Introduction
Breast cancer is a type of malignancy that
originates in breast tissue (1). As
one of the most prevalent types of cancer in females, breast cancer
affects 1 in 8 females during their lives, and is associated with
notable psychological and economic burdens to patients and their
families (2,3). Treatment outcomes for the majority of
patients with breast cancer with initial cytotoxic drug treatments,
including methotrexate, are typically poor due to intrinsic
resistance (4). In addition, the
long-term use of certain drugs, including cyclophosphamide, will
lead to the development of drug resistance, thereby inducing the
development of aggressive malignancies (5,6).
Therefore, the development of novel treatment targets is urgently
required to improve the treatment outcomes of breast cancer.
Leptin is a hormone that is primarily secreted by
adipose cells, which have important roles in regulating energy
balance by inhibiting hunger (7).
Recent studies have demonstrated that upregulated expression level
of leptin gene is also correlated with the development of various
human diseases, such as cardiovascular disease (8), non-alcoholic fatty liver disease
(9) and different types of cancers
(10). The functionality of leptin
in the tumor microenvironment of breast cancer has been well
studied: It has been demonstrated that leptin induces obesity and
contributes to the development of this disease (11). Effects of leptin on breast cell
proliferation remain to be elucidated. The current study aimed to
investigate the role of leptin in the growth of breast cancer.
Materials and methods
Patients
A total of 48 female patients with breast cancer
were recruited from China-Japan Union Hospital (Changchun, China)
from July 2015-January 2017. Patients were aged from 31.0–68.0
years, with a mean age of 48.3±7.6 years. All patients received
surgical resections, and tumor tissues and adjacent healthy tissues
were collected during surgery. At the same time, a total of 37
healthy participants were also selected to serve as a control
group. Age of controls ranged from 29.0–66.0 years, with a mean age
of 46.4±9.1 years. No significant difference in age was observed
between the patient and control groups. The present study was
approved by the Ethics Committee of China-Japan Union Hospital, and
all patients provided written informed consent.
Preparation of serum samples
Fasting blood (20 ml) was extracted from all
patients and controls. Blood was stored at room temperature for 1
h, followed by centrifugation at 1,000 × g for 15 min at room
temperature to collect serum. Serum samples were stored at −80°C
prior to further use.
ELISA
Serum leptin was detected using an ELISA kit (cat.
no. KAC2281; Thermo Fisher Scientific, Inc., Waltham, MA, USA). All
operations were performed in strict accordance with the
manufacturer's protocol. Serum leptin was normalized to ng/ml.
Cell lines and cell culture
The normal human breast cell line Hs 578Bst, and the
breast cancer cell lines MCF-7 and MDA-MB-231 were purchased from
American Type Culture Collection (ATCC; Manassas, VA, USA). Hs
578Bst cells were cultured with ATCC Hybri-Care medium (cat no.
46-X) containing 1.5 g/l sodium bicarbonate, 30 ng/ml mouse
epidermal growth factor (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA).
MCF-7 cells were cultured with ATCC-formulated Eagle's minimum
essential medium (cat no. 30–2003) containing 0.01 mg/ml human
recombinant insulin (Sigma-Aldrich; Merck KGaA) and 10% fetal
bovine serum (Sigma-Aldrich; Merck KGaA). MDA-MB-231 cells were
cultured with ATCC-formulated Leibovitz's L-15 medium (cat no.
30-2008) containing 10% fetal bovine serum (Sigma-Aldrich; Merck
KGaA). Cells were cultured at 37°C, and harvested during
logarithmic growth phase for subsequent experiments.
