Introduction
Coronary artery disease (CAD) is one of the most
common cardiovascular diseases in the elderly population worldwide
(1). Percutaneous coronary
intervention (PCI) has become the mainstay invasive therapy for CAD
patients (2). Recent advances in
interventional cardiology have highlighted the importance of a
detailed understanding of the tissue characteristics of coronary
atherosclerotic lesions, including the identification of plaque
stability, estimation of lesion covering, and tearing degree pre-
and post-PCI (3,4). Coronary angiography (CAG) has been
considered to be the gold standard diagnostic method for CAD and is
widely accepted as a firm basis for determining treatment
strategies for PCI (5). However,
previous evidence indicated that CAG may not provide accurate
images in certain cases and may fail to reflect specific critical
characteristics of atherosclerotic lesions or vessel-stent
associations in high-risk cases (5–7).
Therefore, novel techniques that fully reflect lesion and stent
features have been developed in interventional cardiology.
Optical coherence tomography (OCT) is a novel
technique that can provide cross-sectional and three-dimensional
imaging in vivo with ultra-high resolution (10–20 µm)
(8–12). It is a particularly attractive
technique because it provides real-time images and microstructural
information on the tissues (13,14).
Furthermore, it can be used to provide a detailed analysis of the
coronary artery wall, including plaque characterization, thin-cap
fibroatheroma (TCFA) and vulnerable plaque identification, and
assessments of the vascular response to PCI, which may be
responsible for acute coronary events (11,12,15–17).
Considerable evidence currently indicates that the early use of OCT
reveals various abnormal vessel reactions associated with stent
implantation, such as stent malapposition, suboptimal stent
deployment, thrombus, tissue prolapse, and edge dissection
(18,19). Finally, OCT is also helpful for
guiding coronary management and interventions, including stent
apposition and the early identification of procedure-associated
complications (17,20). However, the evaluation of OCT
clinical usefulness requires a prospective comparison of the two
techniques in a significant number of patients.
The present study, a comparative prospective study
of the CAG and OCT findings prior to and/or following PCI, was
designed and conducted to investigate whether OCT may provide
further information in addition to the traditional CAG and lead to
substantial changes in PCI treatment strategies.
Materials and methods
Study population
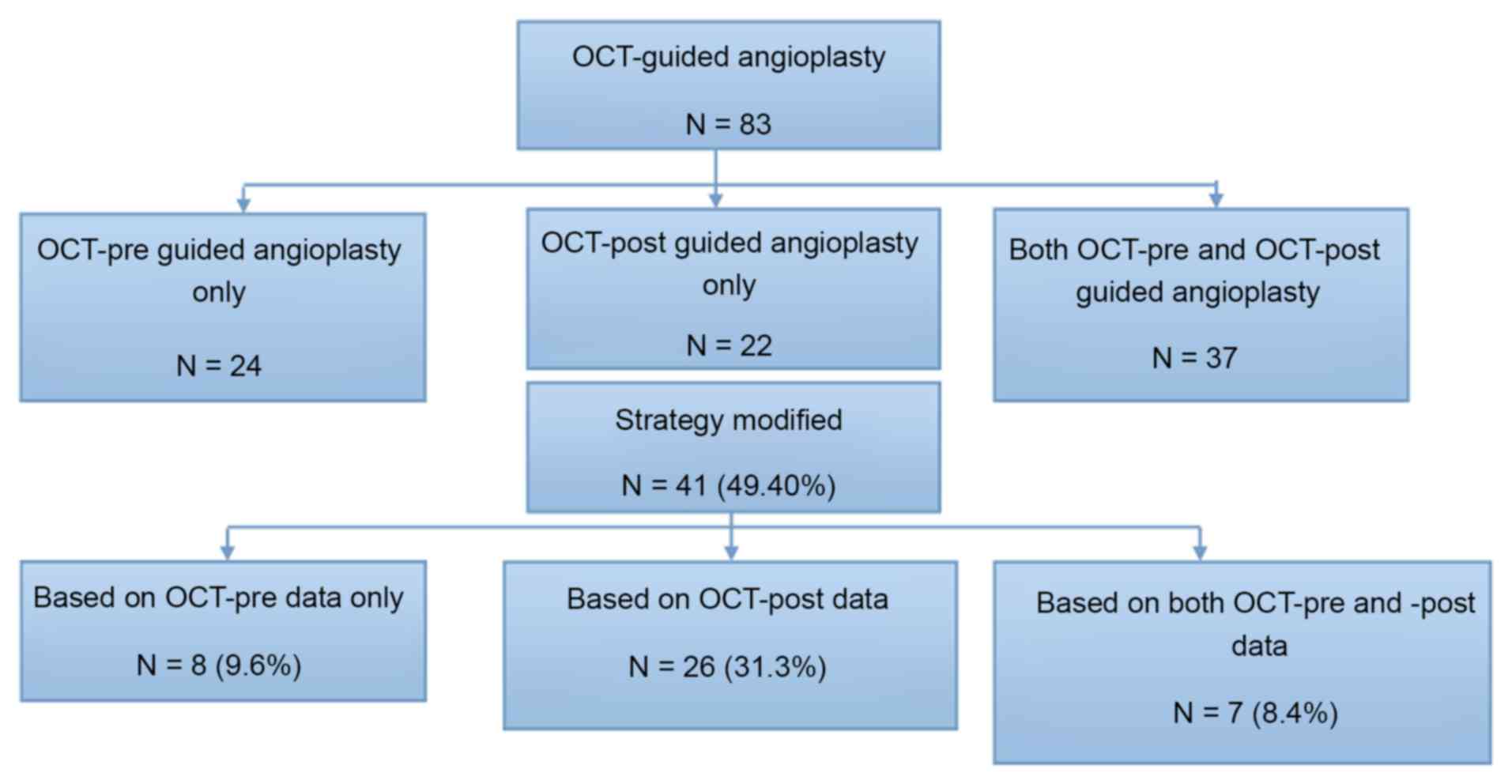
In the present study, 83 consecutive patients
(>18 years old) with CAD scheduled to undergo PCI were recruited
prospectively at the Department of Cardiology, University Hospital
Jean-Minjoz (Besançon, France) between January 2011 and December
2012. The study was designed to separately analyze the treatment
strategy changes that were respectively achieved by OCT performance
when performed before or after angioplasty and to determine the
possible advantages and inconveniences of performing both. OCT was
run before angioplasty, subsequent to the initial CAG in 24
patients (OCT-pre group); or it was run after angioplasty following
angiography in 22 patients (OCT-post group); 37 patients underwent
OCT both before and after angioplasty (Fig. 1). The protocol was approved by the
Ethics Committee of the Regional Health Agency
Bourgogne/Franche-Comté (Comité de Protection des Personnes EST
II), and all patients provided written informed consent for the
OCT-guided and CAG-guided PCI and the follow-up.
