Introduction
Patients with diabetes mellitus typically develop
irreversible renal and ocular tissue impairments due to the high
levels of blood glucose (1,2). Diabetic cataract is one of the major
ocular complications of diabetes mellitus and subcapsular cataract
is considered the distinctive phenotype (3). Notably, a previous study demonstrated
that human lens epithelial cells (LECs) are involved in
modifications of proliferation and apoptosis in diabetic
subcapsular cataract in an aldose reductase 2 (ALR2) transgenic
mouse model (4). It has been
reported that a number of aberrant nucleated cells assemble
underneath the posterior subcapsular region in experimentally
diabetic mice (5). However, the
exact mechanism in diabetic cataract formation remains unclear.
Under physiological conditions, LECs are located in
the less proliferative center zone or the mitotically active
germinative zone of the lens. These LECs regulate the majority of
the homeostatic functions of the lens, including its growth and
differentiation, repair of damage and maintenance of lens
transparency (6). However, under
pathological conditions, LECs may promote the emergence of various
types of cataracts, including posterior capsule opacity (PCO),
anterior subcapsular cataract (ASC) and diabetic cataract (7).
Transforming growth factor (TGF)-β is a pleiotropic
growth factor that controls cell growth, proliferation and
migration, morphological plasticity and epithelial-mesenchymal
transition (EMT) in tumor cells and specific epithelial cells
(8–11). Furthermore, TGF-β exerts its
biological effects through two membrane-bound receptors, designated
type II and activin receptor-like kinase 5 (ALK5) (6). In the TGF-β family, TGF-β1 and TGF-β2
are expressed abundantly in the human lens and ocular media
(12), and are considered key
factors that regulate LEC proliferation, migration and EMT in ASC
and PCO (13,14). Pathological studies regarding
posterior subcapsular cataract (PSC) have indicated that various
abnormal nucleated cells and the extracellular matrix accumulate
underneath the capsule, resulting in transformation of the
epithelial cell phenotype into fibroblastic cells (13–15).
These studies suggested EMT of LECs may be an important initial
step in the development of PCO and ASC, in which TGF-β is a major
inducing factor. Du et al (16) studied the mechanism of high glucose
(HG)-induced lens opacity accompanied by lens fibrosis in mice. The
study reported that the TGF-β2/phosphoinositide 3-kinase/protein
kinase B axis served an important role in HG-induced EMT of ex
vivo human LECs and the inhibition of TGF-β delayed the EMT of
LECs. Kim et al (17)
identified that aldose reductase (AR) and TGF-β exerted vital
actions in a rat diabetic cataract model. The study indicated that
the inhibition of AR resulted in the decrease of mRNA expression
levels of TGF-β and fibronectin (FN) in HG-induced ex vivo
human LECs. In another study, Kim et al (18) reported that the expression levels of
TGF-β and FN were increased in HG-induced ex vivo human LECs
and the expression levels of TGF-β2, Smad2/3, p38 mitogen-activated
protein kinase (MAPK) and FN were decreased when the inhibitor
KIOM-79 was applied. The results suggest that TGF-β2/p38MAPK and
TGF-β2/Smad2/3 signaling pathways may serve key roles in EMT of
LECs.
Lu et al (19)
reported that rat lenses cultured in HG conditions exhibited
increased expression levels of TGF-β, E-cadherin and α-smooth
muscle actin (SMA). These results suggest that TGF-β may be a key
molecule in EMT of HG-induced LECs and diabetic cataract. However,
it remains unclear whether EMT of LECs is involved in PSC.
c-Src kinase, converting into p-Src418
when activated, is a non-receptor intracellular tyrosine kinase
that mediates a variety of cellular responses (20). c-Src may be activated by oxidative
stress, osmotic stress, ultraviolet radiation and inflammation,
which are associated with cataract formation (21–23). In
various types of tumor cells, activated c-Src serves a principal
role in regulating EMT properties, cell adhesion, proliferation and
migration (24–27) and tumor metastasis (28,29).
Notably, in the ocular media, activated c-Src is considered a
predominant factor in the formation of cortical cataracts (30,31).
Previously, it was revealed that activated c-Src mediated the EMT
of LECs in normal culture medium, resulting in the occurrence of
PCO in vitro (32).
In the present study, HLE-B3 cells were employed
in vitro to investigate the roles of c-Src and TGF-β in the
EMT of LECs. Furthermore, the association between c-Src and TGF-β
was assessed.
