Introduction
Coronary atherosclerotic heart disease (CHD), also
known as coronary heart disease, is caused by coronary
atherosclerosis that leads to occlusion and stenosis of the
coronary arteries leading to myocardial ischemia and hypoxia in
patients (1,2). It is the most common cardiovascular
disease for the elderly, and untimely treatment will lead to
disability and death, and it is also a kind of disease with high
mortality rate in the elderly (3).
According to statistics, there are as high as 290 million people
suffering from cardiovascular diseases in China, and mortality rate
is higher than 30%, and the mortality and morbidity rates are now
increasing year by year (4,5). Acute myocardial infarction (AMI) is the
most serious type with the highest incidence among coronary heart
disease in the elderly, mainly caused by coronary artery stenosis
due to atherosclerosis, leading to myocardial ischemia, myocardial
injury, and it is the main cause of sudden death clinically. There
are as many as 8.5 million patients annually who die of AMI. At
present, with the aging of the population, people pay more and more
attention to the health problems of the elderly. This is also one
of the problems to be solved immediately (6–8).
Fluvastatin is a reductase inhibitor, which mainly
reduces the synthesis and storage of cholesterol in liver cells,
and also has the effect of lowering blood cholesterol and
low-density lipoprotein (9).
Clinically, fluvastatin is the most widely used lipid-modifying
drug, which has little side-effects and adverse reactions, playing
an important role in atherosclerosis (10). Toll-like receptor 4 (TLR4), an innate
immune receptor, is an important member of the Toll-like receptor
family. It is a class of cytokines that recognizes the early
invasion of pathogens and induces cell differentiation (11). TLR4 plays an important regulatory
role in wound healing, anti-infective immunity and tumor formation.
The relationship between fluvastatin and TLR4 signaling pathway in
cardiomyocytes of myocardial infarction (MI) rats model has not
been reported in domestic and foreign literature yet. In this
study, we investigated the effect of fluvastatin on cardiomyocyte
apoptosis and the effect of fluvastatin on TLR4 signaling pathway
by constructing MI rats, and further explored the protective effect
of fluvastatin on cardiomyocytes.
Materials and methods
Experimental animals
Eighty healthy Wistar rats, male, 6–9 weeks old,
weighing 180–300 g, were purchased from Beijing Vital-Lihua
Experimental Animal Technology Co., Ltd. (Beijing, China). The rats
were fed in separated cages at room temperature 26°C and regular
light, with ambient noise <45 dB, feeding for a week in the
above-mentioned environment. The study was approved by the Ethics
Committee of Qilu Hospital of Shandong University (Jinan,
China).
Experimental model construction and
grouping
Eighty rats were randomly divided into normal
control group (n=20), sham operation group (n=20), IM group (n=20)
and fluvastatin treatment group (n=20). Rat IM model was
established: Rats were anesthetized with 10% chloral hydrate (300
µl/g); tracheal intubation was used for assisted respiration in
rats; the thoracic and pericardium were opened by surgery to expose
the heart; the needle was inserted at the left atrial appendage;
the coronary artery was ligated below the pulmonary cone by using
5/0 thread. The anterior part of the local ventricle was white
after the ligation; while in the sham operation group, the
threading was not ligated. After operation, fluvastatin treatment
group was given intragastric administration of [30 mg/(kg·d)], and
the other groups were given intragastric administration of an equal
volume of normal saline, each group received gavage for 1 week.
Sample processing
On day 7 of lavage, 10% KCI (3 ml) was injected
through the jugular vein. When the heart stopped beating, the chest
was opened immediately and the heart was cut, placed in saline for
cleaning. The left and right atrial appendage and blood vessels
were removed and the heart was dried with filter paper; the heart
was cut into two parts in the papillary muscle plane; 200 mg tissue
was obtained near the apical non-infarcted area for the
determination of TLR4 protein; the other part was placed in a −80°C
refrigerator for preservation.
