex
(bark) |
Phellodendronamurense | 20 |
Berberinechloride |
| 07 | PulsatillaKoreana
(root) |
Pulsatillakoreana | 20 | Anemonin,
saponin |
| 08 |
SophoraeSubprostratae Radix (root) |
Sophoratonkinensis | 20 | Oxymatrine |
| 09 | Aucklandiae Radix
(root) |
Aucklandialappa | 10 | Dehydrocostus
lactone |
| 10 | Aquilariaagallocha
(bark) |
Aquilariaagallocha | 10 | Tannic acid |
Experimental animals
Male Sprague-Dawley (SD) rats, aged five weeks, were
purchased from SLC, Inc. (Shizuoka, Japan). All animals received
food and water ad libitum and were housed under standard
laboratory conditions at an ambient temperature of 22±3°C with
humidity of 60±5% under a daily 12/12 h light/dark schedule. All
animals were handled according to the Animal Welfare Guidelines
issued by the Korean National Institute of Health and the Korean
Academy of Medical Sciences for the care and use of laboratory
animals. This study was conducted with the approval of the
Institutional Animal Care and Use Commitee of Dongguk University
(IACUC; No. 130387).
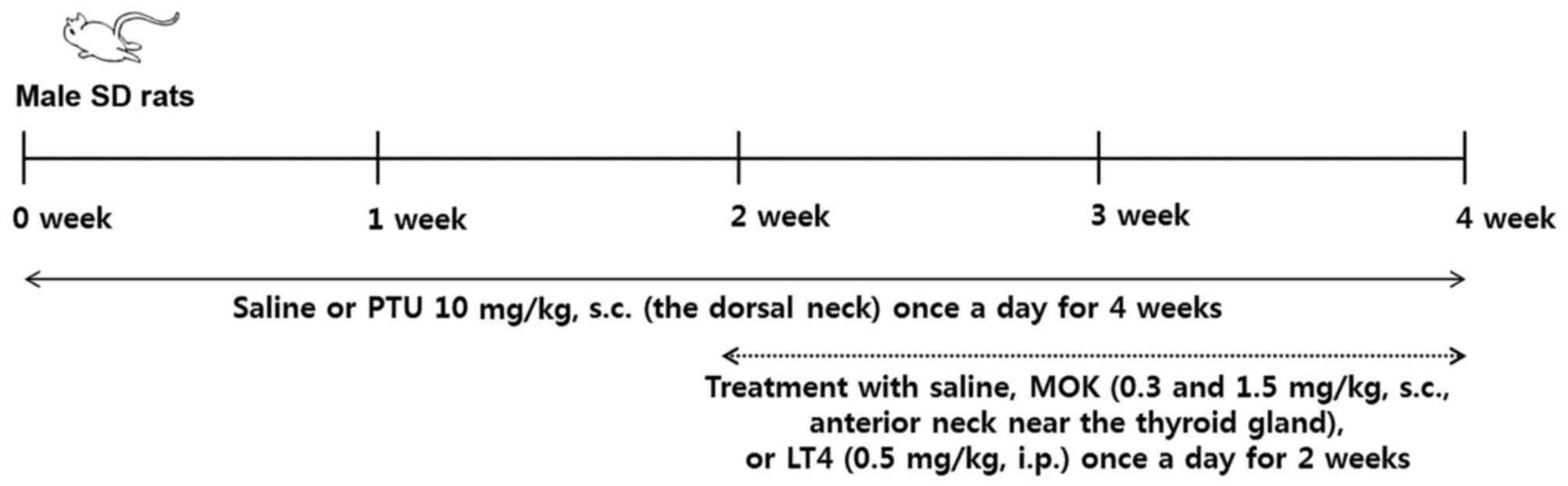
Induction of hypothyroidism
For the induction of hypothyroidism, we used the
method based on previous reports (11–13) with
minor modification (Fig. 1). PTU
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 10 mg/kg/body
weight (BW) was dissolved in 0.3 ml saline, and the rats were given
a daily subcutaneous injection of PTU into the dorsal neck for 28
days. In normal rats, saline was subcutaneously injected at a
volume of 0.3 ml/animal, instead of PTU. Two weeks later, MOK
pharmacopuncture at 0.3 and 1.5 mg/kg was administered
subcutaneously into the anterior neck near the thyroid gland at a
volume of 0.15 ml/animal; the compound was dissolved in saline and
administered once daily from day 15 to day 28 after the induction
of hypothyroidism. The rats in the control group were injected with
an equal volume of saline by the same method. LT4 at 0.5 mg/kg
(Sigma-Aldrich; Merck KGaA) was used as a reference drug. The rats
were randomly divided into four groups of five animals each: normal
group (Normal), PTU-induced hypothyroidism control group
(PTU+Vehicle), MOK pharmacopuncture 0.3 ml-treated group (PTU+Low
MOK), MOK pharmacopuncture 1.5 ml-treated group (PTU+High MOK), and
LT-administered group (PTU+LT4).
Measurement of BW and food and water
intake
All animals were observed daily for clinical signs
for 4 weeks from the first injection day. The BW and food
consumption of each rat were measured at the initiation of
treatment and once a week during the treatment period. The amounts
of food and water intake were averaged every week during the
treatment period.
Measurement of body temperature
Rectal temperature was measured once a week in all
animals using a Thermalert TH-8 (Physitemp Instruments, Clifton,
NJ, USA) monitor with a (RET-2) rectal probe attached to the
thermocouple. White petrolatum (Gallipot, St. Paul, MN, USA) was
applied to the probe before insertion. The probe was inserted 3 cm
into the rectum while the rat was gently restrained. A steady
readout was obtained within 30 s of probe insertion.
Serological analysis
Blood samples were collected by cardiac puncture
under isoflurane (1.5 to 3.0%) anesthesia, and the rats were
sacrificed on day 36 after the primary immunization. Blood was
clotted for 2 h at room temperature (RT) and centrifuged at 5,000 ×
g for 10 min at 4°C to obtain serum.
The levels of thyroid-stimulating hormone (TSH), T3,
and T4 were measured in the sera of rats using commercially
available enzyme-linked immunosorbent assay (ELISA) kits according
to the manufacturer's recommendations (Cusabio, Wuhan, China). The
concentration of each hormone was calculated from the standard
curve for each hormone in the ELISA kits. Serum aspartate
transaminase (AST), alanine transaminase (ALT), total cholesterol,
HDL-cholesterol, LDL-cholesterol, triglyceride (TG), and glucose
levels were measured with an automated blood analyzer (FDC7000i;
Fujifilm Corporation, Tokyo, Japan)) and an ELISA reader (ASYS
Hitech GmbH, Eugendorf, Austria).
Histological analysis
On day 36, all rats were sacrificed by anesthesia
after serum collection. Thyroid tissues were removed from the mice
for histological examination. Thyroid tissues were fixed in 4%
paraformaldehyde solution, decalcified with Calci-Clear Rapid
(National Diagnostics, Atlanta, GA, USA), embedded in paraffin, and
longitudinally cut into 5-µm serial sections. The sections were
then stained with hematoxylin and eosin (H&E) to assess
morphological changes of the thyroid glands. To observe
histopathological changes in more detail, the mean thyroid
follicular sizes were calculated using ImageJ [National Institutes
of Health (NIH), Bethesda, MD, USA].