Materials and methods
Cell culture, reagents and
treatments
Immortalized human lens epithelial cells (HLE-B3)
cells were obtained from Dr Zhang Wei at the Beijing Institute of
Ophthalmology (Beijing, China). HLE-B3 cells were maintained in
Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal
bovine serum (HyClone; GE Healthcare Life Sciences, Logan, Utah,
USA), penicillin (100 units/ml) and streptomycin (100 µg/ml). All
cells were maintained at 37°C in a humidified incubator containing
5% CO2. Cells were treated with 0.25% trypsin-0.02% EDTA
solution and passaged at 75–80% confluence.
Stock solutions of PP1, a c-Src inhibitor, and
SB431542, an ALK5 inhibitor, (Enzo Life Sciences, Inc.,
Farmingdale, NY, USA) were prepared in dimethyl sulfoxide and
diluted with cell culture media to obtain a final concentration of
10 µmol/l. Antibodies against c-Src (cat. no. 2123), E-cadherin
(cat. no. 3195), β-tubulin (cat. no. 2128) and β-actin (cat. no.
4970) were obtained from Cell Signaling Technology, Inc. (Danvers,
MA, USA). Antibodies against TGF-β1 (cat. no. ab92486),
p-Src418 (cat. no. ab4816), α-SMA (cat. no. ab5694),
ALK5 (cat. no. ab31013) and GAPDH (cat. no. ab181602) were obtained
from Abcam (Cambridge, MA, USA). Anti-TGF-β2 antibody (cat. no.
ABE586) was obtained from EMD Millipore (Bedford, MA, USA).
A preliminary study was performed to optimize the
time and glucose concentration for subsequent experiments. HLE-B3
cells were subsequently divided into three groups: HG DMEM
(glucose, 35.5 mM), HG DMEM with PP1 (10 µmol/l) and HG DMEM with
SB431542 (10 µmol/l). The glucose concentration for the normal
glucose (NG) group was 5.5 mM. The time-dependent effects were
measured at 0, 3, 6 and 12 h for further experiments. The HLE-B3
cells in exponential growth phase were seeded at a cell density of
1×105 cells/ml, cultured at 37°C, 5% CO2 and
then assessed at 0, 3 and 6 h.
Short hairpin (sh)RNA knockdown
assay
shRNA for human c-Src (GenBank no. NM: 005417) and
random control lentiviral plasmid (pLKO.1-puro Control Vector) were
obtained from GenePharma (GenePharma Co. Ltd, Beijing, China). The
human HLE-B3 cells were cultured in the 60 mm diameter culture dish
under 37°C, 5% CO2 condition. When cells growth covered
50–60% of the dish, the cells were transfected with 5 µg
shRNA-c-Src or 5 µg shRNA-vector using Lipofectamine 2000
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's recommendations.
Following 72 h of incubation, transfection efficiency was measured
by determining the green fluorescent protein expression ratio via
fluorescence microscopy (Olympus BX53; Olympus Corporation, Tokyo,
Japan; data not shown).
In c-Src knockdown experiments, 5×106
cells/ml HLE-B3 cells were divided into two groups: shRNA-vector
and shRNA-c-Src. Cells were cultured in HG DMEM (35.5 mM) for a
total of 12 h, and the time-dependent effects were monitored at 0,
3, 6, 9 and 12 h. The knockdown of c-Src was confirmed via western
blot analysis.
Western blot analysis
Total protein in HLE-B3 cells was extracted using
radioimmunoprecipitation assay lysis buffer with protease inhibitor
cocktail set1 (cat. no. 539131) and phosphatase inhibitor cocktail
set V (cat. no. 524629; all EMD Millipore). Protein concentration
was determined using BCA protein assay reagent (Beyotime Institute
of Biotechnology, Shanghai, China). Proteins (30 µg/lane) were
separated using 10% SDS-PAGE and electrophoretically transferred
onto nitrocellulose filter membranes (EMD Millipore). Membranes
were blocked in 4°C overnight with 5% non-fat milk and incubated
with various primary antibodies, including rabbit anti-c-Src
monoclonal antibody (1:10,000), rabbit anti-p-Src418
monoclonal antibody (1:1,000), rabbit anti-TGF-β1 polyclonal
antibody (1:100), rabbit anti-TGF-β2 polyclonal antibody (1:1,000),
rabbit anti-E-cadherin polyclonal antibody (1:1,000), rabbit
anti-α-SMA polyclonal antibody (1:1,000), rabbit anti-ALK5
polyclonal antibody (1:200), rabbit anti-β-actin monoclonal
antibody (1:1,000), rabbit anti-β-tubulin monoclonal antibody
(1:1,000) and rabbit anti-GAPDH monoclonal antibody (1:10,000) at
4°C for 18 h. Following 2 h incubation at room temperature with
horseradish peroxidase-conjugated secondary antibody (1:1,000; cat.
no. 7074; Cell Signaling Technology, Inc.), the protein bands were
quantified using a Bio-Rad chemiluminescent gel imaging system and
standardized to β-actin, β-tubulin or GAPDH protein expression
using Image Lab™ software (version 6.0; both Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Determination of TGF-β1 and TGF-β2
secretion using ELISA
An ELISA kit (eBioscience; Thermo Fisher Scientific,
Inc.) was employed to evaluate the secretion of TGF-β1 (cat. no.