Cell proliferation assay
Cells from each cell line were transferred into
96-well plates containing the corresponding aforementioned medium
with 5×103 cells per well. Following incubation at 37°C
for 3–5 h, cell adhesion was reached and 100 µl Dulbecco's modified
Eagle's medium (Sigma-Aldrich; Merck KGaA) was added. Cells were
cultured at 37°C with the different concentrations of leptin (0,
25, 50 and 100 mM; Sigma-Aldrich; Merck KGaA), and 10 µl cell
counting kit-8 solution (Sigma-Aldrich; Merck KGaA) was added at
24, 48 72 and 96 h later. Following incubation for another 4 h,
optical density values at 450 nm were measured using a microplate
reader. For Wnt inhibitor PNU-74654 (20 µM; Sigma-Aldrich; Merck
KGaA) treatment, 20 µM PNU-74654 and 100 mM leptin was added to the
culture medium under the same conditions as aforementioned.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tumor tissues, adjacent
healthy tissue, serum and in vitro cultured cells using
TRIzol reagent (Thermo Fisher Scientific, Inc.), and cDNA was
synthesized via reverse transcription using Oligo(dT)15 primer
(Shanghai Sangong Pharmaceutical Co., Ltd., Shanghai, China), dNTPs
(Sigma-Aldrich; Merck KGaA) and Avian Myeloblastosis Virus reverse
transcriptase (New England BioLabs, Inc., Ipswich, MA, USA) and its
buffer (New England BioLabs, Inc.). The temperature protocol for
reverse transcription was: 25°C for 5 min, 55°C for 20 min and 75°C
for 15 min. The following primers were used in qPCR: Leptin,
forward 5′-CAAGCAGTGCCTATCCAGA-3′ and reverse
5′-AAGCCCAGGAATGAAGTCCA-3′; and GAPDH forward
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse 5′-TTGATTTTGGAGGGATCTCG-3′.
A 25 µl reaction mixture was prepared using SYBR™ Green Master Mix
(Thermo Fisher Scientific, Inc.) according to manufacturer's
protocol and the reaction conditions were as follows: 94°C for 3
min, followed by 40 cycles of 94°C for 10 sec, 55°C for 30 sec and
72°C for 25 sec, and 72°C for 10 min. PCR products were subjected
to agarose gel electrophoresis and results were observed using the
ChemiDoc™ and GelDoc™ imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA USA). Quantity One® 1-D Analysis Software
V4.6.7 (Bio-Rad Laboratories, Inc.) was used to analyze the results
with GAPDH endogenous control using 2−ΔΔCq method
(12).
Western blotting
Cells of the MG-63 and MDA-MB-231 cell line were
treated with 0 (control cells; C) or 100 mN leptin (leptin group).
Cell lysis buffer (cat. no. P0013K; Beyotime Institute of
Biotechnology, Haimen, China) was then used to extract total
protein from these cells. Total protein concentration was
determined via bicinchoninic acid assay. A total of 20 µg protein
from each sample was subjected to electrophoresis using 10%
SDS-PAGE, and were subsequently transferred to a polyvinylidene
difluoride membrane (Bio-Rad Laboratories, Inc.). Blocking was
performed by incubating membranes with 5% skimmed milk at room
temperature for 2 h. Following washing, membranes were incubated
with primary antibodies against β-catenin (1:1,200; cat. no.
ab16051; Abcam, Cambridge, UK) and endogenous control β-actin
(1:1,000; cat. no. SAB5500001; Sigma-Aldrich; Merck KGaA) overnight
at 4°C. Following washing, membranes were incubated with
horseradish peroxidase conjugated anti-rabbit immunoglobulin G
secondary antibodies (1:1,000; cat. no. MBS435036; MyBioSource, San
Diego, CA, USA) at 37°C for 1 h. Enhanced chemiluminescence
(SuperSignal; Thermo Fisher Scientific, Inc.) was performed to
detect the signals and Quantity One® 1-D Analysis
Software V. 4.6.7 (Bio-Rad Laboratories, Inc.) was used to measure
grayscale. This experiment was repeated three times.
Statistical analysis
Data were analyzed using SPSS 19.0 (IBM, Corp.,
Armonk, NY, USA). Comparisons between two groups were performed
using Student's t-test and comparisons among multiple groups were
performed using one-way analysis of variance followed by a post-hoc
LSD test. P<0.05 was considered to indicate a statistically
significant difference.