Western blot analysis
To investigate the effects of MOK pharmacopuncture
on the oxidation of liver, heart, and brain tissues, as well as
expressions of the transient receptor potential cation channel
subfamily V member 1 (TRPV1) protein in dorsal root ganglion (DRG)
and brain tissues, we conducted western blot analysis. Briefly,
livers, brains, and DRG tissues were harvested from each group,
minced, and homogenized with an electric homogenizer in 5 volumes
of extraction buffer (100 mM Tris, pH 7.4, 150 mM sodium chloride
(NaCl), 1 mM ethylene glycol-bis (β-aminoethyl
ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1 mM ethylenediamine
tetraacetic acid (EDTA), 1% Triton X-100, and 0.5% sodium
deoxycholate). The tissue lysates were placed on a shaker at 4°C
for 1 h and centrifuged at 10,000 × g for 5 min. Protein
concentrations were determined by the Bradford assay (Bio-Rad,
Hemel Hempstead, UK). A total of 30 µg/ml of protein was separated
on a 10 to 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel and
then transferred to a nitrocellulose membrane (EMD Millipore,
Billerica, MA, USA). Each membrane was incubated for 1 h with 5%
skim milk in TBS-T buffer (0.1 M Tris-HCl, pH 7.4, 0.9% NaCl, 0.1%
Tween-20) to block non-specific binding and incubated with primary
anti-superoxide dismutase 2 (SOD2), catalase (CAT) and TRPV1
antibodies (Cell Signaling Technology, Inc., Danvers, MA, USA), and
anti-β-actin antibody (Sigma-Aldrich; Merck KGaA) antibodies. The
membranes were incubated with peroxidase-conjugated affinity goat
anti-rabbit IgG (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Each protein was detected using a chemiluminescence detection
system according to the manufacturer's instructions (ECL; Amersham,
Berkshire, UK). The band intensity was quantified by densitometric
analysis using ImageJ software (NIH).
Measurement of total glutathione (GSH)
levels
The contents of total glutathione was measured in
the sera of all animals using the GSH/glutathione disulfide (GSSG)
assaykit (Cell Biolabs, Inc., San Diego, CA, USA) based on the
presence of GSH reductase that reduces GSSG to GSH in the presence
of nicotinamide adenine dinucleotide phosphate-oxidase (NADPH).
Subsequently, the chromogen reacts with the thiol group of GSH to
produce a colored compound that absorbs at 405 nm). Data were
expressed as µM of GSH per gram of liver tissue.
Isolation of splenocytes
Briefly, spleen tissues were rapidly harvested from
SD rats, minced, and passed through stainless steel mesh to obtain
single-cell suspension. Spleen cells were suspended with 1X
phosphate-buffered saline (PBS), centrifuged at 5,000 × g for 5
min, and cell pellets were harvested. Erythrocytes were removed
using red blood cell lysis buffer (Sigma-Aldrich; Merck KGaA).
Reverse transcription-polymerase chain
reaction (RT-PCR) assay
Total RNA was isolated from splenocytes from the
rats using TRIzol reagent, and cDNA synthesis from total RNA with a
mixture including oligo-dT primer, 5X RT buffer (Promega
Corporation, Madison, WI, USA), 0.5 mM dNTP, 3 mM MgCl2,
RNase inhibitor, and Improm-II™ reverse transcriptase (2U) was
carried out at 25°C for 5 min and 42°C for 60 min. The reaction was
terminated at 70°C for 10 min. PCR was carried out using specific
primers for the target genes and PCR mixture [2 µl cDNA, 4 µM 5′
and 3′ primers, 10X buffer (10 mM Tris-HCl, pH 8.3), 50 mM KCl,
0.1% Triton X-100, 25 mM MgCl2, 250 µM dNTPs, and 1 U
Taq polymerase] under the following incubation conditions: 30 sec
denaturation at 94°C, 30 sec annealing at 58 to 60°C, 1 min
extension, and 10 min final extension at the end of 30 cycles. The
primer sequences were as follows: 5′-TCAACAACCCACAGGTCCAG-3′
(sense), 5′-CTTCCTGAGGCTGGATTCCG-3′ (anti-sense) for IFN-γ
(XM_006532446.3); 5′-AGATGGATGTGCCAAACGTCCTCA-3′ (sense),
5′-AATATGCGAAGCACCTTGGAAGCC−3′ (anti-sense) for IL-4 (NM_021283.2);
5′-GGACAACATACTGCTAACCGAC-3′ (sense), 5′-TGGATCATTTCCGATAAGGCTTG-3′
(anti-sense) for IL-10 (NM_010548.2); 5′-GGCCCTTCTCCAGGACAGA-3′
(sense), 5′-GCTGATCATGGCTGGGTTGT-3′ (anti-sense) for Foxp3
(NM_001199348.1); and 5′-CTCGTGGAGTCTACTGGTGT-3′ (sense),
5′-GTCATCATACTTGGCAGGTT-3′ (anti-sense) for glyceraldehyde
3-phosphate dehydrogenase (GAPDH, NM_001289726.1) as a control for
PCR. Band intensity was quantified by automated densitometric
analysis (ChemiDoc MP Imaging System; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
GraphPad Prism (GraphPad Software, Inc., La Jolla,
CA, USA) was used for statistical analysis. Data were expressed as
mean ± SEM (standard error of mean) of three separate experiments
and were analyzed for statistical significance using ANOVA,
followed by Tukey's test for multiple comparison. Null hypotheses
of no difference were rejected for P<0.05.
Results
Effect of MOK pharmacopuncture on
physiological parameters in hypothyroidism rats
BWs were significantly decreased in PTU-induced
hypothyroidism rats (P<0.05, 0.001 and 0.001, respectively) from
2, 3, and 4 weeks after initial PTU treatment as compared with that
in the normal group. MOK pharmacopuncture did not inhibit
PTU-induced loss of BW (Fig. 2A);
however, significant inhibition (P<0.001) of BW loss was
observed in the LT4-treated group as a reference group from 2 weeks
after initial treatment as compared with the PTU-control group. We
also observed the changes in food and water intake for 4 weeks.
Food/water consumption significantly decreased from 3 and 4 weeks
after initial PTU treatment compared with that of the normal group.
However, no significant differences in food (Fig. 2B) and water consumption (Fig. 2C) were found in the MOK
pharmacopuncture at 0.3 and 1.5 mg/kg in PTU-induced hypothyroidism
rats. LT4-treated group was also showed a significant increase in
food and water consumption compared with the control group.
Effect of MOK pharmacopuncture on the
levels of thyroid hormones in hypothyroidism rats
As shown in Fig. 3, a
significant increase in serum TSH level (P<0.001) and decrease
in serum T3 (P<0.01) and T4 (P<0.001) levels were detected in
PTU-induced hypothyroidism rats compared with normal controls. MOK
pharmacopuncture at 0.3 and 1.5 mg/kg in hypothyroidism rats
significantly (P<0.01, respectively) decreased serum TSH levels
(Fig. 3A) and significantly
(P<0.01 and P<0.05) increased serum T4 levels (Fig. 3C). The LT4-treated group also showed
a significant (P<0001) decrease in TSH levels (P<0.001) and a
significant (P<0.001) increase in T4 levels (P<0.001)
compared with the control group. In T3 levels, MOK pharmacopuncture
at low concentration in hypothyroidism rats significantly
(P<0.05) increased, but MOK pharmacopuncture at high
concentration or LT4 treatment did not show a significant decrease
(Fig. 3B).
 | Figure 3.Effects of MOK pharmacopuncture on
the levels of thyroid hormones in PTU-induced hypothyroidism rats.
MOK pharmacopuncture was subcutaneously administered once daily for
2 weeks, and the levels of (A) TSH, (B) T3, and (C) T4 in the sera
of rats were measured by ELISA, respectively. Data are presented as
mean ± standard deviation (n=5 per each group). **P<0.01, and
***P<0.001 vs. normal; #P<0.05,
##P<0.01, and ###P<0.001 vs. control.
Normal, normal group; PTU+Vehicle, control group; PTU+Low MOK, MOK
0.3 ml/kg-treated group in control; PTU+High MOK, MOK 1.5
mg/kg-treated group in control; and PTU+LT4, L-Thyroxine 0.5
mg/kg-treated group as a reference drug. |
Effect of MOK pharmacopuncture on
serological parameters in hypothyroidism rats
In PTU-induced hypothyroidism rats, a significant
decrease in serum glucose (P<0.001, Fig. 4A) and TG (P<0.05; Fig. 4B) levels and an increase in serum
total cholesterol (P<0.001; Fig.