Cardiomyocyte apoptosis detection
TUNEL assay kit was used to detect the myocardial
apoptotic cells in each group, and the operation was performed
strictly according to the instructions of the kit. Apoptotic
cardiomyocytes nuclei stained yellow or yellow-brown, and normal
myocardial nuclei stained blue. The cells were counted using light
microscope (BX-42; Olympus, Tokyo, Japan). Five fields were
randomly selected for each slide, and the apoptotic cells in the
field of vision were counted. The apoptosis rate (AI) = total
apoptosis/total cell number × 100%.
Western blot method
The spare cells were extracted and added into the
cell lysis solution. The RIPA lysis buffer (Sigma-Aldrich, St.
Louis, MO, USA) was placed on the ice for 1 h. After shaking
vigorously for 1–2 times every 10 min, the cells were centrifuged
at 9,350 × g for 10 min at 4°C. The supernatant was taken and the
protein concentration was measured by the BCA method. A total of 30
µg of total protein was extracted and subjected to a buffer. After
denaturation at 94°C for 3 min, the protein was separated by 12%
SDS-PAGE electrophoresis and transferred to a PVDF membrane at a
constant current of 350 mA. The membrane was placed in TBS buffer
with 5% skim milk in room temperature and in the dark for blocking
for 1 h, and placed overnight at 4°C with the corresponding
antibody; rinsed 3 times with TBS buffer on the next day for 5–10
min each, goat anti-rabbit and goat anti-rat IgG were marked with
HRP (1:2,000), then washed with TBS buffer three times for 5–10 min
each, fixed and developed by using a color-developing system, and
light-emitting imaging technology was used for exposure imaging and
grayscale scanning.
Reverse transcription-qPCR (RT-qPCR)
detection of tissue TLR4
The spare tissue was added with TRIzol reagent,
shook and placed at room temperature for 30 min for complete lysis.
Total RNA was extracted from each group of cells according to the
kit manual, and the extraction process was performed strictly
according to the instructions. The extracted RNA was analyzed by UV
spectrophotometer (Hitachi, Tokyo, Japan) and protein
electrophoresis to determine its concentration and purity. The
extracted total RNA was reverse-transcribed according to the
instructions of the reverse transcription kit. The extracted cDNA
samples were stored at −20°C. TLR4 primers designed by Shanghai
Sangon Biological Engineering Corp., (Shanghai, Chian) are shown in
Table I. PCR reaction system was
designed according to the instructions, and the total system was
12.62 µl, using DEPC to make up to 20 µl. PCR reaction conditions:
94°C pre-denaturation 10 min; 94°C 45 sec, 60°C 45 sec, 72°C 45
sec, a total of 40 cycles. The manufacturer's software was used for
amplification data analysis, and β-actin was used as an internal
reference gene.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | TLR4 | β-actin |
|---|
| Upstream |
5′-AGCAGAGGAGAAAGCATCTATGATGC-3′ |
5′-AGCAGAGAATGGAAAGTCAAA-3′ |
| Downstream |
5′-GGTTTAGGCCCCAGAGTTTTTCTCC-3′ |
5′-ATGCTGCTTACATGTCTCGAT-3′ |
Statistical analysis
SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. The data were expressed as mean
± SD, and the mean value of multiple groups were compared by
one-way ANOVA. Comparison between each two groups were by
Student-Newman-Keuls (SNK) q-test or LSD-t. P<0.05 was
considered statistically significant.
Results
Modeling results
In this experiment, among a total of 80 rats, 60
were performed with coronary artery ligation, and 38 survived 4
days after the operation; the other 20 rats were normal control
group.
RT-qPCR detection of the expression of
TLR4 mRNA in the cells
In this experiment, we detected the expression of
TLR4 mRNA by RT-qPCR in rat cardiomyocytes of four groups, and the
results showed that TLR4 mRNA was expressed in all four groups. The
expression of TLR4 in the normal control group was 0.902±0.140 and
the TLR4 expression in the sham operation group was 1.214±0.274.