Results
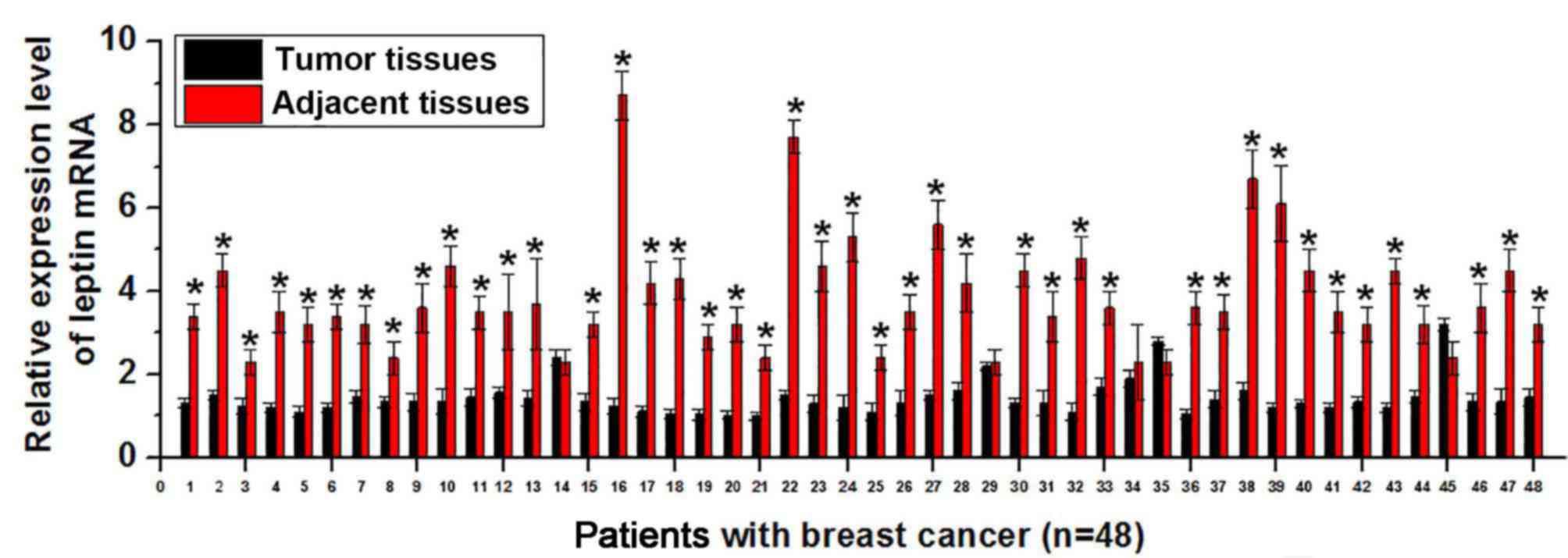
Expression of leptin mRNA in tumor
tissues and adjacent healthy tissues of patients with breast
cancer
Expression of leptin is upregulated in different
human diseases, including cardiovascular diseases (8) and non-alcoholic fatty liver disease
(9). Therefore, expression of leptin
mRNA in tumor tissues and adjacent healthy tissues was detected via
RT-qPCR. As presented in Fig. 1,
expression of leptin mRNA was significantly higher in tumor tissues
than in adjacent healthy tissues in 43 of 48 patients with breast
cancer (P<0.01. This suggests that increased expression level of
leptin is associated with the development of breast cancer.
Serum leptin in patients with breast
cancer and healthy controls
Serum leptin was detected by ELISA. Results
indicated that levels of serum leptin were significantly higher in
patients with breast cancer than in healthy controls (P<0.05;
Fig. 2).
Leptin treatment promoted breast
cancer cell proliferation
As presented in Fig.
3A, expression level of leptin mRNA was significantly lower in
the normal human breast cell line Hs 578Bst than in the breast
cancer cell lines MCF-7 and MDA-MB-231 (P<0.05). Different
concentrations of leptin were used to treat breast cancer cells and
effects of leptin on cell proliferation were detected via cell
proliferation assay. As presented in Fig. 3, the cell proliferation ability of
the MCF-7 (Fig. 3B) and MDA-MB-231
(Fig. 3C) cell lines were markedly
increased by leptin treatment in a dose-dependent manner. However,
leptin treatment had no marked effect on the cell proliferation
ability of the normal human breast cell line Hs 578Bst (data not
shown).