Outcomes
The primary study objective was to determine the
percentage of patients in whom an alteration in the procedural
treatment strategy was decided based on the information obtained
from OCT imaging. This was defined as a change in one or more of
the following parameters: i) Additional balloon inflation; ii)
implantation of Additional stent(s); iii) use of glycoprotein (GP)
IIb/IIIa inhibitors; iv) use of thrombus aspiration; v) use of
rotational atherectomy; vi) avoiding stenting.
The secondary objective was to compare the
percentages of patients in whom OCT revealed the presence of one or
more of the following parameters: Thrombus burden, plaque rupture,
spontaneous dissection, and identified calcification (11). These parameters identified
respectively by OCT-pre and CAG-pre recordings were compared.
Following angioplasty, the incidences of stent malapposition,
suboptimal stent deployment, suboptimal lesion coverage, and edge
dissection were also compared as recorded by OCT-post vs.
post-angioplasty fluoroscopy.
Safety outcomes were assessed according to the
procedural duration and perioperative outcomes of OCT, including
coronary no-reflow phenomenon as described by Berg and Buhari
(21), coronary perforation,
occlusive dissection, coronary spasm, stent occlusion, and
PCI-associated myocardial infarction (MI).
CAG procedure
CAG was performed via femoral or radial artery
access in all patients. Unfractionated heparin (2,500 U; Pharmacia
& Upjohn, London, UK) was administered prior to CAG, and a 6F
guiding catheter was inserted toward the coronary ostium. Adequate
views of the region of interest that avoided vessel foreshortening
and side-branch overlap were obtained subsequent to the
administration of an intracoronary bolus of nitroglycerin (200 µg;
Nitronal Injection, Pohl-Boskamp GmbH & Co., Hohenlockstedt,
Germany). Classical qualitative angiographic criteria (11) were used, and the quantitative CAG
procedure was performed by experienced personnel using standard
methodology (22,23).
OCT evaluation
OCT and CAG examinations were consecutively
performed during PCI when a patient fulfilled the following
criteria: i) Suitable coronary artery anatomy for OCT evaluation as
instructed by the international consensus of clinicians (9,24,25); ii)
stable hemodynamics; iii) absence of severe co-morbid conditions,
including severe renal or liver dysfunction, or other
co-morbidities, such as cancer; iv) no contraindications to
iodinated contrast media, aspirin, and/or clopidogrel. However, the
OCT was not examined if CAG quality was sufficient for the
physician to make a suitable treatment decision. OCT images were
acquired using the FD-OCT Optis system (Lightlab Imaging
Incorporated, Westford, MA, USA) and 6F guide catheter compatible
Dragonfly Duo and Dragonfly Optis catheter (Lightlab Imaging
Incorporated). The catheter was introduced into the coronary artery
via a standard 0.014-inch angioplasty wire, after prior injection
of an intracoronary bolus of nitroglycerin. To remove all blood
adequately from the imaging site, nonocclusive flushing was
performed using continuously injected contrast medium via an
automated power injector, and the OCT catheter was pulled back at a
speed of 18 mm/sec to guarantee sufficient time to acquire images
of a 54-mm long segment (frame density: 10 frames/mm). When poor
image quality was obtained, the pullback was repeated subsequent to
modification of the flushing intensity or probe position. The data
were then digitally stored for offline analysis (25,26). OCT
images were analyzed online and offline using Lightlab software
(V1.13, Lightlab Imaging Incorporated). All OCT images were
analyzed in University Hospital of Besancon by 2 independent
operators blinded to the angiographic findings and procedural
strategy. Discordant OCT analyses were resolved by consensus.
