Introduction
In recent years, lung cancer has become one of the
most common malignant tumors threatening human health (1), and its incidence rate has been on the
rise (2,3). Therefore, it is imperative to know how
to accurately distinguish malignant lung lesions (MLLs) from benign
lung lesions (BLLs).
Young women, pregnant women, and critically ill
patients cannot tolerate chest X-rays and computed tomography (CT)
because of radiation, although they are first-line imaging
modalities for assessing the morphological features of pulmonary
lesions (PLs) (4–6). When a PLL is present and
space-occupied, the acoustic windows of the lung tissue are
available, which makes the ultrasonic wave pass. Hence, after
selecting the appropriate detection angles for PLLs below the
pleura, optimal images can be obtained during transthoracic
ultrasound (TUS) examination (7,8). The
advantages of US compared to X-ray or CT are its easy operation,
lower cost, and lack of exposure to radiation. In addition,
US-guided biopsy is as accurate as CT-guided biopsy for the
diagnosis of PLLs or pleural lesions. Real-time guidance can be
performed using US, making the procedure faster and free of
ionizing radiation (9).
In recent years, US elastography has been widely
applied to the differential diagnosis of thyroid, breast, liver,
and prostate lesions (10–13). It can reflect the intrinsic features
of the lesions. The tissue stiffness of malignant lesions is
usually harder than those that are benign (14,15). US
elastography is mainly categorized as strain elastography (SE) and
shear wave elastography (SWE), in which the information about the
tissue stiffness can be obtained by pressure or shear force that
deforms the tissue directly or indirectly (16). The tissue stiffness obtained by
manual compression can be affected by different operators, leading
to inter-observer differences. However, acoustic radiation force
impulse (ARFI) elastography that includes an ARFI imaging mode and
a point share wave elastography (p-SWE) mode can conduct
qualitative or quantitative measurement objectively by assessing
the elastogram in grayscale or the shear wave velocity (SWV) value
of the lesions (17).
Sperandeo et al (18) reported that lung SE enables good
non-invasive imaging of PLs, as it provides information on their
stiffness and improves the accuracy and yield of fine needle
aspiration biopsy (FNAB). But there are few reports about the
usefulness of ARFI elastography in diagnosing PLLs. As such, this
study purported to assess the value of TUS elastography in
distinguishing malignant from benign PLLs.
Materials and methods
Study population
This retrospective study was approved by the Ethics
Committee of this tertiary hospital (the Second Affiliated Hospital
of Harbin Medical University, Harbin, China) and the written
informed consent of patients was obtained. From January 2013 to
January 2015, 201 consecutive patients with PLLs found by chest
X-ray or CT examination were enrolled in this institution (the
Second Affiliated Hospital of Harbin Medical University). The
exclusion criteria were as follows: i) Trouble breathing (n=20);
ii) the lesions were covered by bone tissue (n=15); iii) no
pathological result (n=42); and iv) accompanied with pleural
effusion (n=33). If there were multiple lesions, the largest was
incorporated into the study. Finally, conventional US and
elastography examinations (including SE and ARFI elastography) were
performed on the remaining 91 patients. The final pathological
results were obtained by surgery or biopsy.
Conventional US, SE, ARFIimaging, and
p-SWE
Conventional US and US elastography examinations
were performed by a radiologist with at least 10 years of
experience (H.W.) in ultrasonic examination with the US machine
(Siemens Acuson S2000; Siemens Medical Solutions, Eschborn,
Germany) and the 4C1 convex vibration probe (frequency range,
1.5–4.0 MHz). The focus and gain of images were adjusted to obtain
optimal images. The probe was held perpendicularly to the skin
surface on the intercostal space and parallel to the ribs
throughout the examinational process. Using a 2-dimensional US, the
PL was assessed for its size, position within the lobe, shape,
margin, internal echogenicity, echotexture, and presence of air
bronchogram. Then the color Doppler US mode was applied and the
gain was adjusted to the optimal level so background color signals
did not display any noise throughout the process. Afterwards, SE
was applied with mild manual compression. The region of interest
(ROI) box was aimed at the whole lesion and some peripheral normal
tissues. The radiologist kept the probe motionless for 5 sec to
obtain optimal elastic images. The US image was on the left side of
the screen while the SE image was on the right side and
color-coded. ARFI imaging was performed after SE. The ROI box was
placed in the same position as SE. The ARFI image was on the right
side of the screen and gray-coded. After that, the p-SWE mode was
initiated. A 6×5 mm ROI box was placed at the solid portion without
air covering the lesion. The SWV value was displayed on the right
side of the screen. Seven consecutive measurements were performed
at the same depth of each lesion. Finally, the average value was
obtained after removing the maximum and minimum values.