4C), LDL-cholesterol (P<0.001, Fig. 4D), AST (P<0.05; Fig. 4E), and ALT (P<0.01; Fig. 4F) levels were observed compared with
those of the normal group. The MOK pharmacopuncture group at high
concentration showed significantly increased glucose levels
(P<0.05) and decreased levels of total cholesterol (P<0.01),
LDL-cholesterol (P<0.001), and AST (P<0.01) compared with
those of the control group. In the LT4-treated group, a significant
increase in glucose (P<0.01) and TG (P<0.05) levels and a
decrease in total cholesterol (P<0.001), and LDL-cholesterol
(P<0.001) were detected compared with those of the control
group, but did not change the levels of AST and ALT.
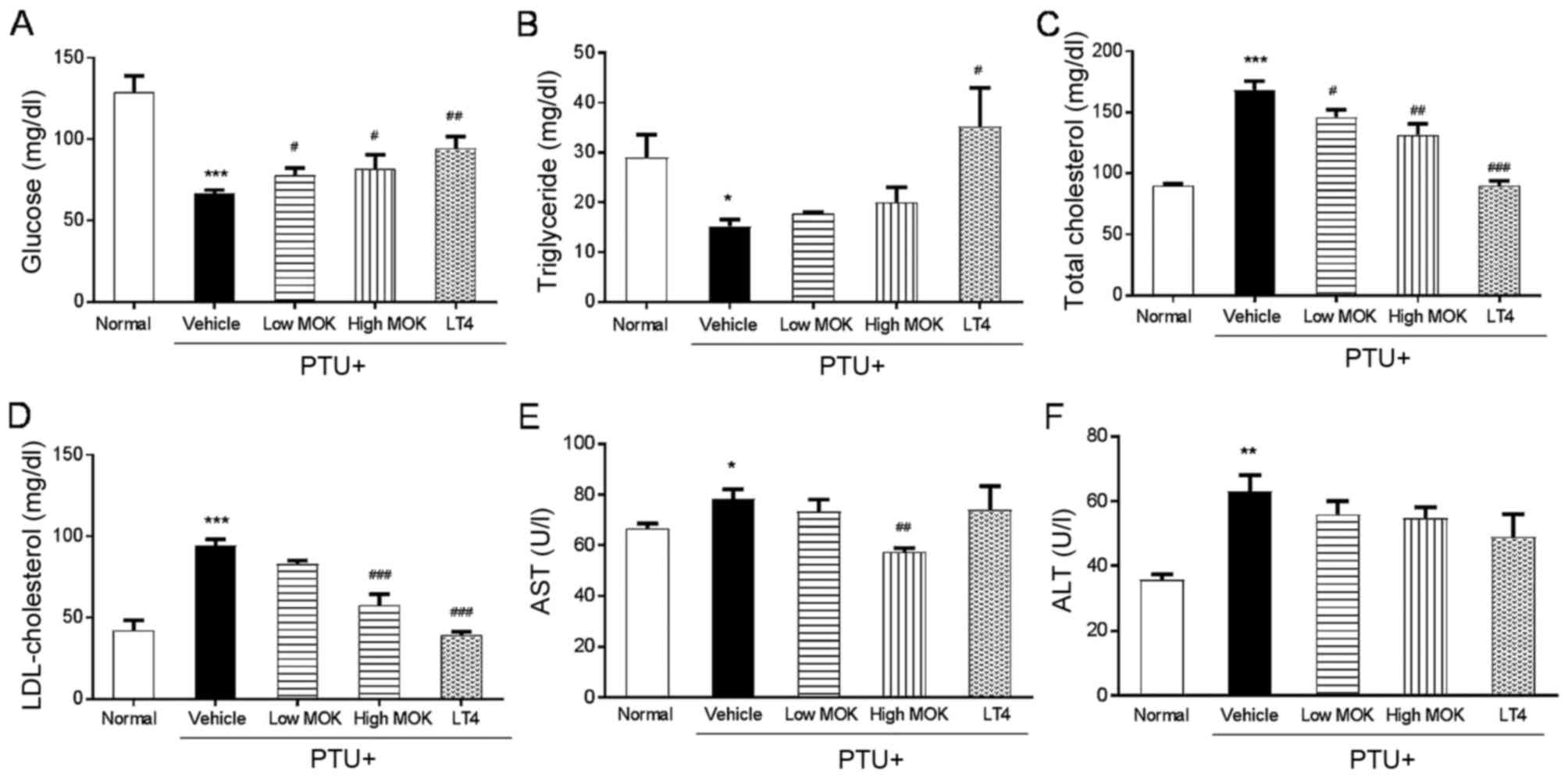 | Figure 4.Effects of MOK pharmacopuncture on
the changes of serological parameters in PTU-induced
hyperthyroidism rats. MOK pharmacopuncture was subcutaneously
administered once daily for 2 weeks, and the levels of (A) glucose,
(B) triglyceride, (C) total cholesterol, (D) LDL-cholesterol, (E)
AST, and (F) ALT in the sera of rats were measured by automatic
blood biochemical analyzer. Data are presented as mean ± standard
deviation (n=5 per each group). *P<0.05, **P<0.01, and
***P<0.001 vs. normal; #P<0.05,
##P<0.01, and ###P<0.001 vs. control.
Normal, normal group; PTU+Vehicle, control group; PTU+Low MOK, MOK
0.3 ml/kg-treated group in control; PTU+High MOK, MOK 1.5
mg/kg-treated group in control; and PTU+LT4, L-Thyroxine 0.5
mg/kg-treated group as a reference drug. |
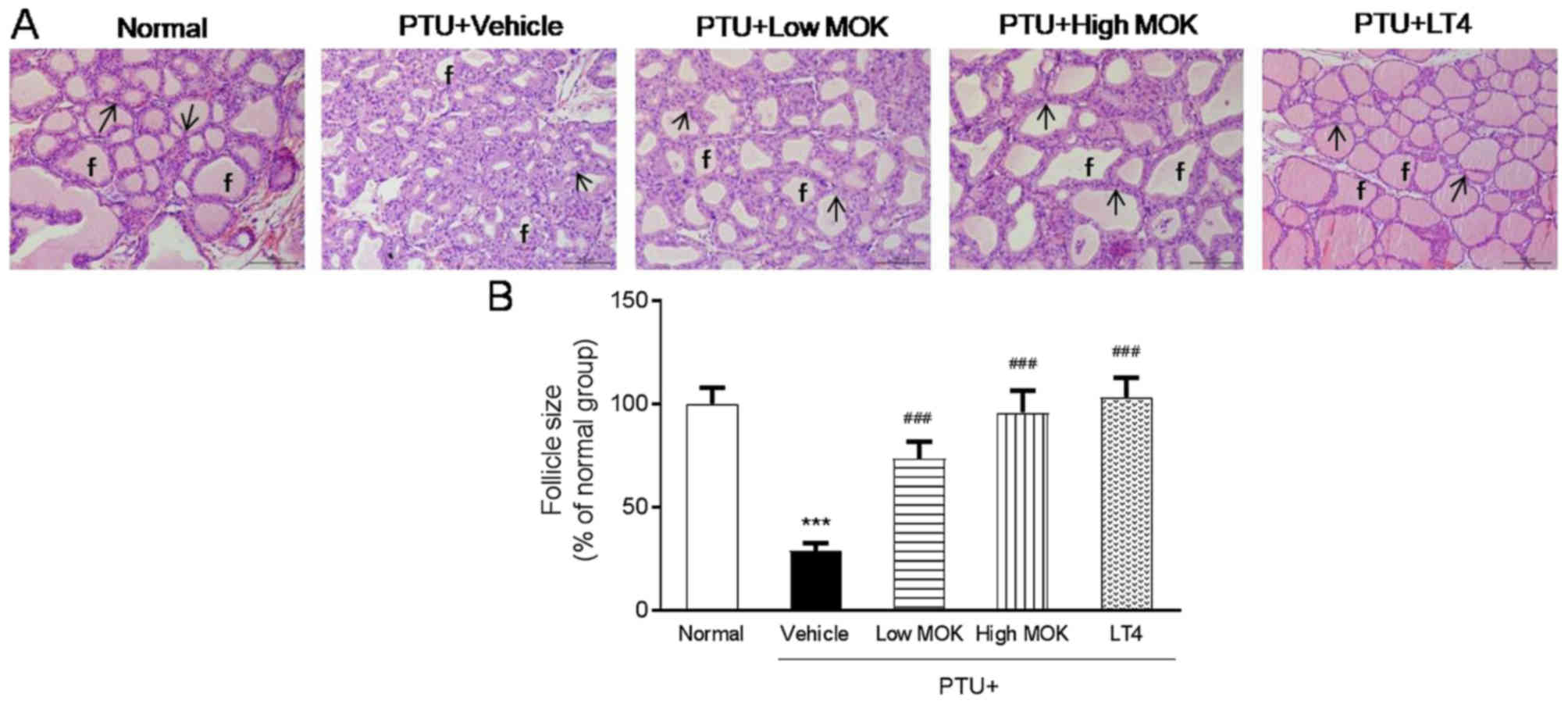
Effects of MOK pharmacopuncture on
histopathological changes in the thyroid gland of hypothyroidism
rats
PTU treatment induced the destruction of the thyroid
gland with marked and noticeable hypertrophic changes related
hyperplasia of follicular cells and decrease in follicular
diameters and colloids. In PTU-induced hypothyroidism rats,
increase in total thyroid gland thickness and decrease in mean
follicular size were also observed compared with those of the
normal group (Fig. 5). However, MOK
pharmacopuncture at 0.3 and 1.5 mg/kg or LT4 treatment was shown to
decrease the histopathological changes, such as hyperplasia of
follicular cells and related hypertrophic changes (Fig. 5A). In addition, MOK pharmacopuncture
at 0.3 and 1.5 mg/kg significantly increased the follicular size
(P<0.001, respectively) compared with that of the control group
(Fig. 5B).