TLR4 expression in IM group was 3.412±0.879 and TLR4 expression in
fluvastatin treatment group was 1.864±0.467. Compared with IM
group, TLR4 mRNA expression in sham operation group, fluvastatin
treatment group and normal control group were significantly
increased, and comparison in each group was statistically
significant (P<0.05), indicating that fluvastatin can reduce the
expression of TLR4mRNA in cardiomyocytes. There was statistical
significance between fluvastatin treatment group and sham operation
group and normal control group (P<0.05); there was no
statistical difference between normal control group and sham
operation group (P>0.05) (Table
II and Fig. 1).
 | Table II.Protein and mRNA expression. |
Table II.
Protein and mRNA expression.
| Group | No. | TLR4 mRNA | TLR4 protein |
|---|
| Normal control | 20 |
0.902±0.140a |
0.384±0.034a |
| Sham operation | 20 |
1.214±0.274a,b |
0.391±0.048a,b |
| Fluvastatin
treatment | 20 |
1.864±0.467a–c |
0.423±0.074a–c |
| IM | 20 | 3.412±0.879 | 0.574±0.098 |
Western blot analysis
In this experiment, the expression of TLR4 protein
in rat cardiomyocytes was detected by western blot analysis. It was
observed that TLR4 protein was expressed in all four groups.
Compared with IM group, the expression of TLR4 protein in normal
control group, sham operation group and fluvastatin treatment group
were all significantly decreased, and the differences were
statistically significant (P<0.05). The difference between the
normal control group and the fluvastatin group, sham operation
group was statistically significant (P<0.05), and there was no
significant difference between the normal control group and the
sham operation group (P>0.05) (Table
II).
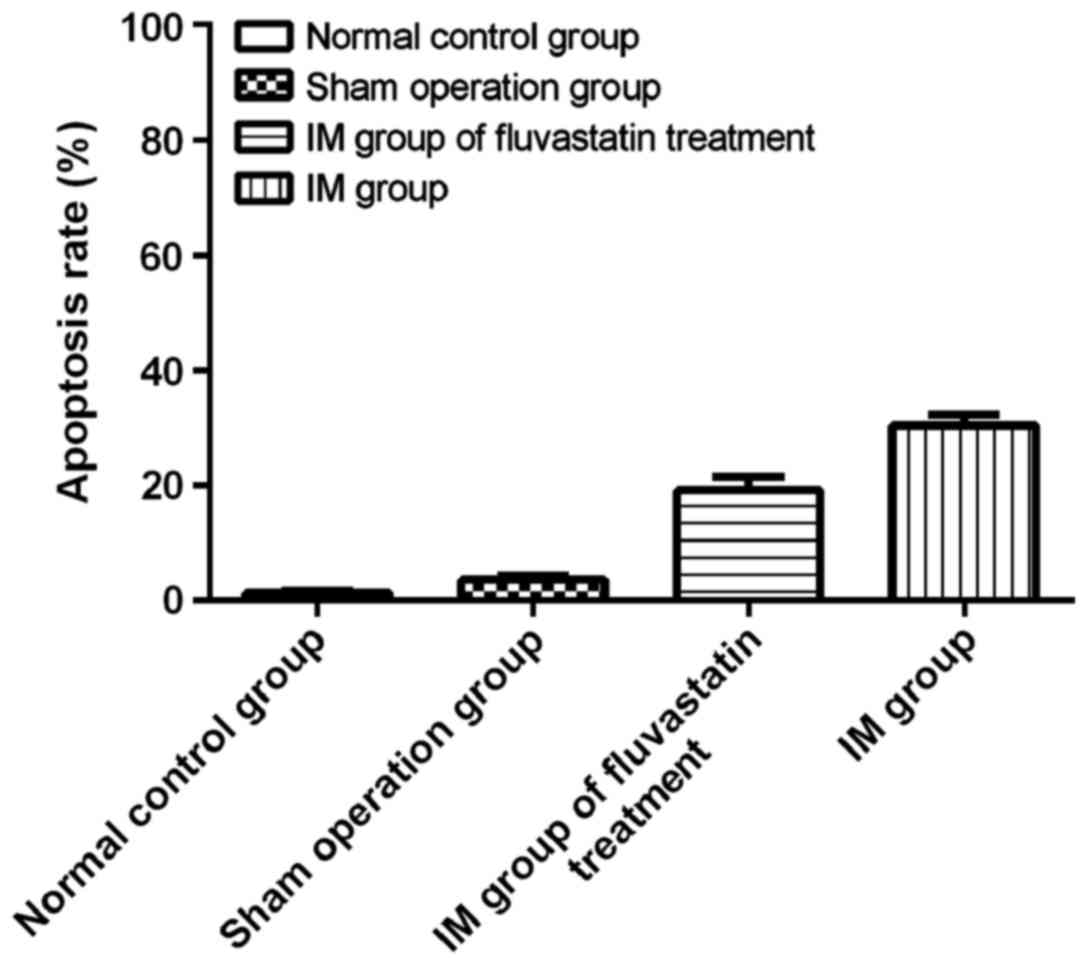
Cardiomyocyte apoptosis
In this experiment, the apoptosis of rat
cardiomyocytes was detected by TUNEL method. The nuclei of
TUNEL-positive apoptotic cells were brownish yellow and the nuclei
of TUNEL-negative was blue. It was observed that in normal control
group, no apoptotic cardiomyocytes were found; a very small amount
of cardiomyocytes in sham operation group occurred apoptosis; the
amount of apoptosis cells in IM group increased significantly
compared with normal control group and sham operation group
(30.4±3.1%), and the difference was statistically significant;
compared with the IM group, fluvastatin treatment could