US-guided core needle biopsy
Before US-guided core needle biopsy (US-CNB), all
patients signed the informed consent forms. The procedure was
performed by 2 experienced radiologists (Q.J. and H.W.) using a US
machine (Philips iU22; Philips Ultrasound, Inc., Reedsville, PA,
USA), a Bard automatic biopsy gun, and a biopsy needle (16 G; Bard
Peripheral Vascular, Inc., Tempe, AZ, USA). Tissue samples were
taken under the guidance of US. Biopsy was repeated 3–4 times
according to the specimen quality. Afterward, pathological
diagnosis was performed by 1 pathologist with 20 years' work
experience who was blinded to previous US findings.
Image interpretation
The images were interpreted by 2 investigators (H.W.
and Y.L.) who were blinded to the pathological results. The size of
the lesions was defined by the longest diameter measured on US,
with <5 cm and ≥5 cm. The shape of the lesions was defined as
having regular contours (triangular or rounded) and irregular
contours (non-triangular or non-rounded). The margin was defined as
smooth (at least 50% of the lesion was visible) and rough (at least
50% of the lesion was invisible). The internal echogenicity of the
lesions was defined as homogeneous
hyperechoic/isoechoic/hypoechoic/anechoic or heterogeneous
hyperechoic/isoechoic/hypoechoic/anechoic. The lesions were grouped
as with or without air bronchogram (punctiform or linear
hyperechoic artifacts within the lesions) (19). The vascularity of the lesions was
classified into abundant (≥2 blood vessel signals) and non-abundant
(<2 blood vessel signals). As current international
recommendations do not yet provide a specific way to classify the
stiffness of PLL, we customized the classifications. SE imaging was
described by the color scale of the ROI, with red, green, and blue
delineating from soft to hard, and SE was classified into 4 grades.
ARFI imaging was classified into 4 grades from soft to hard
according to the grayscale (Table
I).
 | Table I.Scoring systems for strain
elastography imaging and acoustic radiation force impulse imaging
in the peripheral pulmonary lesions. |
Table I.
Scoring systems for strain
elastography imaging and acoustic radiation force impulse imaging
in the peripheral pulmonary lesions.
| Score | SE imaging | ARFI-imaging |
|---|
| 1 | Shown homogeneously
in red and green | Displayed <25%
areas of dark gray or black |
| 2 | Shown predominantly
in green, a few blue areas or spots | Displayed 25–50%
areas of dark gray or black |
| 3 | Shown predominantly
in green, or with equal areas in blue | Displayed 50–75%
areas of dark gray or black |
| 4 | Shown predominantly
in blue with or without a few green areas | Displayed >75%
areas of dark gray or black |
Hematoxylin and eosin (H&E)
staining
Lung tissues were fixed in formalin, cleared in
xylene (both from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
and embedded in paraffin. The paraffin-embedded lung tissue samples
were examined by conventional light microscopic examination: 5 µm
sections were stained with H&E, and assessed in a blinded
manner. An automatic microscope (Provis AX-70) with a camera
(Olympus Corporation, Tokyo, Japan) was used to capture the
microscopic images of the lung samples. The morphometric analysis
was performed using ImageJ software version 1.60 (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All statistical analyses were carried out using SAS
9.13 statistical software (SAS Institute Inc., Shanghai, China).
The geometric mean and standard deviation were used to describe
measurement data and compared between groups using Student's
t-test. Frequency and percentage were used to describe counting
data and compared between groups using the Chi-square test or
Fisher's exact test. A receiver operating characteristic (ROC)
curve was created to analyze the accuracy of each variable.
P<0.05 was considered statistically significant.