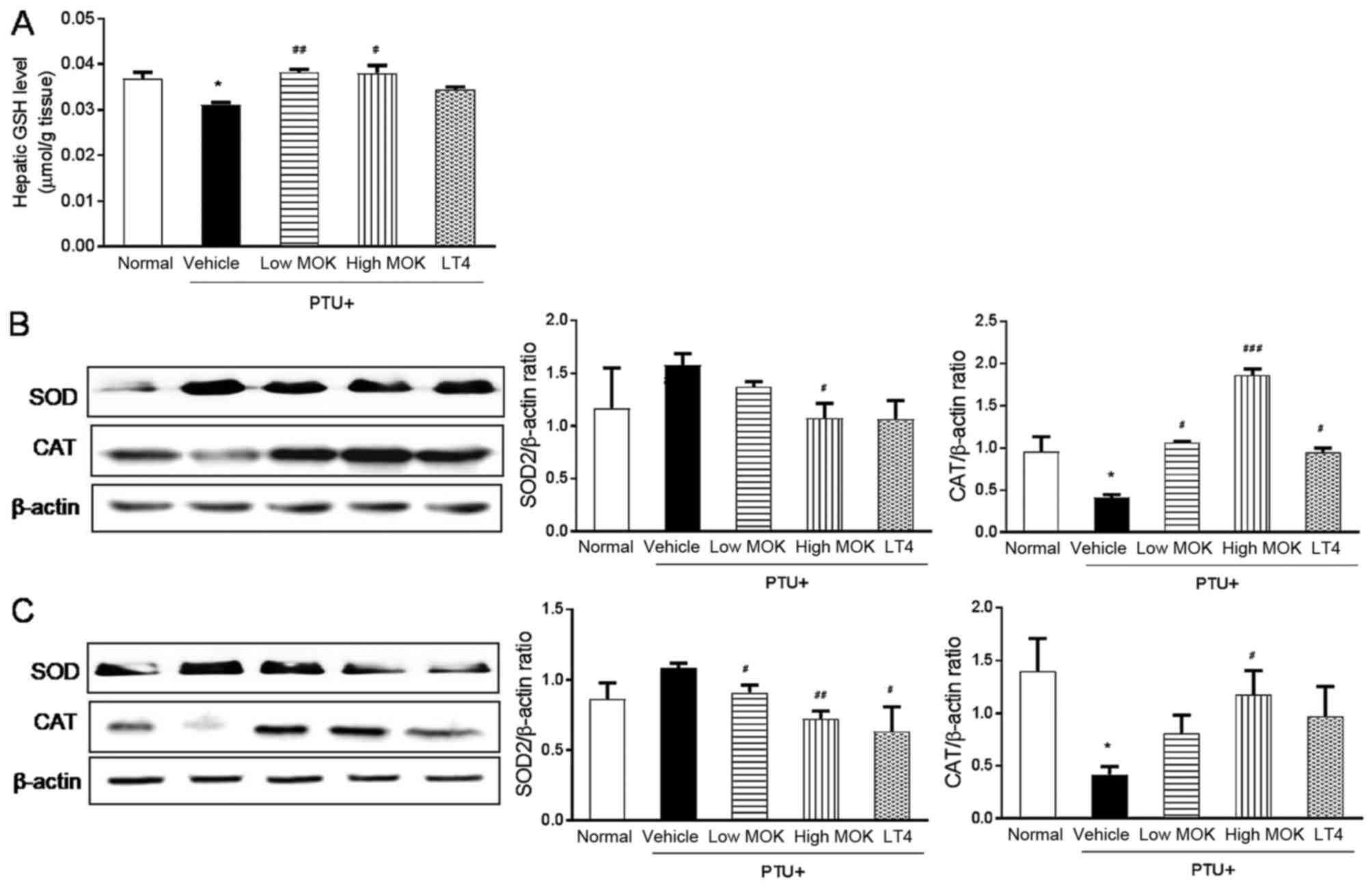
Effect of MOK pharmacopuncture on
oxidation in the liver and brain of hypothroidism rats
To investigate the effect of MOK pharmacopuncture on
oxidative damage in hypothyroidism, we measured the levels of the
antioxidant substance GSH in the liver tissues of hyperthyroidism
rats and the expression of the antioxidant enzymes SOD and CAT in
both liver and brain tissues. As shown in Fig. 6A, the level of GSH was significantly
(P<0.05) reduced in the liver tissues of PTU-induced
hypothyroidism rats and significantly increased in the rats treated
with MOK pharmacopuncture at 0.3 (P<0.01) and 1.5 mg/kg
(P<0.05). Next, the expression of SOD protein was increased in
hypothyroidism rats and significantly decreased in both liver
(P<0.05; Fig. 6B) and brain
tissues (P<0.01; Fig. 6C)
compared with that of the control group after MOK pharmacopuncture
at 1.5 mg/kg. CAT expression was significantly (P<0.05)
decreased in liver and brain tissues. The hypothyroidism-induced
decrease in CAT was significantly increased in the liver
(P<0.001) and brain tissues (P<0.05) by MOK pharmacopuncture
at 1.5 mg/kg.
Effect of MOK pharmacopuncture on body
temperature and TRPV1 expression in hypothyroidism rats
To investigate the regulatory effect of body
temperature in hypothyroidism, we measured the core body
temperature, and the expression of the thermoregulator, TRPV1
channel in the DRG and brain tissues by western blot, respectively.
In PTU-induced hypothyroidism rats, the body temperature from 2, 3,
and 4 weeks after initial PTU treatment was significantly lower
than the normal group (P<0.001) in a time-dependent manner
(Fig. 7A). MOK pharmacopuncture at
0.3 and 1.5 mg/kg resulted in a significantly (P<0.01,
respectively) higher body temperature than that of the control
group from 1 to 2 weeks after initial treatment. In the LT4-treated
group, the body temperature was also significantly (P<0.001)
higher than those of the PTU control group and normal rats. In
LT-4-treated group, it was shown a significant increase of body
temperature in hypothyroidism rats.