Definitions and recommendations for
modifying treatment strategies
Various features were determined during the OCT
examination according to previously published consensus opinions
and studies (9,23–25). Any
inner-layer plaque profile discontinuity was considered a plaque
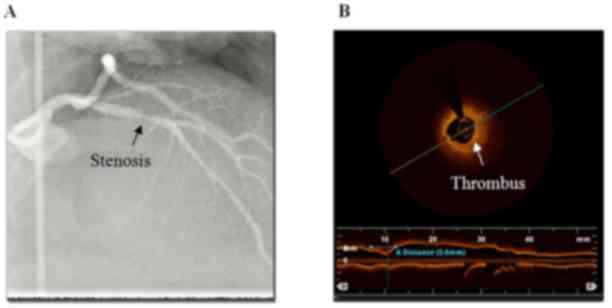
rupture. A thrombus was defined as any intraluminal mass of ≥200 µm
without vessel wall surface continuity or a highly backscattered
luminal protrusion in continuity with the vessel wall resulting in
signal-free shadowing. Dissection was confirmed as the presence of
a linear rim of tissue with a width of ≥200 µm that was evidently
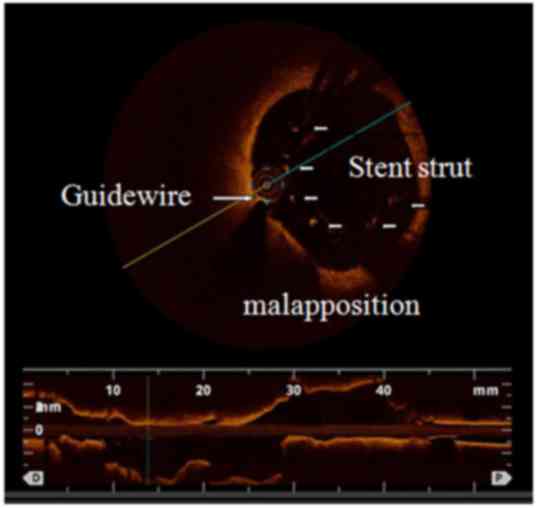
separated from the vessel wall or plaque. Stent malapposition was
identified as a distance between the stent and lumen that was
greater than the sum of strut thickness plus abluminal polymer
thickness; this was considered to be significant if the stent-lumen
distance was >200 µm. Suboptimal stent expansion was deemed to
be present when the ratio of in-stent minimal lumen area (MLA) to
average reference area was <80%.
In accordance with previously published studies
(24,26), the following actions and decision
changes were recommended when the OCT examination detected
abnormalities that were not originally recognized by the
angiography results: Edge dissection and narrowing of the
referenced lumen required additional stent implantation; stent
under-expansion and malapposition required further dilation of the
previously implanted stent with a non- or semi-compliant balloon;
stent implantation was not indicated in patients with confirmed
stenosis of <50% at the thrombosis site without dissection or
plaque rupture; platelet GP IIb/IIIa receptor antagonist
administered to patients with OCT-detected thrombosis who did not
originally receive this medication; thrombus aspiration was
indicated in patients with major thrombosis burdens; or guidewire
relocation was conducted when the original guidewire was inserted
into the false lumen of the dissection or out of the struts of the
stent. The clinician decided whether to perform additional
interventions.
Statistical analysis
Continuous variables are presented as mean ±
standard deviation, whereas categorical variables are expressed as
absolute number and percentage. Intergroup differences were
assessed using Fisher's exact test or Student's t-test when
appropriate. All calculations were performed using SPSS software
(version 11.5; SPSS Inc., Chicago, IL, USA), and values of
P<0.05 were considered to indicate a statistically significant
difference.
Results
Clinical baseline characteristics
The baseline characteristics of all patients are
listed in Table I. All patients (38
men, 45 women; mean age, 65.8±11.3 years old) were diagnosed with
CAD. The comorbidities, concurrent medications and potential
numbers of affected coronary arteries are shown in Tables I and II. Among the 83 patients, 13 with
ST-segment elevation myocardial infarction (STEMI) (15.7%), 19 with
non-STEMI (22.9%), 22 with stable angina (26.5%), 10 with unstable
angina (12.0%), 11 with silent ischemia (13.3%), and 8 with
elective percutaneous coronary intervention (9.6%) underwent
coronary angiography. More than 50% of the patients had
multi-vessel disease (54.3%), including 33 with two-vessel disease
(39.8%) and 12 with three-vessel disease (14.5%); only 38 had
single-vessel disease (45.7%). Three typical cases demonstrating
the advantages of the OCT examination compared with CAG are shown
in detail in Figs. 2–4.
 | Table I.Baseline patient characteristics
(n=83). |
Table I.
Baseline patient characteristics
(n=83).
|
Characteristics | n | % |
|---|
| Sex |
|
|
|
Male | 38 | 45.8 |
|
Female | 45 | 54.2 |
| Hypertension | 49 | 59.04 |
| Dyslipidemia | 48 | 57.83 |
| Diabetes
mellitus | 13 | 15.66 |
| Smoker | 37 | 44.58 |
| Obesity | 21 | 25.3 |
| Family history of
CVD | 20 | 24.10 |
| Prior history |
|
|
|
Myocardial infarction | 38 | 45.8 |
| Heart
failure | 4 | 4.82 |
|
Stroke | 3 | 3.61 |
| Renal
insufficiency | 11 | 13.25 |
|
Valvular heart disease | 9 | 10.84 |
|
Angioplasty | 38 | 45.8 |
|
CABG | 2 | 2.41 |
| Indication for
coronary angiography |
|
|
|
STEMI | 13 | 15.7 |
|
NSTEMI | 19 | 22.9 |
| Stable
angina | 22 | 26.5 |
|
Unstable angina | 10 | 12.0 |
| Silent
ischemia | 11 | 13.3 |
|
Elective PCI | 8 | 9.6 |
| Extent of
disease |
|
|
|
Single-vessel disease | 38 | 45.7 |
|
Two-vessel disease | 33 | 39.8 |
|
Three-vessel disease | 12 | 14.5 |
 | Table II.Treatment of the included patients
pre- and/or post-angioplasty (n=83). |
Table II.