Results
This study involved 50 males and 41 females, with an
average age of 55.11±11.11 years (range, 24–86 years). The mean
diameters of the BLLs and MLLs were 58.17±20.18 and 72.05±24.27 mm,
respectively (range, 20.1–141.4 mm). The pathological results were
confirmed by US-CNB procedures in 80 patients and by surgery in 11
patients. There were 36 benign lesions (32 chronic inflammatory
lesions, 4 tuberculosis) and 55 malignant lesions (33
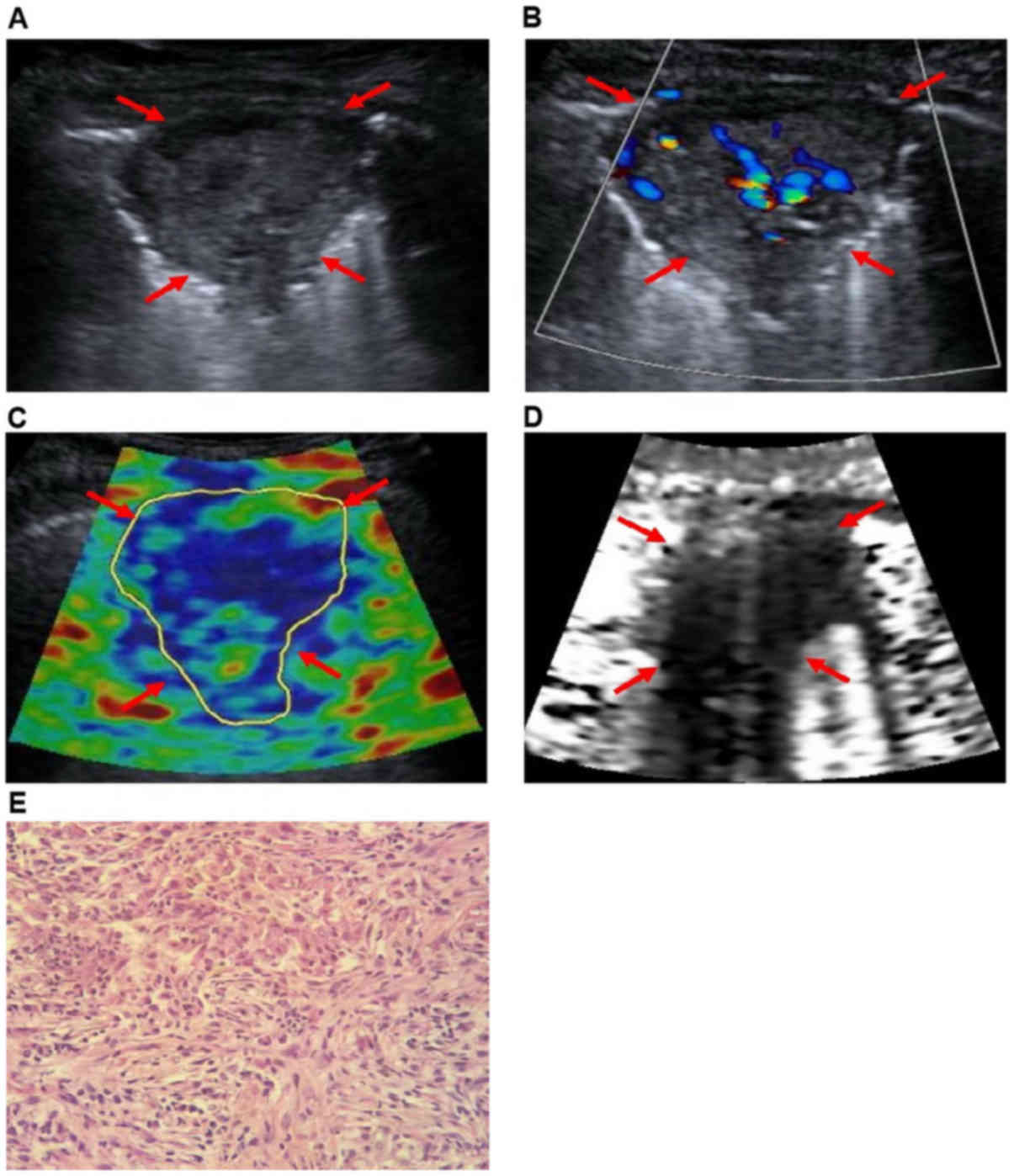
adenocarcinomas, 21 squamous cell carcinomas (Fig. 1), and 1 small cell lung cancer).
Female sex (P=0.009) and older age (P=0.002) were predictive of
malignancy. The location of the lesions and smoking history were
not relevant factors in this study (P>0.05; Table II).
 | Table II.Basic characteristics of patients with
peripheral pulmonary lesions. |
Table II.