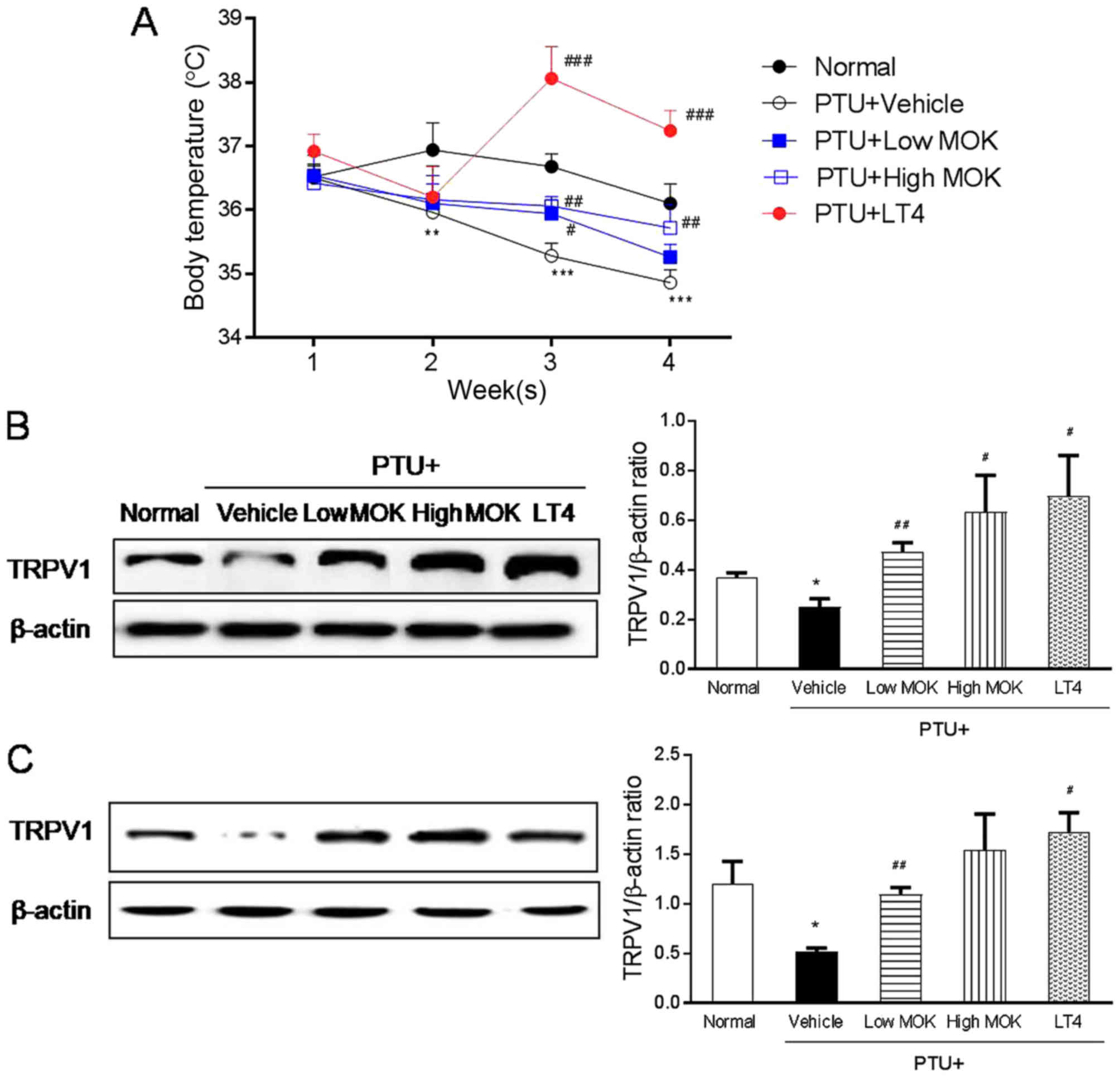 | Figure 7.Effect of MOK pharmacopuncture on the
changes in body temperature and the expression of TRPV1 protein in
PTU-induced hypothyroidism rats. MOK pharmacopuncture was
subcutaneously administered once daily for 2 weeks, and the body
temperature was measured by (A) rectal thermometer once a week. The
production of TRPV1 protein was determined in (B) DRG and (C) brain
tissues isolated from PTU-induced hypothyroidism rats using western
blot. Data are presented as mean ± standard deviation (n=5 per each
group). *P<0.05, **P<0.01, and ***P<0.001 vs. normal;
#P<0.05, ##P<0.01, and
###P<0.001 vs. control. Normal, normal group;
PTU+Vehicle, control group; PTU+LowMOK, MOK 0.3 ml/kg-treated group
in control; PTU+High MOK, MOK 1.5 mg/kg-treated group in control;
and PTU+LT4, L-Thyroxine 0.5 mg/kg-treated group as a reference
drug. |
The expression of TRPV1 was significantly decreased
in the DRG (Fig. 7B) by MOK
pharmacopuncture at 0.3 (P<0.01) and 1.5 mg/kg (P<0.05) and
in the brain at 0.4 mg/kg (P<0.01, Fig. 7C) of hypothyroidism rats compared
with the normal group. The treatment of LT4 also significantly
decreased TRPV1 expression in both DRG (P<0.01) and brain
tissues (P<0.01).
Effects of MOK pharmacopunctureon the
expression of IL-4, IL-10, Foxp3, and IFN-γ in the spleen of
hypothyroidism rats
To understand the action mechanism of MOK
pharmacopuncture on Th1/Th2 immune response, we measured the serum
levels of IFN-γ, Th1 cytokine, IL-4, and Th2 cytokine in
hypothyroidism rats by ELISA and the expression of IFN-γ, IL-4,
IL-10, and Foxp3 mRNA in the spleen tissues by RT-PCR. Spleen
weight was significantly (P<0.01) decreased in hypothyroidism
rats compared with that of the normal group, and this decrease was
significantly increased by MOK pharmacopuncture at 0.3 (P<0.01)
and 1.5 mg/kg (P<0.01) or LT4 treatment (P<0.05; Fig. 8A). Next, MOK pharmacopuncture
significantly decreased at 0.3 (P<0.01) and 1.5 mg/kg
(P<0.01) in the sera of hypothyroidism rats and significantly
increased the IL-4 levels at 0.3 (P<0.01) and 1.5 mg/kg
(P<0.05). MOK pharmacopuncture decreased the expression of IFN-γ
mRNA, but increased the expression of IL-4 mRNA in the spleen
tissues of hypothyroidism rats (Fig.
8C). Further, MOK pharmacopuncture significantly increased the
expression of IL-10 and Foxp3 mRNA in the spleen tissues of
hypothyroidism rats.