significantly reduce the apoptosis of cardiomyocytes (19.2±3.8%),
and the difference was statistically significant (P<0.05); there
was no difference between normal control group and sham operation
group (P>0.05) (Fig. 2).
Discussion
Apoptosis, also known as apoptosis, is a form of
active death that occurs when genes are regulating cells. It plays
a very important role in the growth and development of normal
tissues and in the development of diseases (12,13).
There are many factors that can lead to apoptosis of cells. There
are studies which have shown that the main factors of apoptosis
were hypoxia, persistent ischemia and reperfusion (14). It has been reported (15) that ischemia-induced perfusion injury
in rabbit models leads to apoptosis in rabbit cardiomyocytes, and
other studies (16) have shown that
cardiomyocytes apoptosis was found in the myocardium of patients
with acute IM and end-stage heart failure. Cell apoptosis is
directly controlled by genes that control apoptosis, and
extracellular signals regulate cells and eventually cause cell
death. There are many reasons for the apoptosis of myocardial cells
after IM such as neurohormone system, inflammatory cytokines and CO
(17). Because of the coexistence of
multiple factors, it is very difficult to distinguish the role
played by IM in a certain period of time and one stimulus. We found
through animal experiments that after myocardial infarction,
myocardial apoptosis occurs in rats at different time-points after
IM, and cardiomyocyte apoptosis is mediated by multiple death
signals and regulated by different genes. The mechanism of
different genes on myocardial apoptosis is not yet fully
understood.
TLR4, as a natural immune receptor, its mRNA
expression was found for the first time in animal models of
cardiomyocytes, and Kim et al (18) pointed out that activation of TLR4
signaling pathway can induce cardiomyocyte apoptosis, and the use
of TLR4 blocking antibody to block TLR4 eventually leads to
diminished cardiomyocyte apoptosis. It has been reported (19) that after TLR4 activation after acute
IM, a large number of inflammatory factors are expressed. The
release of inflammatory cytokines is related to cardiomyocyte
apoptosis, and TLR4 signaling pathway-mediated apoptosis may be
related to the release of inflammatory cytokines under
TLR4-mediated conditions (20).