Basic characteristics of patients with
peripheral pulmonary lesions.
| Basic
characteristic | Benign, n (%) | Malignant, n (%) | P-value |
|---|
| Sex |
|
Female | 10 (24.4) | 31 (75.6) | 0.009a |
| Male | 26 (52.0) | 24 (48.0) |
|
| Age (years) |
|
<50 | 10 (71.4) | 4 (28.6) | 0.002a |
|
50–69 | 24 (40.0) | 36 (60.0) |
|
| ≥70 | 2 (11.8) | 15 (88.2) |
|
| Smoking history |
| No | 10 (37.0) | 17 (63.0) | 0.749 |
| Yes | 26 (40.6) | 38 (59.4) |
|
| Location (in
lung) |
|
Right | 18 (35.3) | 33 (64.7) | 0.348 |
| Left | 17 (42.5) | 23 (57.5) |
|
Conventional US features
On conventional US, characteristics such as a lesion
diameter ≥5 cm, irregular contours, presence of air bronchogram,
and non-abundant vascularity showed significant differences between
benign and malignant lesions (P<0.05; Table III). In addition, using 5 cm as a
cut-off value for the lesion diameter led to a sensitivity of 87.3%
and a specificity of 38.9%. The margin and echogenicity of lesions
presented no significant differences (P>0.05).
 | Table III.Ultrasound characteristics of benign
and malignant peripheral pulmonary lesions. |
Table III.
Ultrasound characteristics of benign
and malignant peripheral pulmonary lesions.
| Characteristic | Benign, n (%) | Malignant, n (%) | P-value |
|---|
| Diameter of tumor
(cm) |
|
<5 | 14 (66.7) | 7
(33.3) | 0.006b |
| ≥5 | 22 (31.4) | 48 (68.6) |
|
| Shape |
| Regular
contour | 20 (51.3) | 19 (48.7) | 0.048a |
| Irregular
contour | 16 (30.8) | 36 (69.2) |
|
| Margin |
|
Smooth | 6
(60.0) | 4
(40.0) | 0.161 |
|
Rough | 30 (37.0) | 51 (63.0) |
|
| Echogenicity
(hypoechoic/hypo-anechoic) |
|
Homogeneous | 6
(66.7) | 3
(33.3) | 0.147 |
|
Heterogeneous | 30 (36.6) | 52 (63.4) |
|
| Air
bronchogram |
|
Absent | 10 (26.3) | 28 (73.7) | 0.029a |
|
Present | 26 (49.1) | 27 (50.9) |
|
| Vascularity |
|
Non-abundant | 17 (30.9) | 38 (69.1) | 0.037a |
|
Abundant | 19 (52.8) | 17 (47.2) |
|
US elastography features
For elastography features (Table IV), no significant difference was
found in SE between BLLs and MLLs (P=0.542). But significant
differences were found in ARFI imaging and p-SWE between BLLs and
MLLs (P<0.05). On ARFI imaging scores, the lesions with 1–2 and
3–4 were classified as benign and malignant, respectively, and
corresponding sensitivity, specificity, positive predictive value
(PPV), and negative predictive value (NPV) were 83.6% (46/55),
52.8% (19/36), 73.0% (46/63), and 67.9% (19/28), respectively.
Using this classification method, 9 false negative lesions and 17
false positive lesions were found. The SWV values of the MLLs were
higher than those of the BLLs (2.47±0.92 vs. 1.85±0.92 m/sec;
P=0.0022). According to the ROC curve, the area under the curve
(AUC) of p-SWE was 0.709. With a cut-off value of 1.951 m/sec, the
sensitivity and the specificity for diagnosis of malignancy were
70.9% (39/55) and 69.4% (25/36), respectively, with a PPV of 78.0%
(39/50) and an NPV of 61.0% (25/41) attached.
 | Table IV.Ultrasound elastography features of
benign and malignant peripheral pulmonary lesions. |
Table IV.
Ultrasound elastography features of
benign and malignant peripheral pulmonary lesions.
| Elastography
features | Benign, n (%) | Malignant, n
(%) | P-value |
|---|
| SE-imaging
score |
|
| 0.542 |
| Score
1 | 10 (71.4) | 4
(28.6) |
|
| Score
2 | 17 (33.3) | 34 (66.7) |
|
| Score
3 | 5
(29.4) | 12 (70.6) |
|
| Score
4 | 4
(44.4) | 5
(55.6) |
|
| ARFI-imaging
score |
|
|
<0.001b |
| Score
1 | 15 (93.8) | 1 (6.3) |
|
| Score
2 | 4
(33.3) | 8
(66.7) |
|
| Score
3 | 15 (37.5) | 25 (62.5) |
|
| Score
4 | 2 (8.7) | 21 (91.3) |
|
| p-SWE
[SWV(m/sec)] | 1.85±0.92 | 2.47±0.92 | 0.002a |
Discussion
US is not a familiar tool for thoracic radiologists
due to its infrequent use. However, it still offers several
advantages to both the radiologists and patients. Some studies have
showed that US guidance is as accurate as CT guidance in obtaining
adequate samples from PLLs (19,20).