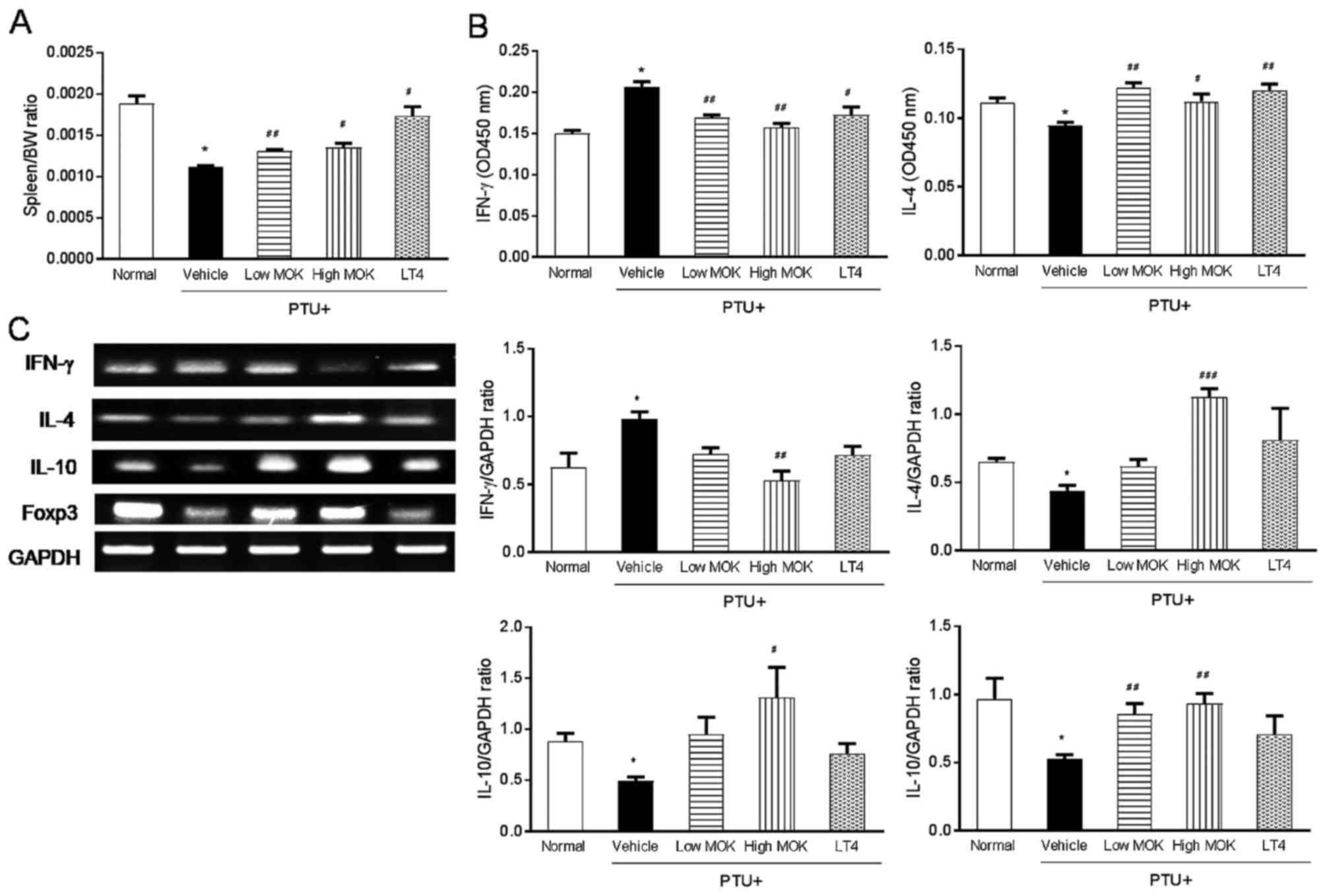 | Figure 8.Effects of MOK pharmacopuncture on
the expression of IL-4, IL-10, Foxp3, and IFN-γ in the spleen of
PTU-induced hypothyroidism rats. MOK pharmacopuncture was
subcutaneously administered once daily for 2 weeks, and the weight
of the spleen (A) in PTU-induced hypothyroidism rats was measured.
Relative organ weights to body weights were measured. (B) The serum
levels of IFN-γ and IL-4 in hypothyroidism rats by ELISA and (C)
the expression of IFN-γ, IL-4, IL-10, and Foxp3 mRNA in the spleen
tissues by RT-PCR, respectively. Data are presented as mean ±
standard deviation (n=5 per each group). *P<0.05 vs. normal;
#P<0.05, ##P<0.01, and
###P<0.001 vs. control. Normal, normal group;
PTU+Vehicle, control group; PTU+Low MOK, MOK 0.3 ml/kg-treated
group in control; PTU+High MOK, MOK 1.5 mg/kg-treated group in
control; and PTU+LT4, L-Thyroxine 0.5 mg/kg-treated group as a
reference drug. |
Discussion
Pharmacopuncture is a new form of acupuncture
treatment in TKM; it is also known as acupoint injection in TCM,
and it has better efficacy than oral administration because the
drug does not pass through the digestive system. Therefore,
pharmacopuncture is commonly applied in Korean clinics. This method
has often been used for the regulation of immune imbalance in TKM.
MOK is a polyherbal medicine for immuno-pharmacopuncture, and MOK
pharmacopuncture is used to treat patients with thyroid diseases
such hyperthyroidism and hypothyroidism. It is believed that MOK
pharmacopuncture has a good effect on immune regulation in thyroid
diseases, but its scientific evidence has been little studied. In
our previous study, we found that MOK showed an anti-inflammatory
effect in LPS-stimulated macrophages (8) and a modulatory effect on Th1/Th2 immune
response in ConA-stimulated splenocytes (9). In the present study, we confirmed the
therapeutic effect of MOK pharmacopuncture on PTU-induced
hypothyroidism in rats through regulation of the imbalance of
thyroid hormones, body temperature, and antioxidation. MOK
pharmacopuncture is clinically applied with MOK extract at 0.3 to
0.8 mg/ml in acupoints of thyroid region of the patients (45 kg BW)
twice a week for 3 months according to the guideline of KIPA. In
this study, we used MOK extract at 0.3 and 1.5 mg/ml in rats once a
day for 2 weeks after induction of hypothyroidism.
Because thyroid hormones are known to play a
fundamental role in the regulation of various types of metabolism
in the body, their insufficient release can induce hypothyroidism
with inhibition of basic body metabolism, decrease in catabolic
actions, accumulation of tissue glycoproteins, and increase in BW
(3,14). In our study, hypothyroidism was
induced in rats by injection of the PTU as a representative
inhibitor of thyroid functions (11–13). It
has been reported that PTU-induced hypothyroidism rats showed
absolute reduction of T3 and T4 levels and the increase in TSH,
similar to human hypothyroidism (11,15).
Therefore, laboratory evaluation of TSH, T3, and T4 levels is
considered the best screening test for hypothyroidism (16). We also found marked and noticeable
increase in TSH and decrease in T3 and T4 levels in PTU-induced
hypothyroidism rats.
Patients with diabetes and hyperglycemia have a
higher prevalence of thyroid disorders than the normal population
(17). Hypothyroidism is also
accompanied by a variety of abnormalities in plasma lipid
metabolism, including elevated TG and LDL cholesterol
concentrations (18). In our study,
PTU-induced hypothyroidism rats showed a significant decrease in
serum glucose and TG levels, but a significant increase in serum
total cholesterol, LDL-cholesterol, AST and ALT levels. MOK
pharmacopuncture in hypothyroidism rats increased glucose levels
and decreased lipid accumulation in both low and high doses,
suggesting that MOK pharmacopuncture can regulate the
hypothyroidism-induced metabolism abnormality similar to LT4
treatment. Thyroid hormones were found to affect lipid
concentration, hepatic metabolism, and the synthesis of cholesterol
(17,18). The abnormalities of lipoprotein
metabolism commonly involved with hypothyroidism are elevated
levels of total cholesterol and LDL-cholesterol. Elevated
cholesterols can induce the development of lethal cardiovascular
diseases as side effects of hypothyroidism (18,19).
These abnormal blood lipid levels in hypothyroidism are ameliorated
by LT4 treatment (17,20,21). In
our study, MOK pharmacopuncture significantly decreased the levels
of total cholesterol and LDL-cholesterol in both low and high
doses. These results suggest that MOK pharmacopuncture can reduce
the risk of diabetes and cardiovascular diseases through the
regulation of lipid accumulation similar to LT4 treatment.
The liver is the main target organ of thyroid
hormone; therefore, hypothyroidism is commonly accompanied with
hepatic damage (22). Thyroid
hormones are known to play an essential role in hepatocyte
proliferation of rat liver (23).
Its serious damage was accompanied to the thyroid hormones
imbalances regardless of hypothyroidism. Clinical diagnosis of
disease and damage to the structural integrity of liver is also
commonly assessed by monitoring the status of serum AST and ALT
activities (24). In our study, PTU
treatment significantly increased serum levels of AST and ALT, and
they were significantly inhibited by L-thyroxin and MOK
pharmacopuncture in both low and high concentrations.