Treatment of the included patients
pre- and/or post-angioplasty (n=83).
|
| Prior to procedure,
n (%) | Following
procedure, n (%) | P-value |
|---|
| Aspirin | 83 (100) | 83 (100) | NS |
|
Thienopyridines |
|
|
|
|
Clopidogrel | 71 (85.5) | 71 (85.5) | NS |
|
Prasugrel | 10 (12.1) | 10 (12.1) | NS |
|
Ticagrelor | 2 (2.4) | 2 (2.4) | NS |
| Anticoagulant |
|
|
|
|
Unfractionated heparin | 77 (92.8) | 77 (92.8) | NS |
|
Enoxaparin | 2 (2.4) | 2 (2.4) | NS |
|
Bivalirudin | 4 (4.8) | 4 (4.8) | NS |
| Glycoprotein
IIb/IIa inhibitor |
|
|
|
|
Tirofiban | 1 (1.2) | 3 (3.6) | 0.37 |
|
Abciximab | 2 (2.4) | 10 (12.0) | 0.036 |
|
Eptifibatide | 2 (2.4) | 11 (13.3) | 0.018 |
Qualitative data provided by OCT and
CAG prior to and following angioplasty
The qualitative analysis results of the CAG and OCT
images pre- and post-PCI are shown in Table III. A flowchart of the OCT-guided
angioplasty and OCT-based changes in treatment strategy is also
provided in Fig. 1. Among the 83
patients, a total of 61 patients underwent OCT before angioplasty
(24 patients received only OCT pre-guided angioplasty and 37
patients received OCT pre- and post-guided angioplasty) and 59
patients underwent OCT following angioplasty (22 patients received
only OCT post-guided angioplasty and 37 patients received OCT pre-
and post-guided angioplasty). Prior to PCI, compared with CAG, OCT
was more sensitive for plaque rupture (0 vs. 10 cases; P=0.007),
diagnosing thrombus (9 vs. 20 cases; P=0.0162), dissection (4 vs.
12 cases; P=0.0289), and calcification (15 vs. 49 cases;
P<0.001). Subsequent to PCI, compared with CAG, OCT was again
more sensitive for the diagnosis of thrombus (1 vs. 24 cases;
P<0.001), stent edge dissection (5 vs. 32 cases; P<0.001),
stent malapposition (1 vs. 42 cases; P<0.001), intimal tissue
protrusion (8 vs. 49 cases;P<0.001), suboptimal stent
expansion (15 vs. 29 cases; P=0.0065), stent incomplete lesion
coverage (11 vs. 20 cases; P=0.0467) and stent struts coverage
bifurcation (0 vs. 35 cases; P<0.001).
 | Table III.Qualitative data provided by CAG and
OCT pre- and post-angioplasty. |
Table III.
Qualitative data provided by CAG and
OCT pre- and post-angioplasty.
| A, Pre-angioplasty
(n=61) |
|---|
|
|---|
| Variable | CAG, n (%) | OCT, n (%) | P-value |
|---|
| Plaque rupture | 0 (0.0) | 10 (16.4) | 0.0007 |
| Thrombus | 9 (14.8) | 20 (32.8) | 0.0162 |
| Dissection | 4 (6.6) | 12 (19.7) | 0.0289 |
| Calcification | 15 (24.6) | 49 (80.3) | <0.0001 |
| Guidewire into the
false lumen | 0 (0.0) | 1 (1.7) | NS |
| Guidewire through
outside of stent | 0 (0.0) | 1 (1.7) | NS |
|
| B,
Post-angioplasty (n=59) |
|
|
Variable | CAG, n
(%) | OCT, n
(%) | P-value |
|
| Thrombus | 1 (1.7) | 24 (40.7) | <0.0001 |
| Dissection | 5 (8.5) | 32 (54.2) | <0.0001 |
| Stent
malapposition | 1 e(1.7) | 42 (71.19) | <0.0001 |
| Intimal tissue
protruding | 8 (13.6) | 49 (83.1) | <0.0001 |
| Suboptimal stent
expansion | 15 (25.4) | 29 (49.2) | 0.0065 |
| Incomplete Lesion
coverage | 11 (18.6) | 20 (33.9) | 0.0467 |
| Stent coverage
bifurcation | 40 (67.8) | 40 (67.8) | NS |
| Stent strut
coverage bifurcation | 0 (0.0) | 35 (59.3) | <0.0001 |
Quantitative characteristics provided
by OCT and CAG prior to and following angioplasty
The target vessel and lesion characteristics
detected pre- and post-PCI are shown in Table IV. Prior to PCI, the lesion diameter
and ratio of diameter stenosis identified by OCT were significantly
different from those measured by CAG (1.7±0.6 mm vs. 1.3±0.6 mm and
47.1±0.4% vs. 57.0±16.9%, respectively; P<0.001); after PCI, the
stent diameter and ratio of diameter stenosis on OCT were
significantly different from those measured by CAG (3.0±0.6 mm vs.