This initial study demonstrated that the diagnostic information of
PLLs may be provided by ARFI elastography in addition to
radioactive techniques or invasive procedures, while SE was not
significant for diagnosis.
Conventional US was capable of detecting lung
lesions only when they were located in the peripheral regions of
the organ. As there was no typical neoplastic pattern on US, needle
biopsy of these lesions was mandatory (21). Sheth et al (22) reported that small PLs appeared
hypoechoic and larger PLs were more heterogeneous. However, some
studies (23,24) showed that wedge shape, isoechoic or
hypoechoic, and air bronchograms might appear on peripheral
pulmonary consolidations. In our study, we found that a lesion
diameter greater than or equal to 5 cm, irregular contour, presence
of air bronchogram, and non-abundant vascularity were predictive
factors of malignant PLLs, whereas the margin and echogenicity of
lesions were not. An explanation may be that overlapping US
characteristics between malignant and benign lesions existed due to
the various types of PLL pathologies. In addition, the sensitivity
and specificity of US characteristics varied considerably,
30.9–87.3% and 16.7–55.6%, respectively, and the results were not
satisfactory. Therefore, additional methods should be adopted to
diagnose PLLs.
SE was introduced to assess the tissue stiffness by
hand or using cardiovascular pulsation or respiratory motion to
achieve compression. However, some studies revealed that it was
difficult for radiologists to control the pressure using manual
compression in SE examination, thus leading to considerably low
reproducibility (25,26). In our study, we also found the same
problem due to the limitation of cardiovascular pulsation or
respiratory motion, which made the compression inconsistent.
Furthermore, no significant differences on SE were found between
BLLs and MLLs. In the study by Sperandeo et al (21), which performed TUS elastography on 95
patients with PLs, only squamous cell lung carcinoma displayed
increased stiffness. In their study, TUS elastography was limited
in characterizing lesions, which was similar to our result.
Additionally, 1 lesion with a score of 2 proved to be
adenocarcinoma; a possible pathological explanation might be that
the cells contained many acini and had intracellular mucus.
To the best of our knowledge, this research was the
first preliminary study to detect the diagnostic performance of
ARFI imaging and p-SWE in PLLs. ARFI elastography was more
effective in diagnosing PLLs, with the advantages of free-hand
compression and semiquantitative or quantitative measurements. In
our study, the ARFI score of the malignant PLLs was higher than
that of the benign ones (P=0.011), indicating higher stiffness.
When score 3 was applied as cut-off value, the corresponding
sensitivity was 83.6% (46/55), which was higher than chest
radiography (73.5%) (27). Thus, a
malignant lesion may be suggested before surgery on the condition
that it obtained a score of 3 or more on ARFI imaging. Under such
circumstances, we found that tuberculosis (a total of 4 cases) had
a score of 3 and 1 chronic inflammation had a score of 4 on ARFI
imaging. This phenomenon might be explained by the formation of
lung fibrous band structures in tuberculosis and the proliferation
of fibrosis in chronic inflammation, which might increase the
tissue stiffness (Fig. 2). However,
our study was limited by a small sample size; there is a need for a
separate study with more tuberculosis and chronic pneumonia
patients for further analysis.
Compared to SE, p-SWE was applied to evaluate the
tissue stiffness by generating shear wave, with the advantages of a
smaller subjective influence of the operator, independent elastic
image quality, and quantitative measurement. In our study, the mean
SWV values of the malignant lesions were higher than those of the
benign lesions (2.47±0.92 vs. 1.85±0.92 m/sec; P=0.002). With a
cut-off value of 1.951 m/sec, the sensitivity, specificity, PPV,
and NPV for diagnosing MLLs were 70.91, 69.44, 78.00, and 60.98%,
respectively. It was observed that p-SWE was more effective than SE
in distinguishing malignant from benign lesions. Although the
sensitivity of p-SWE was not as good as low-dose CT, their
specificity was similar (73.4%). Furthermore, the specificity of
p-SWE was not as good as that of chest radiography, but the
sensitivity was similar (27). s
P-SWE has provided a new method for evaluating the hardness of
PLLs. However, false positives and false negatives also existed in
p-SWE examination. Among the benign lesions, 1 chronic inflammatory
lesion had a mean SWV of 4.344 m/sec, which may indicate the
existence of fibrosis in the lesion. In addition, 1 small cell lung
cancer had a mean SWV of 1.202 m/sec and an ARFI imaging score of
2, which might be explained by the complex heterogeneity of tumor
cells (28).