BMS249-4) and TGF-β2 (cat. no. BMS254) according to the
manufacturer's protocol. Three samples of each group were
examined.
Statistical analysis
All data were analyzed using SPSS statistical
analysis software, version 16.0 (SPSS, Inc., Chicago, IL, USA).
Data were expressed as the mean ± standard deviation. For multiple
group comparisons at different time points, statistical analysis
was performed using repeated measures analysis of variance followed
by Fisher's Least Significant Difference post hoc test. For groups
at the same time point, the Student's t-test was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
HG conditions induce EMT of LECs
To investigate the effect of HG conditions on LECs,
the protein expression levels of EMT marker protein E-cadherin and
interstitial cell marker protein α-SMA in HLE-B3 cells under HG and
NG conditions were studied using western blot analysis. Results
indicated that the protein expression levels of E-cadherin were
marginally decreased whereas α-SMA protein expression levels were
marginally increased under normal glucose (NG) conditions between 0
to 12 h. However, a time-dependent decrease in E-cadherin
expression levels and a time-dependent increase in α-SMA expression
levels was indicated when HLE-B3 cells were exposed to HG
conditions. The differences were significant at 6 and 12 h compared
with 0 h under the same conditions (P<0.05). Additionally,
results indicated that the protein expression levels of E-cadherin
were significantly decreased whereas α-SMA protein expression
levels were significantly increased at 6 and 12 h in HLE-B3 cells
under HG conditions compared with that in HLE-B3 cells under NG
conditions at the same time points (P<0.05; Fig. 1). These results suggested that LECs
may slowly trans-differentiate into mesenchymal cells under HG
conditions.
HG conditions activate c-Src
To further investigate the influence of HG
conditions on the activity of c-Src, the protein expression levels
of p-Src418 were determined using western blot analysis
under NG and HG conditions. The results demonstrated that there was
little p-Src418 expression observed following 12 h incubation under
NG conditions. By contrast, the protein expression levels of
p-Src418 were elevated and reached a peak at 6 h of
culture in HG conditions, which was followed by a decrease in
p-Src418 expression at 12 h (Fig. 2). Statistical analysis indicated that
the protein expression levels of p-Src418 were
significantly increased under HG conditions at 3, 6 and 12 h
compared with at 0 h under HG conditions (P<0.05). Furthermore,
the p-Src418 protein expression levels of HLE-B3 cells
at the corresponding time points in the HG conditions were
significantly higher than those indicated under NG conditions
(P<0.05), which suggested that the activation of c-Src was