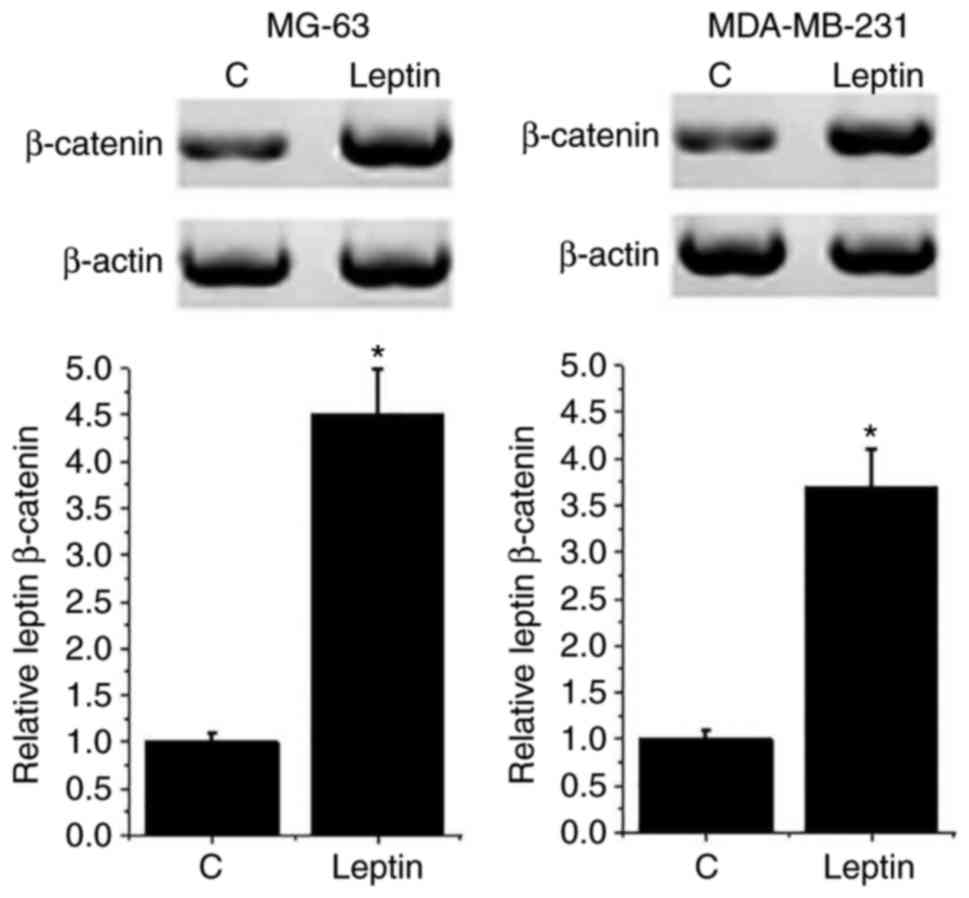
Leptin treatment increased expression
level of β-catenin in breast cancer cells
The Wnt/β-catenin pathway serves pivotal roles in
the development of different types of cancer, including ovarian
(13) and colorectal cancer
(14). Therefore, the effects of
leptin on the Wnt/β-catenin pathway were detected by western
blotting. As presented in Fig. 4,
the expression level of β-catenin was significantly increased in
both breast cancer cell lines following 100 mM leptin treatment
compared with C cells (P<0.05). However, leptin treatment had no
significant effect on β-catenin expression in the normal human
breast cell line Hs 578Bst (data not shown). These results suggest
that leptin may promote the growth of breast cancer by activating
the Wnt/β-catenin pathway.
Wnt pathway inhibitor inhibited the
enhancing effects of leptin on proliferation of breast cancer
Wnt inhibitor PNU-74654 was used to treat breast
cancer cells in culture with 100 mM leptin and culture medium. As
presented in Fig. 5, 100 mM leptin
markedly promoted the proliferation of both breast cancer cell
lines. However, Wnt inhibitor markedly ameliorated this effect in
both breast cancer lines.
Discussion
Leptin is a type of hormone that can be transported
within the human body to participate in a variety of physiological
and biochemical processes. In contrast with the function of ghrelin
as a ‘hunger hormone’, leptin is a ‘satiety hormone’ that inhibits
feelings of hunger to regulate energy balance (15). Previous studies have demonstrated
that the increased expression level of leptin is usually
accompanied with the development of various human diseases,
including cardiovascular disease and non-alcoholic fatty liver
disease (8,9). Leptin expression was significantly
upregulated in esophageal cancer, and increased expression level of
leptin predicts poor prognosis (16). Besides the direct roles of leptin in
pathological processes, increased expression level of leptin in
tumor tissue is also responsible for the development of drug
resistance in the treatment of certain types of human cancer, such
as gastro-esophageal adenocarcinomas (17). Treatment of breast cancer is also
challenged by drug resistance (18,19). In
the present study, the expression level of leptin mRNA was
significantly higher in tumor tissues than in adjacent healthy
tissues of 43 out of 48 patients with breast cancer. In addition,
serum level of leptin protein was also significantly higher in
patients with breast cancer than in normal controls. A previous
study has demonstrated that leptin is highly expressed in breast
cancer tissues with drug resistance (20). Those data suggest that increased
leptin expression may be associated with the development of breast
cancer, and leptin may also serve as a target to improve treatment
outcomes of breast cancer by reducing chemotherapy resistance.