There were some limitations in this study. First,
the selection bias was unavoidable due to its retrospective nature.
Second, the inter-observer and intra-observer agreements were not
analyzed, although some studies have shown that ARFI elastography
has good reproducibility in multiple organs of the body (29,30).
Third, considering the small sample size of our study, prospective
research on a large sample is necessary.
In conclusion, our study indicated that US,
especially ultrasound elastography, is able to provide doctors with
identification methods of PLLs.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Scientific
Research of Heilongjiang Province Health and Family Planning
Commission (grant no. 2014-334).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW was a major contributor in performing the
experiments and writing the manuscript. YL collated and analyzed
the ultrasonic images. QJ conducted the data analysis, and HZ
analyzed the pathological results. XZ led this study, performed the
pathological biopsies of the lungs and gave final approval of the
version to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This retrospective study was approved by the Ethics
Committee of this tertiary hospital (the Second Affiliated Hospital
of Harbin Medical University, Harbin, China) and the written
informed consent of patients was obtained.
Patient consent for publication
Informed consent was obtained from all individual
participants included in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goozner M: A tale of two countries: Lung
cancer care in Brazil and China. J Natl Cancer Inst. 104:1621–1623.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim CH, Lee YC, Hung RJ, McNallan SR, Cote
ML, Lim WY, Chang SC, Kim JH, Ugolini D, Chen Y, et al: Exposure to
secondhand tobacco smoke and lung cancer by histological type: A
pooled analysis of the International Lung Cancer Consortium
(ILCCO). Int J Cancer. 135:1918–1930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Liu Y, Wang H, Hnizdo E, Sun Y, Su
L, Zhang X, Weng S, Bochmann F, Hearl FJ, et al: Long-term exposure
to silica dust and risk of total and cause-specific mortality in
Chinese workers: A cohort study. PLoS Med. 9:e10012062012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strauss GM, Gleason RE and Sugarbaker DJ:
Chest X-ray screening improves outcome in lung cancer. A
reappraisal of randomized trials on lung cancer screening. Chest.
107 6 Suppl:270S–279S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yankelevitz DF: Point: Should lung cancer
screening by chest CT scan be a covered benefit? Yes. Chest.
147:287–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Field JK, Devaraj A, Duffy SW and Baldwin
DR: CT screening for lung cancer: Is the evidence strong enough?
Lung Cancer. 91:29–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adamietz BR, Fasching PA, Jud S,
Schulz-Wendtland R, Anders K, Uder M, Wüst W, Rauh C and
Meier-Meitinger M: Ultrasound elastography of pulmonary lesions-a
feasibility study. Ultraschall Med. 35:33–37. 2014.PubMed/NCBI
|
|
8
|
Volpicelli G: Lung sonography. J
Ultrasound Med. 32:165–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sconfienza LM, Mauri G, Grossi F, Truini
M, Serafini G, Sardanelli F and Murolo C: Pleural and peripheral
lung lesions: Comparison of US- and CT-guided biopsy. Radiology.
266:930–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rhymer JC: Elastography in the detection
of prostatic cancer. Clin Radiol. 58:3372003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu JM, Xu XH, Xu HX, Zhang YF, Zhang J,
Guo LH, Liu LN, Liu C and Zheng SG: Conventional US, US elasticity
imaging, and acoustic radiation force impulse imaging for
prediction of malignancy in thyroid nodules. Radiology.
272:577–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo LH, Wang SJ, Xu HX, Sun LP, Zhang YF,
Xu JM, Wu J, Fu HJ and Xu XH: Differentiation of benign and
malignant focal liver lesions: Value of virtual touch tissue
quantification of acoustic radiation force impulse elastography.