stimulated by HG conditions.
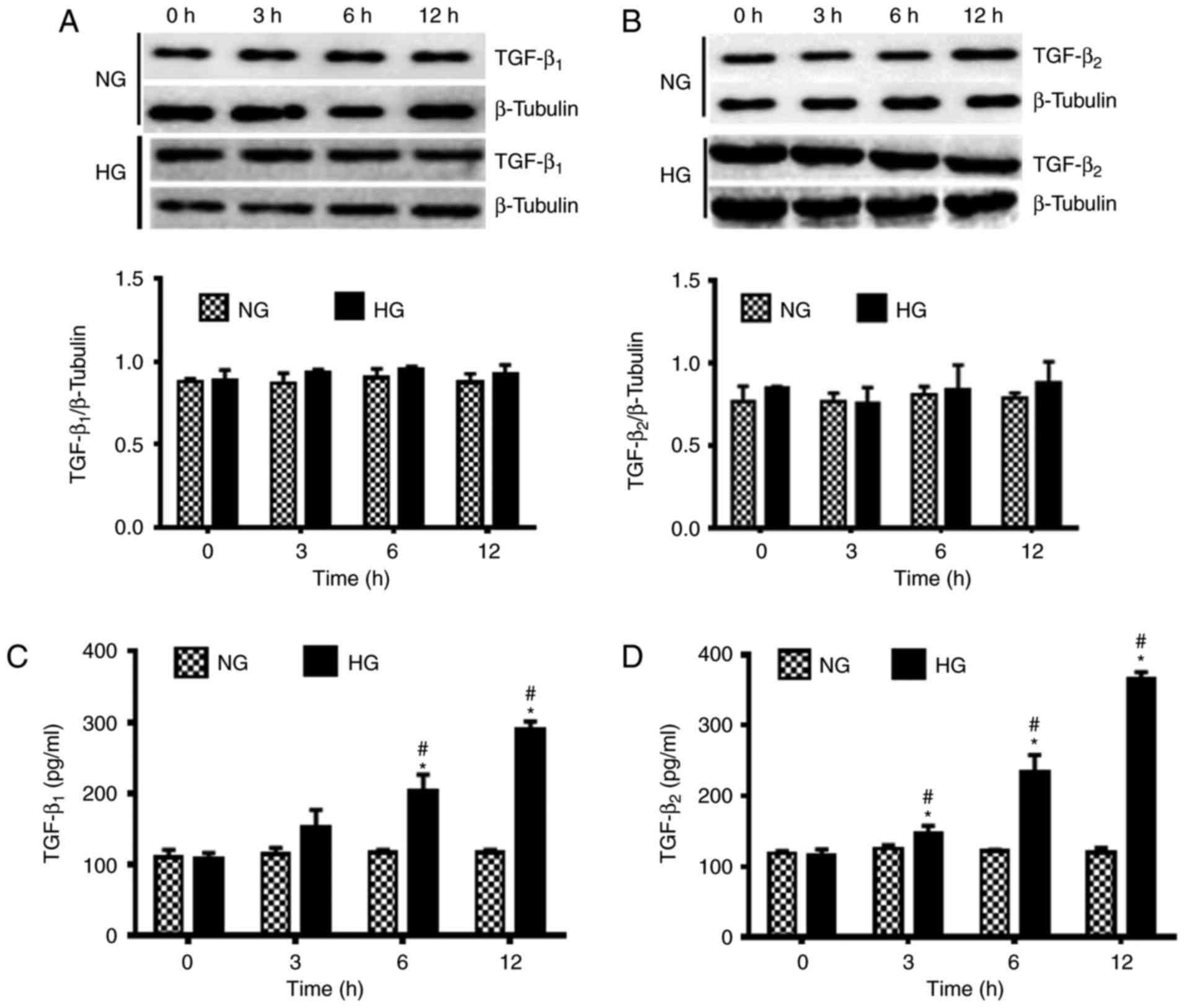
HG conditions activate TGF-β
The influence of HG conditions on the activation of
TGF-β was observed using western blot analysis. There were no
notable changes in the protein expression levels of TGF-β1 and
TGF-β2 under NG conditions. However, under HG conditions the
expression levels of TGF-β1 and TGF-β2 were marginally increased
over 12 h (Fig. 3A and B). These
findings suggested that HG conditions exerted little impact on the
protein expression levels of TGF-β1 and TGF-β2.
The secretion of TGF-β1 and TGF-β2 was also measured
under NG and HG conditions using ELISA. Results revealed that the
secretion of TGF-β1 and TGF-β2 under NG conditions was not
significantly altered over 12 h. However, a significant increase in
the secretion of TGF-β1 at 6 and 12 h and TGF-β2 at 3, 6 and 12 h
was observed under HG conditions compared with 0 h under the same
conditions (P<0.05; Fig. 3C and
D). Furthermore, significantly increased secretion of TGF-β1 at
6 to 12 h and TGF-β2 at 3, 6 to 12 h was indicated under HG
conditions compared with those indicated under NG conditions at the
same time points (P<0.05).
Effect of c-Src and TGF-β on EMT of
LECs
To further investigate the regulatory effects of
c-Src and TGF-β in EMT of LECs, SB431542 and PP1 were added to the
HG media. PP1 was used to investigate the regulatory effect of
c-Src on TGF-β, while SB431542 was used as the positive control.
The protein expression levels of c-Src and TGF-β were measured
using western blot analysis and TGF-β secretion was analyzed using
ELISA. As indicated in Fig. 4, the
protein expression levels of p-Src418, TGF-β1 and TGF-β2
in HLE-B3 cells under HG conditions and PP1 were significantly
decreased compared with those indicated in HLE-B3 cells under HG
conditions alone (P<0.05). Notably, the secretion of TGF-β1 and
TGF-β2 was significantly decreased in HLE-B3 cells under HG
conditions and PP1 compared with cells under HG conditions alone at
6 h (P<0.05; Fig. 5). These
findings suggest that active c-Src promotes the activation of
TGF-β. Under HG conditions, SB431542, which is an inhibitor of
TGF-β, significantly downregulated the protein expression levels
and secretion of TGF-β1 and TGF-β2 (P<0.05) but exerted no
significant effect on p-Src418 expression levels
compared with that in HLE-B3 cells under HG conditions alone
(Figs. 4A-C and 5).
 | Figure 4.Effects of 10 µmol/l c-Src inhibitor
PP1 and 10 µmol/l ALK5 inhibitor SB431542 on the protein expression
levels of p-Src418, TGF-β1, TGF-β2, ALK5, E-cadherin and
α-SMA under HG conditions at 0 to 6 h. Protein expression levels of
(A) p-Src418, (B) TGF-β1, (C) TGF-β2, (D) ALK5, (E)
E-cadherin and (F) α-SMA were determined using western blot
analysis. Data are presented as the mean ± standard deviation.