Leptin promotes proliferation of both normal tissue
cells and cancer cells. In a previous study of prostate cancer,
Somasundar et al (21)
reported that increased expression of leptin was closely correlated
with the increased proliferation ability of cancer cells, and
downregulation of leptin expression significantly reduced the
proliferation rate of prostate cancer cells, indicating that leptin
may serve as a potential target for the treatment of prostate
cancer. In another study, Wang et al (22) reported that increased expression of
leptin promoted cell proliferation and inhibited cell apoptosis of
colorectal carcinoma, which in turn accelerated tumor growth. In
the present study, leptin increased the proliferation rate of two
breast cancer cell lines in a dose-dependent manner, indicating
that leptin promotes the growth of breast cancer by stimulating
cancer cell proliferation.
Wnt/β-catenin is a key player in the development of
various types of cancer. In a previous study of lung cancer, Teng
et al (23) reported that
Wnt/β-catenin signal transduction served pivotal roles in
regulating the proliferation and differentiation of cancer stem
cells. Activation of the Wnt/β-catenin pathway is enriched in
different types of breast cancers, and increased expression level
of β-catenin typically indicates poor treatment outcomes (24); therefore, the Wnt/β-catenin pathway
is considered to be a potential target for the treatment of breast
cancer (25). In the present study,
treatment with 100 mM leptin significantly increased the expression
level of β-catenin in breast cancer cell lines MCF-7 and
MDA-MB-231, indicating that leptin can activate the Wnt/β-catenin
pathway in breast cancer. In addition, treatment with leptin
inhibitor markedly reduced the enhancing effects of leptin on the
proliferation of breast cancer cells. Those data suggest that
leptin can promote the proliferation of breast cancer cells at
least partially by activating the Wnt/β-catenin pathway.
In conclusion, leptin expression level was increased
in breast cancer tissues compared with adjacent healthy tissues.
Serum level of leptin protein was significantly higher in patients
with breast cancer than in normal controls. Leptin promoted the
proliferation of breast cancer cells and activated the
Wnt/β-catenin pathway, whereas treatment with leptin inhibitor
markedly reduced the enhancing effects of leptin on the
proliferation of breast cancer cells. These findings suggest that
leptin can promote breast cancer growth by activating the
Wnt/β-catenin pathway. However, the present study was also limited
by some shortcomings; for example, nuclear translocation of
β-catenin in breast cancer cells was not confirmed by
immunocytochemistry. The present authors intend to present this
data in a future study. In addition, leptin gene knockdown and
overexpression breast cancer cell lines will also be established to
further confirm the conclusions of the present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and LeZ designed the present study; XL, SW and
LiZ performed experiments; XL, HZ and LZ analyzed the data; and LeZ
wrote the manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of China-Japan Union Hospital (Changchun, China), and all
patients provided written informed consent.
Consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Holohan C, van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Housman G, Byler S, Heerboth S, Lapinska
K, Longacre M, Snyder N and Sarkar S: Drug resistance in cancer: An
overview. Cancers (Basel). 6:1769–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Longley DB and Johnston PG: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Driscoll L and Clynes M: Biomarkers and
multiple drug resistance in breast cancer. Curr Cancer Drug
Targets. 6:365–384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ellis LM and Hicklin DJ: Resistance to
targeted therapies: Refining anticancer therapy in the era of
molecular oncology. Clin Cancer Res. 15:7471–7478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sorrentino A, Liu CG, Addario A, Peschle
C, Scambi G and Ferlini C: Role of microRNAs in drug resistant
ovarian cancer cells. Gynecol Oncol. 111:478–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan H, Guo J and Su Z: Advances in
understanding the interrelations between leptin resistance and
obesity. Physiol Behav. 130:157–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wallace AM, McMahon AD, Packard CJ, Kelly
A, Shepherd J, Gaw A and Sattar N: Plasma leptin and the risk of
cardiovascular disease in the west of Scotland coronary prevention
study (WOSCOPS). Circulation. 104:3052–3056. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Polyzos SA, Aronis KN, Kountouras J,
Raptis DD, Vasiloglou MF and Mantzoros CS: Circulating leptin in
non-alcoholic fatty liver disease: A systematic review and
meta-analysis. Diabetologia. 59:30–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stolzenberg-Solomon RZ, Newton CC,
Silverman DT, Pollak M, Nogueira LM, Weinstein SJ, Albanes D,
Männistö S and Jacobs EJ: Circulating leptin and risk of pancreatic
cancer: A pooled analysis from 3 cohorts. Am J Epidemiol.