In general, hypothyroidism is accompanied by a
decrease in the basic body metabolism, and internal respiration. In
return, it induces inhibition of lipid peroxidation and weak
increase in the endogenous antioxidant enzymes such as SOD and CAT
against the release of harmful reactive oxygen species (ROS) and
hydrogen peroxide (H2O2) in hepatic tissue.
Recently, several trials have been conducted to determine the
potent and less toxic natural origin antioxidants for use in
hypothyroidism treatment (25–27). In
our study, MOK pharmacopuncture significantly decreased the GSH
content and CAT activity and slightly increased SOD activity in the
liver and brain tissues of hypothyroidism rats similar to LT4
treatment. These results indicate that MOK pharmacopuncture can
protect liver and brain tissues against hypothyroidism-induced
oxidative stress.
In this study, we also found that MOK
pharmacopuncture regulated body temperature in hypothyroidism rats
through inhibition of the thermoregulator TRPV1 channel. Higher
rectal temperature has been found to be induced in LT4-induced
hyperthyroidism rats (28), while
lower temperature is found in PTU-induced hypothyroidism rats
(15). In our study, a decrease in
body temperature was observed in PTU-induced hypothyroidism rats,
and that was increased by MOK pharmacopuncture. Our sensory nerves
use specialized ion channel proteins to report environmental
temperatures, most notably, but not exclusively, TRP ion channels
(29–31). TRPV1 channels in sensory nerves
respond to heat and to capsaicin, an alkaloid from ‘hot’ peppers,
which binds to open the channel and thus depolarizes the neuron and
fires action potentials (32). Drugs
that block TRPV1 input to the brain provoke hypothalamic-mediated
changes in metabolism that elevate body temperature (33,34). It
is also known that the DRG neurons in rats are sensitive to
capsaicin (34,35). In our study, the regulation of body
temperature by MOK pharmacopuncture was linked to the regulation of
TRPV1 in DRG and brain tissues. These results suggest that MOK
pharmacopuncture can regulate the change in body temperature
through the regulation of the thermo-regulating protein TRPV1 on
hypothyroidism similar to LT4 treatment.
In the body, the spleen is an important immune
organ, and splenocytes consist of different white blood cell types
such as T and B lymphocytes, dendritic cells, and macrophages,
which have different immune functions (36,37).
Thus, in the drug efficacy study, the immune modulatory evaluation
of splenocytes provides an understanding of the influence on T and
B cells (36). In our study, we also
evaluated the immune modulatory effects of MOK pharmacopuncture,
wherein the changes of Th1/Th2 cytokines were investigated in the
splenocytes of hypothyroidism rats. Th cytokines from the CD4+ Th
lymphocytes are thought to regulate the function of the immune
system, including antibody production and cellular immune response
(38). Th cells represent a
functionally heterogeneous population, comprising distinct subsets
termed Th1 and Th2 defined by their cytokine secretion profiles
(39). Th1 cells secrete Th1
cytokines such as IL-2, IFN-γ, IL-12 and TNF-α, while Th2 cells
secrete Th2 cytokines, such as IL-4, IL-10, and Foxp3. The
communication network between Th1 and Th2 cytokines may be
synergistic or antagonistic toward lymphocyte proliferation and
differentiation (40,41). In our study, MOK pharmacopuncture
significantly decreased the levels of IFN-γ as a main Th1 cytokine
and increased the levels of IL-4 as a main Th2 cytokine in the
spleen of PTU-induced hypothyroidism rats. The increase in Th1
cytokine and the decrease in Th2 cytokines have been reported in
hypothyroidism (42). Therefore, our
finding indicates that MOK pharmacopuncture has an immune
modulatory property on imbalance of Th1/Th2, which has been found
to reduce the disease severity of hypothyroidism.
Natural regulatory T (Treg) cells are constitutively
produced in the thymus; they express very high levels of CD25 and
produce IL-10 with the expression of Foxp3 (43–45). The
role of CD4+CD25+FoxP3+ Treg cells has been widely reported in the
prevention of autoimmune diseases and immunopathology in all types
of infections (46,47). In our study, MOK pharmacopuncture
significantly increased the expression of IL-10 and FoxP3 mRNA in
the spleen of PTU-induced hypothyroidism rats. MOK pharmacopuncture
also regulated the imbalance of Th1/Th2 cytokines at high dose,
however, further study is needed, suggesting that MOK
pharmacopuncture can help to suppress autoimmune response. Some
data suggest that the transcription factors such as interferon
regulatory factors (IFRs) are involved in the pathogenesis of many
autoimmune disorders (48). IRF7have
been implicated in metabolic autoimmune disorders including
diabetes and obesity (49). However,
the systemic effects of IRFs on metabolism are largely unknown. In
further study, we will investigate the effects of MOK
pharmacopuncture on hypothyroidism by the metabolic regulation of
IRFs, which suggests a new strategy for treatment of thyroid
autoimmune diseases.
In this study, we firstly demonstrated that MOK
pharmacopuncture has a therapeutic effect on hypothyroidism rats,
suggesting that MOK pharmacopuncture can make a good use for the
treatment of hypothyroidism patients. However, the mechanism of
responsible for the therapeutic effects of MOK and the function of
MOK constituents require further research. In our study, small
groups (n=5 in each group) with approval of IACUC were used,
however, it will be added the numbers of animals for better
understanding of MOK pharmacopuncture for further study.
In conclusions, MOK pharmacopunture in PTU-induced
hypothyroidism rats was found to improve the pathological
progression by normalization of the hypothyroidism-induced thyroid
hormone imbalance, inhibition of lipid accumulation, and
antioxidation, similar to L-thyroxin. The underlying mechanism was
related to the regulation of body temperature by TRPV1 channel
activation and Th1/Th2 cytokine imbalance. This indicates that MOK
pharmacopuncture is a useful therapy for patients with
hypothyroidism in traditional clinics.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
[Ministry of Science, ICT and Future Planning (MSIP); grand no.
NRF-2017R1C1B5076224].
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gaberšček S and Zaletel K: Thyroid
physiology and autoimmunity in pregnancy and after delivery. Expert
Rev Clin Immunol. 7:697–706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chakera AJ, Pearce SH and Vaidya B:
Treatment for primary hypothyroidism: Current approaches and future
possibilities. Drug Des Devel Ther. 6:1–11. 2012.PubMed/NCBI
|
|
3
|
Garber JR, Cobin RH, Gharib H, Hennessey
JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA and Woeber
KA: American Association Of Clinical Endocrinologists And American
Thyroid Association Taskforce On Hypothyroidism In Adults: Clinical
practice guidelines for hypothyroidism in adults: Cosponsored by
the American Association of Clinical Endocrinologists and the
American Thyroid Association. Thyroid. 22:1200–1235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Korean Acupuncture and Moxibustion
Medicine Society: Pharmacopuncture therapyAcupuncture Medicine. Kim
JH: Hanmi Medical Publishing co.; Paju: pp. 204–208. 2016, (In
Korean).
|
|
5
|
Jung C, Jung JH and Lee MS:
PharmacopuncturemedinesA clinical study of immune
pharmacopuncturology. Jung C: Kyungrak Medical Publishing Co.;
Chungnam: pp. 127–133. 2011, (In Korean).
|
|
6
|
Hwang JH: A case report of Hwa-byeong with
MOK Herbal acupuncture therapy. J Immuno-Pharmacopuncture. 2:43–55.
2013.(In Korean).
|
|
7
|
Kim HJ, Gwan R, Han JW, Jung C and Park
KH: Analysis of physioactivities on MOK yakchim. J
Immuno-Pharmacopuncture. 2:17–25. 2013.(In Korean).
|
|
8
|
Hwang JH, Hwang MS and Park YK: MOK, a
pharmacopuncture medicine, reduces inflammatory response through
inhibiting the proinflammatory cytokine production in
LPS-stimulated mouse peritoneal macrophages. Acupuncture. 34:11–21.
2017. View Article : Google Scholar
|
|
9
|
Hwang JH: Effects of MOK, a
pharmacopuncture medicine, on the TH1/TH2 immune response and
antioxidation in Con A-stimulated primary mouse splenocytes.