Med Oncol. 32:682015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ko KH, Jung HK, Kim SJ, Kim H and Yoon JH:
Potential role of shear-wave ultrasound elastography for the
differential diagnosis of breast non-mass lesions: Preliminary
report. Eur Radiol. 24:305–311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cross TJ, Mitchell JD and Cramp ME:
Elastography for the non-invasive assessment of liver disease:
Limitations and future developments. Gut. 58:1171–1172.
2009.PubMed/NCBI
|
|
15
|
Moon HJ, Sung JM, Kim EK, Yoon JH, Youk JH
and Kwak JY: Diagnostic performance of gray-scale US and
elastography in solid thyroid nodules. Radiology. 262:1002–1013.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ophir J, Céspedes I, Ponnekanti H, Yazdi Y
and Li X: Elastography: A quantitative method for imaging the
elasticity of biological tissues. Ultrason Imaging. 13:111–134.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shiina T, Nightingale KR, Palmeri ML, Hall
TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D, et
al: WFUMB guidelines and recommendations for clinical use of
ultrasound elastography: Part 1: Basic principles and terminology.
Ultrasound Med Biol. 41:1126–1147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sperandeo M, Trovato FM, Dimitri L,
Catalano D, Simeone A, Martines GF, Piscitelli AP and Trovato GM:
Lung transthoracic ultrasound elastography imaging and guided
biopsies of subpleural cancer: A preliminary report. Acta Radiol.
56:798–805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lichtenstein D, Mezière G and Seitz J: The
dynamic air bronchogram. A lung ultrasound sign of alveolar
consolidation ruling out atelectasis. Chest. 135:1421–1425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeon KN, Bae K, Park MJ, Choi HC, Shin HS,
Shin S, Kim HC and Ha CY: US-guided transthoracic biopsy of
peripheral lung lesions: Pleural contact length influences
diagnostic yield. Acta Radiol. 55:295–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sperandeo M, Filabozzi P, Varriale A,
Carnevale V, Piattelli ML, Sperandeo G, Brunetti E and Decuzzi M:
Role of thoracic ultrasound in the assessment of pleural and
pulmonary diseases. J Ultrasound. 11:39–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sheth S, Hamper UM, Stanley DB, Wheeler JH
and Smith PA: US guidance for thoracic biopsy: A valuable
alternative to CT. Radiology. 210:721–726. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang PC, Luh KT, Chang DB, Yu CJ, Kuo SH
and Wu HD: Ultrasonographic evaluation of pulmonary consolidation.
Am Rev Respir Dis. 146:757–762. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang PC, Chang DB, Yu CJ, Lee YC, Kuo SH
and Luh KT: Ultrasound guided percutaneous cutting biopsy for the
diagnosis of pulmonary consolidations of unknown aetiology. Thorax.
47:457–460. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim MH, Luo S, Ko SH, Bae JS, Lim J, Lim
DJ and Kim Y: Thyroid nodule parameters influencing performance of
ultrasound elastography using intrinsic compression. Ultrasound Med
Biol. 41:2333–2339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang JM, Won JK, Lee KB, Park IA, Yi A
and Moon WK: Comparison of shear-wave and strain ultrasound
elastography in the differentiation of benign and malignant breast
lesions. AJR Am J Roentgenol. 201:W347–W356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
National Lung Screening Trial Research
Team, . Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan
F, Fagerstrom RM, Gareen IF, Gierada DS, et al: Results of initial
low-dose computed tomographic screening for lung cancer. N Engl J
Med. 368:1980–1991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krohn A, Ahrens T, Yalcin A, Plönes T,
Wehrle J, Taromi S, Wollner S, Follo M, Brabletz T, Mani SA, et al:
Tumor cell heterogeneity in Small Cell Lung Cancer (SCLC):
Phenotypical and functional differences associated with
Epithelial-Mesenchymal Transition (EMT) and DNA methylation
changes. PLoS One. 9:e1002492014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma JJ, Ding H, Mao F, Sun HC, Xu C and
Wang WP: Assessment of liver fibrosis with elastography point
quantification technique in chronic hepatitis B virus patients: A
comparison with liver pathological results. J Gastroenterol
Hepatol. 29:814–819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bob F, Bota S, Sporea I, Sirli R, Petrica
L and Schiller A: Kidney shear wave speed values in subjects with
and without renal pathology and inter-operator reproducibility of
acoustic radiation force impulse elastography (ARFI)-preliminary
results. PLoS One. 9:e1137612014. View Article : Google Scholar : PubMed/NCBI
|