182:187–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andò S, Barone I, Giordano C, Bonofiglio D
and Catalano S: The multifaceted mechanism of leptin signaling
within tumor microenvironment in driving breast cancer growth and
progression. Front Oncol. 4:3402014.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagaraj AB, Joseph P, Kovalenko O, Singh
S, Armstrong A, Redline R, Resnick K, Zanotti K, Waggoner S and
DiFeo A: Critical role of Wnt/β-catenin signaling in driving
epithelial ovarian cancer platinum resistance. Oncotarget.
6:23720–23734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang G, Zhu H, Shi Y, Wu W, Cai H and
Chen X: cir-ITCH plays an inhibitory role in colorectal cancer by
regulating the Wnt/β-catenin pathway. PLoS One. 10:e01312252015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Friedman JM and Halaas JL: Leptin and the
regulation of body weight in mammals. Nature. 395:763–770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Howard JM, Cathcart MC, Healy L, Beddy P,
Muldoon C, Pidgeon GP and Reynolds JV: Leptin and adiponectin
receptor expression in oesophageal cancer. Br J Surg. 101:643–652.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bain GH, Collie-Duguid E, Murray GI,
Gilbert FJ, Denison A, McKiddie F, Ahearn T, Fleming I, Leeds J,
Phull P, et al: Tumour expression of leptin is associated with
chemotherapy resistance and therapy-independent prognosis in
gastro-oesophageal adenocarcinomas. Br J Cancer. 110:1525–1534.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY,
Park BE, Jang Y, Cho SY and Kim HS: Potential role of leptin in
angiogenesis: Leptin induces endothelial cell proliferation and
expression of matrix metalloproteinases in vivo and in vitro. Exp
Mol Med. 33:95–102. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding Y, Cao Y, Wang B, Wang L, Zhang Y,
Zhang D, Chen X, Li M and Wang C: APPL1-mediating leptin signaling
contributes to proliferation and migration of cancer cells. PLoS
One. 11:e01661722016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGlothen TZ, Hogan-Blaylock D, Gillespie
C, Colbert L, Guo G, Sanford G and Gonzalez-Perez RR:
Leptin-Notch-Wnt axis affects drug resistance in breast cancer.
Cancer Res. 72:2012. View Article : Google Scholar
|
|
21
|
Somasundar P, Frankenberry KA, Skinner H,
Vedula G, McFadden DW, Riggs D, Jackson B, Vangilder R, Hileman SM
and Vona-Davis LC: Prostate cancer cell proliferation is influenced
by leptin. J Surg Res. 118:71–82. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang D, Chen J, Chen H, Duan Z, Xu Q, Wei
M, Wang L and Zhong M: Leptin regulates proliferation and apoptosis
of colorectal carcinoma through PI3K/Akt/mTOR signalling pathway. J
Biosci. 37:91–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teng Y, Wang X, Wang Y and Ma D:
Wnt/beta-catenin signaling regulates cancer stem cells in lung
cancer A549 cells. Biochem Biophys Res Commun. 392:373–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khramtsov AI, Khramtsova GF, Tretiakova M,
Huo D, Olopade OI and Goss KH: Wnt/beta-catenin pathway activation
is enriched in basal-like breast cancers and predicts poor outcome.
Am J Pathol. 176:2911–2920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
King TD, Suto MJ and Li Y: The
Wnt/β-catenin signaling pathway: A potential therapeutic target in
the treatment of triple negative breast cancer. J Cell Biochem.
113:13–18. 2012. View Article : Google Scholar : PubMed/NCBI
|