*P<0.05 as indicated. HG, high glucose; TGF-β, transforming
growth factor-β; α-SMA, α-smooth muscle actin; ALK5, activin
receptor-like kinase 5. |
Effect of c-Src on the biological
function of TGF-β in EMT of LECs
The influence of c-Src on the biological function of
TGF-β was assessed by determining the activity of ALK5 using
western blot analysis. The results revealed that the protein
expression levels of ALK5 in HLE-B3 cells cultured under HG
conditions with PP1 or SB431542 were significantly decreased
compared with those in HLE-B3 cells under HG conditions alone at 6
h (P<0.05, Fig. 4D). This was
consistent with the results obtained regarding TGF-β1 and TGF-β2
secretion in HLE-B3 cells cultured under HG conditions with
SB431542. The downregulation of ALK5 suggested that c-Src may
influence the biological function of TGF-β.
c-Src and TGF-β mediate EMT of
LECs
The roles of c-Src and TGF-β in EMT of HLE-B3 cells
induced by HG conditions were determined by measuring the
alterations of EMT protein markers E-cadherin and α-SMA in HLE-B3
cells in the presence of PP1 or SB431542 using western blot
analysis. The protein expression levels of E-cadherin in HLE-B3
cells under HG conditions were decreased in a time-dependent
manner. However, HG conditions and PP1 or SB431542 increased the
protein expression levels of E-cadherin in HLE-B3 cells compared
with that in HLE-B3 cells cultured in HG conditions alone. This
difference was statistically significant at 6 h (P<0.05;
Fig. 4E). Notably, the protein
expression levels of α-SMA in HLE-B3 cells under HG conditions were
increased in a time-dependent manner. However, the protein
expression levels of α-SMA were significantly decreased in HLE-B3
cells treated with PP1 or SB431542 under HG conditions compared
with HLE-B3 cells under HG conditions alone at 6 h (P<0.05,
Fig. 4F). These findings suggested
that PP1 or SB431542 hindered the decrease of E-cadherin and the
increase of α-SMA, which suggested the role of c-Src and TGF-β in
promoting EMT of LECs.
Influence of c-Src knockdown on TGF-β
and EMT of LECs
HLE-B3 cells transfected with shRNA-c-Src or
shRNA-vector were exposed to HG conditions (35.5 mM). As indicated
in Fig. 6, the expression levels of
p-Src418 protein increased in a time-dependent manner up
until 9 h, after which the levels were decreased in the
shRNA-vector group. In the shRNA-c-Src group, the
p-Src418 protein expression levels were significantly
decreased compared with those indicated in the shRNA-vector group
at the same time points (P<0.05; Fig.
6A). The decreased expression level of p-Src418
protein, instead of completely silenced p-Src418,
suggested that the knockdown of c-Src may not be completely
successful.
 | Figure 6.Effects of c-Src knockdown by shRNA
on the protein expression levels of p-Scr418, TGF-β1,
TGF-β2, ALK5, E-cadherin and α-SMA in human lens epithelial B3
cells under HG conditions at 0 to 12 h. Protein expression levels
of (A) p-Scr418 and TGF-β2, (B) TGF-β1, (C) ALK5, (D)
E-cadherin and (E) α-SMA were determined using western blot
analysis. The secretion of (F) TGF-β1 and (G) TGF-β2 in human lens
epithelial B3 cells under HG conditions was determined using ELISA.
Data are presented as the mean ± standard deviation.
#P<0.05 vs. shRNA-Vector group. HG, high glucose;
TGF-β, transforming growth factor-β; shRNA, short hairpin RNA;
α-SMA, α-smooth muscle actin; ALK5, activin receptor-like kinase
5. |
The protein expression levels of TGF-β1 and TGF-β2
in shRNA-c-Src or shRNA-vector groups stimulated by HG conditions
were observed using western blot analysis. In the shRNA-c-Src
group, the expression levels of TGF-β1 protein were increased in a
time-dependent manner up until 9 h, after which the expression
levels were decreased. In the shRNA-c-Src group, TGF-β1 protein
expression levels significantly decrease compared with the
shRNA-Vector group (P<0.05; Fig.
6B). Notably, TGF-β2 protein expression levels in the
shRNA-c-Src and shRNA-vector groups were increased in a
time-dependent manner up until 9 h after which the expression
levels were decreased (Fig. 6A).