Fluvastatin, as the first HMG-CoA reduction inhibitor, has obvious
difference from other statins and its structure is a special
open-ring type. Fluvastatin can produce better pharmacological
activity without metabolic transformation; in addition to
lipid-lowering function, it also has the functions of stabling
plaque, suppressing inflammation and regulating coagulation
(21).
In the present study, we explored the role of
fluvastatin in myocardial cells of IM rats by treating rat IM model
with fluvastatin and activating TLR4 signaling pathway to establish
a model. The expression of TLR4 in cardiomyocytes of 4 groups was
detected by TUNEL, western blot analysis and RT-qPCR. The results
showed that the expression of TLR4 mRNA in all 4 groups of
cardiomyocytes was detected by RT-qPCR, and was significantly
expressed in IM group, which is consistent with the study of Yang
et al (22): The expression
of TLR4 mRNA in cardiomyocytes was significantly increased after
IM. Liu et al (23) have
found that after long-term IM, myocardial cells TLR4 and
pro-inflammatory cytokines are upregulated to promote inflammation
and thus increase heart failure. TLR4 expression in normal control
and sham operation group was significantly different from that in
IM group, while TLR4 expression in myocardial cells treated with
fluvastatin decreased significantly compared with IM group. The
expression of TLR4 protein in 4 groups of rats was detected by
western blot, and the results showed that TLR4 protein expression
in IM group was significantly higher than that in other groups,
which was consistent with the conclusion of Sun et al
(24). It is suggested that
fluvastatin can inhibit the expression of TLR4 to reduce the
apoptosis of cardiomyocytes, thus we detected the apoptosis of
cardiomyocytes by TUNEL method. It was observed that there was a
significant difference in the apoptosis of rat cardiomyocytes
between the fluvastatin treatment group and the IM group (19.2±3.8
vs. 30.4±3.1%, P<0.05), indicating that fluvastatin can regulate
cardiomyocyte apoptosis by inhibiting the expression of TLR4.
This experiment showed that fluvastatin can inhibit
the expression of TLR4, however, the specific mechanism of TLR4
inhibition is unclear, whether fluvastatin modulates TLR4 directly
or indirectly through other proteins needs further study and
experiments, and this experiment was based on the animal model, and
whether different modeling methods and practices will affect the
experimental results also needs to be studied in depth in the
future.
In summary, fluvastatin can decrease the apoptosis
of cardiomyocytes and inhibit IM by increasing the expression of
TLR4-like receptors.
Acknowledgements
Not applicable.
Funding
No funding was received
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ drafted the manuscript. LJ and ZZ were mainly
devoted to collecting and interpreting the data. WL, LW and GQ
helped with RT-qPCR. XJ and ZZ were responsible for statistical
analysis. All authors have read and approved the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Qilu Hospital of Shandong University (Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thiene G, Corrado D and Basso C: Sudden
cardiac death in the young and athletes. Springer Milan.
2016:21–71. 2016.
|
|
2
|
Sankar NM, Ramani SS and Anantharaman R:
Coronary artery disease in women. Indian Heart J. 69:807–808. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forman DE, Alexander K, Brindis RG, Curtis
AB, Maurer M, Rich MW, Sperling L and Wenger NK: Improved
cardiovascular disease outcomes in older adults. F1000Res. 5:1–9.
2016.
|
|
4
|
Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu
S, Li Y, Wang L, Liu Y, Yin P, et al: Cause-specific mortality for
240 causes in China during 1990–2013: A systematic subnational
analysis for the Global Burden of Disease Study 2013. Lancet.
387:251–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan NY: Sudden cardiac death in Asia and
China: Are we different? J Am Coll Cardiol. 67:590–592. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reed GW, Rossi JE and Cannon CP: Acute
myocardial infarction. Lancet. 389:197–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stewart WJ: Atrial myocardial infarction:
A neglected stalker in coronary patients. J Am Coll Cardiol.