2.6±0.5 mm and 10.0±8.3% vs. 14.3.0±8.0%, respectively;
P<0.001). The reference vessel diameter measured by OCT was not
significantly different from that measured by CAG (3.3±1.7 vs.
3.0±0.9 mm and 3.3±1.5 vs. 3.0±0.6 mm, respectively; P>0.050)
both before and after PCI. All vessel cross-sectional areas that
could not be measured directly by CAG were also obtained by
OCT.
 | Table IV.Quantitative characteristics of
potentially affected vessels and lesions as provided by CAG and OCT
pre- and post-angioplasty. |
Table IV.
Quantitative characteristics of
potentially affected vessels and lesions as provided by CAG and OCT
pre- and post-angioplasty.
| A, Pre-angioplasty
results (n=61) |
|---|
|
|---|
| Variable | CAG | OCT | P-value |
|---|
| Lesion diameter
(mm) | 1.3±0.6 | 1.7±0.6 | <0.0001 |
| Reference vessel
diameter (mm) | 3.0±0.9 | 3.3±1.7 | NS |
| Ratio of diameter
stenosis (%) | 57.0±16.9 | 47.1±0.4 | <0.0001 |
| Lesion area
(mm2) | – | 2.8±0.6 | – |
| Reference vessel
area (mm2) | – | 8.7±2.9 | – |
| Ratio of area
stenosis (%) | – | 67.4±0.2 | – |
|
| B,
Post-angioplasty results (n=59) |
|
|
Variable | CAG | OCT | P-value |
|
| Stent diameter
(mm) | 2.6±0.5 | 3.0±0.6 | 0.0002 |
| Reference vessel
diameter (mm) | 3.0±0.6 | 3.3±1.5 | NS |
| Ratio of diameter
stenosis (%) | 14.3±8.0 | 10.0±8.3 | 0.0052 |
| Stent area
(mm2) | – | 7.2±2.6 | – |
| Reference vessel
area (mm2) | – | 9.0±4.6 | – |
| Ratio of area
stenosis (%) | – | 19.9±3.6 | – |
Treatment strategy changes based on
OCT
Alterations in the treatments provided the patients
due to the additional information obtained by OCT images are listed
in Table V. The reason for the
changes in treatment strategies based on the OCT findings were as
follows: Thrombus detection, for which 2 patients were treated with
thrombus aspiration and 8 with GP IIb/IIIa inhibitors, while 4
patients avoided stent implantations; dissection detection, for
which 11 patients received additional stent implantation; stent
malapposition observation, for which 1 patient received additional
stent implantation and 11 received additional balloon inflation;
suboptimal stent expansion, for which 12 patients were treated with
additional balloon inflation; plaque rupture, for which 2 patients
were treated with additional stent implantation; stent incomplete
coverage lesion detection, for which 3 patients received additional
stent implantation; stent coverage bifurcation, for which 2
patients received bifurcation intervention; and guidewire
translocation, for which 2 patients were treated by guidewire
repositioning. Therefore, there were 58 modifications of the
therapeutic strategy in total. Because, in some cases, several
types of changes of strategy intervened in a single patient
treatment changes occurred in 41 patients among the 83 patients
included in the study (i.e., 49.4% of patients). The 41 patients
with the treatment strategy changes included 8 (9.6%, 8/83) whose
therapy modification was based on OCT-pre data only, 26 (31.3%,
26/83) whose therapy modification was based on OCT-post data only,
and 7 (8.4%, 7/83) whose therapy modification was based on both
(Fig. 1).
 | Table V.Treatment strategy changes based on
the optical coherence tomography data (n=83). |
Table V.
Treatment strategy changes based on
the optical coherence tomography data (n=83).
| Number of
person-times | Thrombus
aspiration | Use of GP IIb/IIIa
inhibitors | Additional balloon
inflation | Additional stent
implantation | Avoiding stent
implantation | Guidewire
repositioning | Bifurcation
intervention | Total |
|---|
| Thrombus, n | 2 | 8 |
|
| 4 |
|
| 14 |
| Dissection, n |
|
|
| 11 |
|
|
| 11 |
| Malapposition,
n |
|
| 11 | 1 |
|
|
| 12 |
| Suboptimal stent
expansion, n |
|
| 12 |
|
|
|
| 12 |
| Plaque rupture,
n |
|
|
| 2 |
|
|
| 2 |
| Stent incomplete
coverage lesions, n |
|
|
| 3 |
|
|
| 3 |
| Stent coverage
bifurcation, n |
|
|
|
|
|
| 2 | 2 |
| Guidewire
translocation, n |
|
|
|
|
| 2 |
| 2 |
| Total strategy
modification, n | 2 | 8 | 23 | 17 | 4 | 2 | 2 | 58 |
| Strategy
modification percentage, % | 2.4 | 9.6 | 27.7 | 20.5 | 4.8 | 2.4 | 2.4 | 69.9 |
Safety outcomes
The mean procedural duration was 48.5±23.5 min for
OCT and 12.5±8.1 min for CAG. One patient experienced a coronary
spasm during the OCT examination that was relieved following the
administration of coronary dilative medication. No perioperative
complications of no-reflow, coronary perforation, occlusive
dissection, stent occlusion, or PCI-associated MI were
observed.