However, the TGF-β2 protein expression levels were significantly
increased in the shRNA-vector group compared with the shRNA-c-Src
group at all time points (P<0.05). These findings suggested that
suppression of c-Src significantly reduced the protein expression
levels of TGF-β.
The secretion of HG-stimulated TGF-β1 and TGF-β2
proteins, were measured using ELISA in the shRNA-c-Src and
shRNA-vector groups. In shRNA-c-Src and shRNA-vector groups, the
secretion of TGF-β1 and TGF-β2 increased in a time-dependent manner
without significant differences. Furthermore, the secretion of
TGF-β1 and TGF-β2 was significantly decreased in the shRNA-c-Src
group compared with that in the shRNA-vector group at all time
points (P<0.05; Fig. 6F and G),
which suggested that the suppression of c-Src effectively reduced
the secretion of TGF-β.
Suppression of c-Src inhibits the
biological function of TGF-β in the EMT of LECs
To evaluate whether c-Src activity may influence the
biological function of TGF-β, the expression levels of ALK5
stimulated by HG conditions in transfected HLE-B3 cells were
determined using western blot analysis. In the shRNA-vector and
shRNA-c-Src groups, the protein expression levels of ALK5 were
increased in a time-dependent manner. However, the protein
expression levels of ALK5 were significantly decreased in the
shRNA-c-Src group compared with the shRNA-vector group at 0 to 12 h
(P<0.05; Fig. 6C). These results
suggested that c-Src silencing inhibited the activity of ALK5,
which is the downstream mediator of the TGF-β signaling
pathway.
Suppression of c-Src inhibits EMT of
LECs
The influence of c-Src knockdown on EMT of LECs
induced by HG conditions was investigated by evaluating the
expression of EMT protein markers using western blot analysis. In
the shRNA-vector and shRNA-c-Src groups, the protein expression
levels of E-cadherin were decreased in a time-dependent manner.
However, the expression levels of E-cadherin in the shRNA-vector
group were significantly decreased compared with that in the
shRNA-c-Src group at all time points (P<0.05; Fig. 6D). Notably, the protein expression
levels of α-SMA were increased in the shRNA-vector and shRNA-c-Src
groups in a time-dependent manner. Additionally, the protein
expression levels of α-SMA were significantly decreased in the
shRNA-c-Src group compared with that in the shRNA-vector group at 0
to 12 h (P<0.05; Fig. 6E). These
results indicated that knockdown of c-Src may effectively inhibit
EMT of LECs induced by HG conditions.
Discussion
EMT is a process by which epithelial cells lose
their original characteristics and gain mesenchymal cell properties
during specific physiological and pathological stages, including
organ fibrosis (33,34). Previous studies on the EMT of renal
cells cultured in HG conditions (30 or 60 mM) demonstrated
significant increases of α-SMA and decreases of E-cadherin
(33,34). It was also reported that EMT is the
primary factor in the pathogenesis of renal fibrosis, in which
TGF-β and c-Src are key inducing factors (35–38).
Subcapsular cataract is a common type of diabetic cataract
(39). Studies have revealed that a
number of aberrant nucleated cells assemble underneath the
posterior capsule in animal models of subcapsular cataract
(13–15). However, at present, the exact
mechanism and key trigger factors of diabetic subcapsular cataract
remains unclear. In Rap39 transgenic animal models of diabetic
cataract, the presence of α-SMA was confirmed in the muddy plaque
underneath the anterior capsule of the lens in the early stage of
cataract (4), suggesting that
HG-induced EMT of LECs may be a key mechanism in animal models of
diabetic cataract. The data obtained in the present study suggested
a similar process in EMT of LECs under HG conditions (35.5 mM) as
the protein expression levels of E-cadherin were decreased and the
protein expression levels of α-SMA were increased over time.
The present study indicated that HG conditions
contributed to the increased activity of TGF-β and promoted the
phosphorylation of c-Src. Glomerular mesangial cells incubated with
HG (30 mM) concentrations also demonstrated similar effects on the
levels of p-Src418 (40,41).
Furthermore, it has been reported that exposure of A549 cells to HG
(25 mM) concentrations for 48 h resulted in increased activity of
TGF-β (42). This is in agreement
with the present findings, which indicated that c-Src and TGF-β
were activated during EMT of LECs under HG conditions.
As c-Src and TGF-β were concurrently activated in
EMT of LECs, the roles of c-Src and TGF-β in the EMT were further
investigated in the present study. The results revealed that PP1
and SB431542 delayed the EMT progression in LECs induced by HG
stimulation. Previous studies also suggested that PP1 and SB431542
suppressed the activities of c-Src and TGF-β following HG treatment
in different cell phenotypes (30,31,43,44). In
addition, it was reported that activated c-Src promoted EMT and
c-Src-specific inhibitors blocked EMT in renal tubular epithelial
cells (45). Furthermore, various
studies have reported that TGF-β is a central mediator of EMT and
the inhibition of the TGF-β signaling pathway with SB431542
prevented the progression of EMT (43,44,46).