Acupuncture. 34:39–48. 2017. View Article : Google Scholar
|
|
10
|
Somers SL: Examining anger in
‘culture-bound’ syndromes. Psychiatric Times. 15:1998.
|
|
11
|
Ashwini S, Bobby Z, Sridhar MG and Cleetus
CC: Insulin plant (costus pictus) extract restores thyroid hormone
levels in experimental hypothyroidism. Pharmacognosy Res. 9:51–59.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choudhuryl S, Chainyl GB and Mishro MM:
Experimentally induced hypo- and hyper-thyroidism influence on the
antioxidant defence system in adult rat testis. Andrologia.
35:131–140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rooney AA, Matulka RA and Luebke RW:
Developmental atrazine exposure suppresses immune function in male,
but not female Sprague-Dawley rats. Toxicol Sci. 76:366–375. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zamoner A, Barreto KP, Filho DW, Sell F,
Woehl VM, Guma FC, Pessoa-Pureur R and Silva FR:
Propylthiouracil-induced congenital hypothyroidism upregulates
vimentin phosphorylation and depletes antioxidant defenses in
immature rat testis. J Mol Endocrinol. 40:125–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Subudhi U, Das K, Paital B, Bhanja S and
Chainy GB: Supplementation of curcumin and vitamin E enhances
oxidative stress, but restores hepatic histoarchitecture in
hypothyroid rats. Life Sci. 84:372–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown RS: Autoimmune thyroiditis in
childhood. J Clin Res Pediatr Endocrinol. 5 Suppl 1:S45–S49.
2013.
|
|
17
|
Patricia Wu: Thyroid disease and diabetes.
Clin Diabetes Winter. 18:382000.
|
|
18
|
Mullur R, Liu YY and Brent GA: Thyroid
hormone regulation of metabolism. Physiol Rev. 94:355–382. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rizos CV, Elisaf MS and Liberopoulos EN:
Effects of thyroid dysfunction on lipid profile. Open Cardiovasc
Med J. 5:76–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ito M, Arishima T, Kudo T, Nishihara E,
Ohye H, Kubota S, Fukata S, Amino N, Kuma K, Sasaki I, et al:
Effect of levo-thyroxine replacement on non-high-density
lipoprotein cholesterol in hypothyroid patients. J Clin Endocrinol
Metab. 92:608–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teixeira Pde F, Reuters VS, Ferreira MM,
Almeida CP, Reis FA, Buescu A, Costa AJ and Vaisman M: Lipid
profile in different degrees of hypothyroidism and effects of
levothyroxine replacement in mild thyroid failure. Transl Res.
151:224–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simon-Giavarotti KA, Giavarotti L, Gomes
LF, Lima AF, Veridiano AM, Garcia EA, Mora OA, Fernández V, Videla
LA and Junqueira VB: Enhancement of lindane-induced liver oxidative
stress and hepatotoxicity by thyroid hormone is reduced by
gadolinium chloride. Free Radic Res. 36:1033–1039. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Torres S, Díaz BP, Cabrera JJ, Díaz-Chico
JC, Díaz-Chico BN and López-Guerra A: Thyroid hormone regulation of
rat hepatocyte proliferation and polyploidization. Am J Physiol.
276:G155–G163. 1999.PubMed/NCBI
|
|
24
|
Amin A and Hamza AA: Oxidative stress
mediates drug-induced hepatotoxicity in rats: A possible role of
DNA fragmentation. Toxicology. 208:367–375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bhanja S and Chainy GB: PTU-induced
hypothyroidism modulates antioxidant defence status in the
developing cerebellum. Int J Dev Neurosci. 28:251–262. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Messarah M, Saoudi M, Boumendjel A,
Boulakoud MS and Feki AE: Oxidative stress induced by thyroid
dysfunction in rat erythrocytes and heart. Environ Toxicol
Pharmacol. 31:33–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Das K and Chainy GB: Modulation of rat
liver mitochondrial antioxidant defence system by thyroid hormone.
Biochim Biophys Acta. 1537:1–13. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Subudhi U, Das K, Paital B, Bhanja S and
Chainy GB: Alleviation of enhanced oxidative stress and oxygen
consumption of L-thyroxine induced hyperthyroid rat liver
mitochondria by vitamin E and curcumin. Chem Biol Interact.
173:105–114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ramsey IS, Delling M and Clapham DE: An
introduction to TRP channels. Annu Rev Physiol. 68:619–647. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nilius B, Owsianik G, Voets T and Peters
JA: Transient receptor potential cation channels in disease.
Physiol Rev. 87:165–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu LJ, Sweet TB and Clapham DE:
International Union of Basic and Clinical Pharmacology. LXXVI.
Current progress in the mammalian TRP ion channel family. Pharmacol
Rev. 62:381–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Caterina MJ, Rosen TA, Tominaga M, Brake
AJ and Julius D: A capsaicin-receptor homologue with a high
threshold for noxious heat. Nature. 398:436–441. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gavva NR, Treanor JJ, Garami A, Fang L,
Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, et al:
Pharmacological blockade of the vanilloid receptor TRPV1 elicits
marked hyperthermia in humans. Pain. 136:202–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang H and Siemens J: TRP ion channels in
thermosensation, thermoregulation and metabolism. Temperature
(Austin). 2:178–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu PW, Blair NT and Bean BP: Action
potential broadening in capsaicin-sensitive DRG neurons from
frequency-dependent reduction of Kv3 current. J Neurosci.
37:9705–9714. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cesta MF: Normal structure, function, and
histology of the spleen. Toxicol Pathol. 34:455–465. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bronte V and Mikael MJ: The spleen in
local and systemic regulation of immunity. Immunity. 39:806–818.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaiko GE, Horvat JC, Beagley KW and
Hansbro PM: Immunological decision-making: How does the immune
system decide to mount a helper T-cell response? Immunology.
123:326–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Romagnani S: Biology of human TH1 and TH2
cells. J Clin Immunol. 15:121–129. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Murphy KM and Reiner SL: The lineage
decisions of helper T cells. Nat Rev Immunol. 2:933–944. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abbas AK, Murphy KM and Sher A: Functional
diversity of helper T lymphocytes. Nature. 383:787–793. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ganesh BB, Bhattacharya P, Gopisetty A and
Prabhakar BS: Role of cytokines in the pathogenesis and suppression
of thyroid autoimmunity. J Interferon Cytokine Res. 31:721–731.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Barthlott T, Moncrieffe H, Veldhoen M,
Atkins CJ, Christensen J, O'Garra A and Stockinger B: CD25+ CD4+ T
cells compete with naive CD4+ T cells for IL-2 and exploit it for
the induction of IL-10 production. Int Immunol. 17:279–288. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Setoguchi R, Hori S, Takahashi T and
Sakaguchi S: Homeostatic maintenance of natural Foxp3(+) CD25(+)
CD4(+) regulatory T cells by interleukin (IL)-2 and induction of
autoimmune disease by IL-2 neutralization. J Exp Med. 201:723–735.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wan YY and Flavell RA: Regulatory T-cell
functions are subverted and converted owing to attenuated Foxp3
expression. Nature. 445:766–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sakaguchi S: Naturally arising
Foxp3-expressing CD25+CD4+ regulatory T cells in immunological
tolerance to self and non-self. Nat Immunol. 6:345–352. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Belkaid Y: Regulatory T cells and
infection: A dangerous necessity. Nat Rev Immunol. 7:875–888. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Richez C, Barnetche T, Miceli-Rechard C,
Blanco P, Moreau JF, Rifkin I and Scharverbeke T: Role for
interferon regulatory factors in autoimmunity. Joint Bone Spine.
77:525–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang XA, Zhang R, Zhang S, Deng S, Jiang
D, Zhong J, Yang J, Wang T, Hong S, Guo S, et al: Interferon
regulatory factor 7 deficiency prevents diet-induced obesity and
insulin resistance. Am J Physiol Endocrinol Metab. 305:E485–E495.
2013. View Article : Google Scholar : PubMed/NCBI
|