Discussion
Although CAG is widely considered the gold standard
for the diagnosis of CAD as well as a primary examination for
guiding PCI procedures and judging coronary intervention success
(5,27,28),
studies have suggested that its use alone may miss important
information. Such studies have claimed that treatment strategies
would be modified if the OCT examination was used with or instead
of CAG (27,29). A previous study demonstrated that OCT
use may lead to a change in procedural strategy in 50% of patients
in the patients with non-ST-segment elevation acute coronary
syndromes (11). Therefore, the
present study prospectively compared CAG- and OCT-guided
interventional therapies for CAD performed before and/or after the
angioplasty procedure and confirmed that evaluating CAD patients
with OCT compared with CAG provided additional clinical information
for the diagnosis and guiding of PCI therapy. This finding highly
suggests that OCT may be necessary for complex lesions in which the
correlation between vascular lesions, vessel walls, and stents is
not accurately detected by CAG. Thus, the current study findings
support the results of the previous studies and indicate that the
use of OCT provides crucial information that may modify the
treatment strategies in CAD.
However, the present study had certain limitations.
Firstly, the number of included cases is relatively small since it
was a pilot observational prospective study whose design included
data form only 37 patients with both OCT-pre and post-angioplasty;
the other patient data was for either OCT-pre or post-angioplasty
only. The potential middle- and long-term clinical benefits of
OCT-guided PCI compared with the CAG-guided PCI should be confirmed
in a large randomized controlled trial. The patients included in
the current study were followed up for a limited duration;
therefore, the study is currently unable to evaluate the effect of
OCT-guided PCI on clinical outcomes. To the best of our knowledge,
there is no study comparing the effect of OCT-guided PCI with
angiography-guided PCI on the clinical outcome of patients with
CAD. A recently published trial identified that OCT-guided PCI was
safe and resulted in similar minimum stent area to that of
angiography-guided PCI (16).
Previous studies suggested that, despite the
limitation of OCT images to a depth of 2–3 mm (29), the high resolution of OCT results in
higher sensitivity compared with CAG for identifying lesion
characteristics (30,31); thus, it may be a potentially powerful
tool to guide PCI. The present prospective study confirmed that OCT
was more sensitive for detecting small thrombi along the vessel
walls that are difficult to detect by CAG, particularly when the
thrombus was crushed by balloons following PCI (29,30). In
the present study, 2.4% of patients required thrombus aspiration,
9.6% required administration of a GP IIb/IIIa receptor antagonist,
and 4.8% avoided stent implantation when a thrombus with <50%
stenosis was identified by OCT, which would be neglected and be
considered stenosis by angiography. In addition, OCT appeared to be
helpful in patients with vascular dissection (30,32). The
current results revealed that OCT compared with CAG indicated a
higher prevalence of dissection as was suggested in previous
studies (33,34).
OCT performed after angioplasty is better able to
demonstrate the stent-vessel wall association as well as visualize
individual stent struts and their distance from the vessel wall
(10,17,27,35). The
present study results indicated that dissections were not
identified by CAG; however, they were detected by OCT. Considering
that stent malapposition is an important reason for late stent
thrombosis (33,34), OCT-guided PCI may reduce the risk of
late thrombosis, although this finding requires further
confirmation in large randomized controlled trials. OCT is also
capable of detecting plaque rupture and intracoronary thrombus
(36). Although CAG and OCT can both
be used to locate lesions and estimate their pre- and post-PCI
severity, OCT is more accurate in comparison with CAG for these
purposes (33,34). The present study observed that the
reference vessel diameter measured by OCT was larger compared with
that measured by CAG. Therefore, the data provided by OCT may
assist physicians to select a larger stent than when guided by CAG,
thus avoiding stent malapposition.
In the current study, the use of OCT enabled the
detection of guidewire translocation in two patients. The guidewire
had entered the false lumen of the dissection in one patient, while
it had passed through the stent mesh and became positioned between
the struts and vascular intima in the other case. Evidently,
OCT-guided PCI may reduce these risks by the early detection of
guidewire translocation. However, whether these advantages actually
translate into clinical benefits has yet to be determined by
long-term studies.
A previous study indicated that OCT may not increase
periprocedural complications, including coronary no-reflow,
coronary perforation, occlusive dissection, and stent occlusion
(37). However, those complications
were not observed in the current study when either imaging
procedure was performed.
In conclusion, the evaluation of CAD patients with
OCT compared with CAG provided additional clinical information for
the diagnosis and guidance of PCI therapy. Therefore, for cases
with unclear images prior to and following angioplasty, it is
suggested that an OCT examination should be conducted to further
clarify the correlation between vascular lesions, vessel walls, and
stents. However, future prospective long-term studies are required
to confirm whether the systematic application of OCT, which
significantly increases the procedure time as it is currently
performed, would improve the long-term prognosis of these
patients.
Acknowledgements
The authors would like to thank Professor Emeritus
Dominique Angèle Vuitton (University Bourgogne Franche-Comté,
Besancon, France) for her help in correcting the manuscript.