These studies support the present findings, which indicated that
c-Src and TGF-β mediate the EMT of LECs.
The present study indicated that PP1 directly
inhibited the activity of c-Src and TGF-β in HLE-B3 cells following
HG stimulation. However, although SB431542 also significantly
inhibited the activity of TGF-β, SB431542 had no significant effect
on activity of c-Src. It is likely that PP1 may induce the indirect
inhibition on TGF-β by the PP1-induced inhibition of c-Src
activity. Previous studies have reported that PP1 and PP2 inhibited
TGF-β-mediated cell invasion and migration in pancreatic ductal
adenocarcinoma cells and non-small-cell lung carcinoma cells via
directly targeting ALK5, suggesting that PP1 and PP2 may be dual
inhibitors of TGF-β and c-Src in vitro (47,48).
This is consistent with the speculations from the present study.
Furthermore, incubation with SB431542 resulted in a significant
decrease in the protein expression level of TGF-β at 6 h, which
suggests that SB431542 may affect the cell growth and the cell
cycle and promote cell apoptosis of HLE-B3 cells (49,50). The
present study has demonstrated that PP1 not only restrained the
c-Src-induced EMT of LECs, but also downregulated TGF-β-mediated
EMT of LECs. However, it is not known whether c-Src participates in
the TGF-β-mediated EMT of LECs.
In order to further assess the association between
c-Src and TGF-β and to confirm the inhibiting effect of PP1 on the
intracellular function of TGF-β, shRNA was used to knockdown the
c-Src gene in HLE-B3 cells. The results demonstrated that, when
phosphorylation of c-Src418 was inhibited by c-Src gene
knockdown, the activities of TGF-β and ALK5 were significantly
inhibited compared with the shRNA-vector group. This suggests that
c-Src may be an upstream molecule that regulates the bioactivity of
TGF-β. In addition, the EMT of HLE-B3 cells was blocked following
c-Src knockdown, which indicates that active c-Src may promote the
EMT of LECs. It has been reported that the activation of c-Src
promoted TGF-β-mediated EMT of glomerular endothelial cells, in
which c-Src is the key regulatory molecule (51). However, studies with regards to c-Src
and TGF-β have provided various results, including the suggestion
that TGF-β may regulate the activity of c-Src in vascular smooth
muscle cells and non-small-cell lung cancer A549 cells (52,53).
Maeda et al (46) indicated
that Src activation was not involved in the processing of
TGF-β-mediated EMT in mammary epithelial cells and pancreatic
ductal adenocarcinoma cells. These different results regarding
c-Src and TGF-β in the regulation of EMT of various cells may be
due to the differences in cell type and stimuli.
In conclusion, the findings of the present study
suggest that c-Src may be an upstream molecule that regulates the
TGF-β-mediated EMT of LECs under HG conditions. Therefore, the
c-Src/TGF-β signal axis, particularly c-Src, may be a potential
target for preventing and treating complications associated with
diabetes mellitus, including diabetic subcapsular cataract.
However, further in vitro investigations on the exact
mechanism and signaling pathway of c-Src and TGF-β are
required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the grants from
the National Natural Science Foundation of China to JZ (grant no.
81370998) and the Innovation Project in Science and Technology of
Shaanxi Province to JZ (grant no. 2012KTCQ03-03).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ designed the experiments. ZHH and FW performed
the experiments. FLW and QL analyzed and interpreted the results of
the experiments. QL was a major contributor in writing the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lang VB, Baretić M and Pavić E: Kidney
disease in diabetic patients-the role of family medicine physician.
Acta Med Croatica. 70:319–324. 2016.(In Croatian). PubMed/NCBI
|
|
2
|
Campos EJ, Campos A, Martins J and
Ambrósio AF: Opening eyes to nanomedicine: Where we are, challenges
and expectations on nanotherapy for diabetic retinopathy.
Nanomedicine. 13:2101–2113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richter GM, Choudhury F, Torres M, Azen SP
and Varma R: Los Angeles Latino Eye Study Group: Risk factors for
incident cortical, nuclear, posterior subcapsular, and mixed lens
opacities: The Los Angeles Latino eye study. Ophthalmology.
119:2040–2047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zablocki GJ, Ruzycki PA, Overturf MA,
Palla S, Reddy GB and Petrash JM: Aldose reductase-mediated
induction of epithelium-to-mesenchymal transition (EMT) in lens.
Chem Biol Interact. 191:351–356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hegde KR and Varma SD: Cataracts in
experimentally diabetic mouse: Morphological and apoptotic changes.