70:2890–2892. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li S, Guo LZ, Kim MH, Han JY and
Serebruany V: Platelet microRNA for predicting acute myocardial
infarction. J Thromb Thrombolysis. 44:556–564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kataoka Y, Andrews J, Puri R, Psaltis P
and Nicholls SJ: Lipid lowering therapy to modify plaque
microstructures. J Atheroscler Thromb. 24:360–372. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruel I, Aljenedil S, Sadri I, de Varennes
É, Hegele RA, Couture P, Bergeron J, Wanneh E, Baass A, Dufour R,
et al: Imputation of baseline LDL cholesterol concentration in
patients with familial hypercholesterolemia on statins or
ezetimibe. Clin Chem. 70:20222017.
|
|
11
|
Zhai Y, Ao L, Cleveland JC, Zeng Q, Reece
TB, Fullerton DA and Meng X: Toll-like receptor 4 mediates the
inflammatory responses and matrix protein remodeling in remote
non-ischemic myocardium in a mouse model of myocardial ischemia and
reperfusion. PLoS One. 10:e01218532015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Sun Y, Yang H, Lu Y and Li L:
Oxidized low-density lipoprotein induces apoptosis in cultured
neonatal rat cardiomyocytes by modulating the TLR4/NF-κB pathway.
Sci Rep. 6:278662016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tixeira R, Caruso S, Paone S, Baxter AA,
Atkin-Smith GK, Hulett MD and Poon IK: Defining the morphologic
features and products of cell disassembly during apoptosis.
Apoptosis. 22:475–477. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Wu Z, Huang C, Zhao Y, Zhou Y,
Zhou X, Lu X, Mao L and Li S: Effect of lipoxin A4 on myocardial
ischemia reperfusion injury following cardiac arrest in a rabbit
model. Inflammation. 36:468–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kleinbongard P, Schulz R and Heusch G:
TNFα in myocardial ischemia/reperfusion, remodeling and heart
failure. Heart Fail Rev. 16:49–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Q, Zhang J, Xu Y, Huang Y and Wu C:
Effect of carvedilol on cardiomyocyte apoptosis in a rat model of
myocardial infarction: A role for toll-like receptor 4. Indian J
Pharmacol. 45:458–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SC, Stice JP, Chen L, Jung JS, Gupta
S, Wang Y, Baumgarten G, Trial J and Knowlton AA: Extracellular
heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res.
105:1186–1195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satoh M, Shimoda Y, Maesawa C, Akatsu T,
Ishikawa Y, Minami Y, Hiramori K and Nakamura M: Activated
toll-like receptor 4 in monocytes is associated with heart failure
after acute myocardial infarction. Int J Cardiol. 109:226–234.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhai Y, Shen XD, OConnell R, Gao F,
Lassman C, Busuttil RW, Cheng G and Kupiec-Weglinski JW: Cutting
edge: TLR4 activation mediates liver ischemia/reperfusion
inflammatory response via IFN regulatory factor 3-dependent
MyD88-independent pathway. J Immunol. 173:7115–7119. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Wang L, Hu X, Zhang P, Chen Y, Liu
X, Xu M, Zhang Y and Zhang M: Effect of rosuvastatin on
atherosclerotic plaque stability: An intravascular ultrasound
elastography study. Atherosclerosis. 248:27–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Yang J, Ding JW, Chen LH, Wang YL,
Li S and Wu H: Sequential expression of TLR4 and its effects on the
myocardium of rats with myocardial ischemia-reperfusion injury.
Inflammation. 31:304–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu L, Wang Y, Cao ZY, Wang MM, Liu XM,
Gao T, Hu QK, Yuan WJ and Lin L: Up-regulated TLR4 in
cardiomyocytes exacerbates heart failure after long-term myocardial
infarction. J Cell Mol Med. 19:2728–2740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Huang J and Song K: BET protein
inhibition mitigates acute myocardial infarction damage in rats via
the TLR4/TRAF6/NF-κB pathway. Exp Ther Med. 10:2319–2324. 2015.
View Article : Google Scholar : PubMed/NCBI
|