Funding
No external funding sources were used for the
present study. Dr Jianfeng Huang was funded in part by the
Scholarship program in Science and Technology Department, Consulate
General of France in Shanghai, China and by Servier International,
Paris, France.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH was involved in the acquisition and analysis of
data, and in the preparation and revision of the manuscript at all
stages; KB, MC and RC were involved in performing the coronary
angiography, including optical coherence tomography in the patients
included in the study and in recording the data; MW and XC were
involved in the analysis of data and preparation of the manuscript;
FE was involved in the design of the study and in the preparation
and revision of the manuscript; FS and NM were involved in the
design of the study, in the submission of the study to the ethical
committee, in performing the coronary angiography in the patients
and in the revision of the manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Ethics Committee of
the Regional Health Agency Bourgogne/Franche-Comté (Comité de
Protection des Personnes EST II), and all patients provided
written informed consent for the OCT-guided and CAG-guided PCI and
the follow-up.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ehara S, Hasegawa T, Nakata S, Matsumoto
K, Nishimura S, Iguchi T, Kataoka T, Yoshikawa J and Yoshiyama M:
Hyperintense plaque identified by magnetic resonance imaging
relates to intracoronary thrombus as detected by optical coherence
tomography in patients with angina pectoris. Eur Heart J Cardiovasc
Imaging. 13:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin GA, Dudley RA, Lucas FL, Malenka DJ,
Vittinghoff E and Redberg RF: Frequency of stress testing to
document ischemia prior to elective percutaneous coronary
intervention. JAMA. 300:1765–1773. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finn AV, Nakano M, Narula J, Kolodgie FD
and Virmani R: Concept of vulnerable/unstable plaque. Arterioscler
Thromb Vasc Biol. 30:1282–1292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sirker J, Pereira RG and Affleck I:
Diffusion and ballistic transport in one-dimensional quantum
systems. Phys Rev Lett. 103:2166022009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakamura M: Angiography is the gold
standard and objective evidence of myocardial ischemia is mandatory
if lesion severity is questionable. - Indication of PCI for
angiographically significant coronary artery stenosis without
objective evidence of myocardial ischemia (Pro). Circ J.
75:204–210; discussion 217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghosn MG, Leba M, Vijayananda A, Rezaee P,
Morrisett JD and Larin KV: Effect of temperature on permeation of
low-density lipoprotein particles through human carotid artery
tissues. J Biophotonics. 2:573–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raffel OC, Merchant FM, Tearney GJ, Chia
S, Gauthier DD, Pomerantsev E, Mizuno K, Bouma BE and Jang IK: In
vivo association between positive coronary artery remodelling and
coronary plaque characteristics assessed by intravascular optical
coherence tomography. Eur Heart J. 29:1721–1728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghosn MG, Syed SH, Befrui NA, Leba M,
Vijayananda A, Sudheendran N and Larin KV: Quantification of
molecular diffusion in arterial tissues with optical coherence
tomography and fluorescence microscopy. Laser Phys. 19:1272–1275.
2009. View Article : Google Scholar
|
|
9
|
Tearney GJ, Regar E, Akasaka T,
Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM,
Chowdhary S, et al: Consensus standards for acquisition,
measurement, and reporting of intravascular optical coherence
tomography studies: A report from the international working group
for intravascular optical coherence tomography standardization and
validation. J Am Coll Cardiol. 59:1058–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wijns W, Shite J, Jones MR, Lee SW, Price
MJ, Fabbiocchi F, Barbato E, Akasaka T, Bezerra H and Holmes D:
Optical coherence tomography imaging during percutaneous coronary
intervention impacts physician decision-making: ILUMIEN I study.
Eur Heart J. 36:3346–3355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meneveau N, Souteyrand G, Motreff P,
Caussin C, Amabile N, Ohlmann P, Morel O, Lefrançois Y,
Descotes-Genon V, Silvain J, et al: Optical coherence tomography to
optimize results of percutaneous coronary intervention in patients
with non-ST-elevation acute coronary syndrome: Results of the
multicenter, randomized DOCTORS Study (Does Optical Coherence
Tomography Optimize Results of Stenting). Circulation. 134:906–917.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Porto I, Mattesini A, Valente S, Prati F,
Crea F and Bolognese L: Optical coherence tomography assessment and
quantification of intracoronary thrombus: Status and perspectives.
Cardiovasc Revasc Med. 16:172–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barlis P, Serruys PW, Gonzalo N, van der
Giessen WJ, de Jaegere PJ and Regar E: Assessment of culprit and
remote coronary narrowings using optical coherence tomography with
long-term outcomes. Am J Cardiol. 102:391–395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Larin KV and Tuchin VV: Functional imaging
and assessment of the glucose diffusion rate in epithelial tissues
in optical coherence tomography. Kvantovaya Elektronika.
38:551–556. 2008. View Article : Google Scholar
|
|
15
|
Bouma BE, Yun SH, Vakoc BJ, Suter MJ and
Tearney GJ: Fourier-domain optical coherence tomography: Recent
advances toward clinical utility. Curr Opin Biotechnol. 20:111–118.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ali ZA, Maehara A, Généreux P, Shlofmitz
RA, Fabbiocchi F, Nazif TM, Guagliumi G, Meraj PM, Alfonso F,
Samady H, et al: Optical coherence tomography compared with
intravascular ultrasound and with angiography to guide coronary
stent implantation (ILUMIEN III: OPTIMIZE PCI): A randomised
controlled trial. Lancet. 388:2618–2628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Vito L, Cattabiani MA, Paoletti G, Yoon
JH, Chisari A, Gramegna M, Versaci F, Castriota F and Prati F:
Comparison between intermediate and severe coronary stenoses and
clinical outcomes of an OCT-guided PCI strategy. J Cardiovasc Med
(Hagerstown). 17:361–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gonzalo N, Serruys PW, Okamura T, Shen ZJ,
Onuma Y, Garcia-Garcia HM, Sarno G, Schultz C, van Geuns RJ,
Ligthart J and Regar E: Optical coherence tomography assessment of
the acute effects of stent implantation on the vessel wall: A
systematic quantitative approach. Heart. 95:1913–1919. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawamori H, Shite J, Shinke T, Otake H,
Sawada T, Kato H, Miyoshi N, Yoshino N, Kozuki A, Hariki H, et al:
The ability of optical coherence tomography to monitor percutaneous
coronary intervention: Detailed comparison with intravascular
ultrasound. J Invasive Cardiol. 22:541–545. 2010.PubMed/NCBI
|
|
20
|
Farooq MU, Khasnis A, Majid A and Kassab
MY: The role of optical coherence tomography in vascular medicine.
Vasc Med. 14:63–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berg R and Buhari C: Treating and
preventing no reflow in the cardiac catheterization laboratory.
Curr Cardiol Rev. 8:209–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stamper D, Weissman NJ and Brezinski M:
Plaque characterization with optical coherence tomography. J Am
Coll Cardiol. 47:C69–C79. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bezerra HG, Costa MA, Guagliumi G, Rollins
AM and Simon DI: Intracoronary optical coherence tomography: A
comprehensive review clinical and research applications. JACC
Cardiovasc Interv. 2:1035–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Windecker S, Hernandez-Antolin RA,
Stefanini GG, Wijns W and Zamorano JL: Management of ST-elevation
myocardial infarction according to European and American
guidelines. Euro Intervention. 10:(Suppl T):. T23–T31.
2014.PubMed/NCBI
|
|
25
|
Demir OM, Alfakih K and Plein S: Current
international guidelines for the investigation of patients with
suspected coronary artery disease. Eur Heart J Cardiovasc Imaging.
15:1422–1424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Incani A, Poon K, Savage M, Pincus M,
Small A, Bett N, Chua R, Mishra A, Walters D and Raffel C.: Dynamic
Changes to the Proximal Reference Segment Luminal Dimension during
Percutaneous Coronary Intervention an Optical Coherence Tomography
(OCT) Study. The Prince Charles Hospital. 21(Supplement 1):
S37–S382012.
|
|
27
|
Barlis P, Regar E, Serruys PW, Dimopoulos
K, van der Giessen WJ, van Geuns RJ, Ferrante G, Wandel S,
Windecker S, van Es GA, et al: An optical coherence tomography
study of a biodegradable vs. durable polymer-coated limus-eluting
stent: A LEADERS trial sub-study. Eur Heart J. 31:165–176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gonzalo N, Serruys PW, Okamura T, Shen ZJ,
Garcia-Garcia HM, Onuma Y, van Geuns RJ, Ligthart J and Regar E:
Relation between plaque type and dissections at the edges after
stent implantation: An optical coherence tomography study. Int J
Cardiol. 150:151–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yonetsu T, Kakuta T, Lee T, Takahashi K,
Kawaguchi N, Yamamoto G, Koura K, Hishikari K, Iesaka Y, Fujiwara H
and Isobe M: In vivo critical fibrous cap thickness for
rupture-prone coronary plaques assessed by optical coherence
tomography. Eur Heart J. 32:1251–1259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kubo T, Imanishi T, Takarada S, Kuroi A,
Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, et al:
Assessment of culprit lesion morphology in acute myocardial
infarction: Ability of optical coherence tomography compared with
intravascular ultrasound and coronary angioscopy. J Am Coll
Cardiol. 50:933–939. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng T, Yundai C, Lian C, Zhijun S,
Changfu L, Jun G and Hongbin L: Assessment of coronary plaque
characteristics by optical coherence tomography in patients with
diabetes mellitus complicated with unstable angina pectoris.
Atherosclerosis. 213:482–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hatsukami TS, Ross R, Polissar NL and Yuan
C: Visualization of fibrous cap thickness and rupture in human
atherosclerotic carotid plaque in vivo with high-resolution
magnetic resonance imaging. Circulation. 102:959–964. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bouma BE, Tearney GJ, Yabushita H,
Shishkov M, Kauffman CR, DeJoseph Gauthier D, MacNeill BD, Houser
SL, Aretz HT, Halpern EF and Jang IK: Evaluation of intracoronary
stenting by intravascular optical coherence tomography. Heart.
89:317–320. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Radu M, Jorgensen E, Kelbaek H, Helqvist
S, Skovgaard L and Saunamaki K: Optical coherence tomography at
follow-up after percutaneous coronary intervention: Relationship
between procedural dissections, stent strut malapposition and stent
healing. EuroIntervention. 7:353–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takano M, Yamamoto M, Inami S, Murakami D,
Ohba T, Seino Y and Mizuno K: Appearance of lipid-laden intima and
neovascularization after implantation of bare-metal stents extended
late-phase observation by intracoronary optical coherence
tomography. J Am Coll Cardiol. 55:26–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kubo T, Xu C, Wang Z, van Ditzhuijzen NS
and Bezerra HG: Plaque and thrombus evaluation by optical coherence
tomography. Int J Cardiovasc Imaging. 27:289–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hayat U, Thondapu V, Ul Haq MA, Foin N,
Jang IK and Barlis P: Optical coherence tomography to evaluate
coronary stent implantation and complications. Coron Artery Dis.
26E(Suppl 1): e55–e68. 2015. View Article : Google Scholar
|