Diabetes Obes Metab. 7:200–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho HG and Yoo J: Rho activation is
required for transforming growth factor-beta-induced
epithelial-mesenchymal transition in lens epithelial cells. Cell
Biol Int. 31:1225–1230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Struck HG, Heider C and Lautenschläger C:
Changes in the lens epithelium of diabetic and non-diabetic
patients with various forms of opacities in senile cataract. Klin
Monbl Augenheilkd. 216:204–209. 2000.(In German). PubMed/NCBI
|
|
8
|
Deckers M, van Dinther M, Buijs J, Que I,
Löwik C, van der Pluijm G and ten Dijke P: The tumor suppressor
Smad4 is required for transforming growth factor beta-induced
epithelial to mesenchymal transition and bone metastasis of breast
cancer cells. Cancer Res. 66:2202–2209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valcourt U, Kowanetz M, Niimi H, Heldin CH
and Moustakas A: TGF-beta and the Smad signaling pathway support
transcriptomic reprogramming during epithelial-mesenchymal cell
transition. Mol Biol Cell. 16:1987–2002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsapara A, Luthert P, Greenwood J, Hill
CS, Matter K and Balda MS: The RhoA activator GEF-H1/Lfc is a
transforming growth factor-beta target gene and effector that
regulates alpha-smooth muscle actin expression and cell migration.
Mol Biol Cell. 21:860–870. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sebe A, Leivonen SK, Fintha A, Masszi A,
Rosivall L, Kähäri VM and Mucsi I: Transforming growth
factor-beta-induced alpha-smooth muscle cell actin expression in
renal proximal tubular cells is regulated by p38beta
mitogen-activated protein kinase, extracellular signal-regulated
protein kinase1,2 and the Smad signalling during
epithelial-myofibroblast transdifferentiation. Nephrol Dial
Transplant. 23:1537–1545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gordon-Thomson C, de Iongh RU, Hales AM,
Chamberlain CG and McAvoy JW: Differential cataractogenic potency
of TGF-beta1, -beta2, and -beta3 and their expression in the
postnatal rat eye. Invest Ophthalmol Vis Sci. 39:1399–1409.
1998.PubMed/NCBI
|
|
13
|
Hales AM, Chamberlain CG and McAvoy JW:
Cataract induction in lenses cultured with transforming growth
factor-beta. Invest Ophthalmol Vis Sci. 36:1709–1713.
1995.PubMed/NCBI
|
|
14
|
Liu J, Hales AM, Chamberlain CG and McAvoy
JW: Induction of cataract-like changes in rat lens epithelial
explants by transforming growth factor beta. Invest Ophthalmol Vis
Sci. 35:388–401. 1994.PubMed/NCBI
|
|
15
|
Saika S, Kono-Saika S, Ohnishi Y, Sato M,
Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, et al:
Smad3 signaling is required for epithelial-mesenchymal transition
of lens epithelium after injury. Am J Pathol. 164:651–663. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du L, Hao M, Li C, Wu W, Wang W, Ma Z,
Yang T, Zhang N, Isaaca AT, Zhu X, et al: Quercetin inhibited
epithelial mesenchymal transition in diabetic rats,
high-glucose-cultured lens, and SRA01/04 cells through transforming
growth factor-β2/phosphoinositide 3-kinase/Akt pathway. Mol Cell
Endocrinol. 452:44–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YS, Kim NH, Jung DH, Jang DS, Lee YM,
Kim JM and Kim JS: Genistein inhibits aldose reductase activity and
high glucose-induced TGF-beta2 expression in human lens epithelial
cells. Eur J Pharmacol. 594:18–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim NH, Kim YS, Jung DH and Kim JS:
KIOM-79 prevents xylose-induced lens opacity and inhibits TGF-beta2
in human lens epithelial cells cultured under high glucose. J
Ethnopharmacol. 130:599–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu Q, Yang T, Zhang M, Du L, Liu L, Zhang
N, Guo H, Zhang F, Hu G and Yin X: Preventative effects of Ginkgo
biloba extract (EGb761) on high glucose-cultured opacity of rat
lens. Phytother Res. 28:767–773. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
MacKay CE and Knock GA: Control of
vascular smooth muscle function by Src-family kinases and reactive
oxygen species in health and disease. J Physiol. 593:3815–3828.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hovater MB and Sanders PW: Effect of
dietary salt on regulation of TGF-β in the kidney. Semin Nephrol.
32:269–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Montagner A, Delgado MB, Tallichet-Blanc
C, Chan JS, Sng MK, Mottaz H, Degueurce G, Lippi Y, Moret C,
Baruchet M, et al: Src is activated by the nuclear receptor
peroxisome proliferator-activated receptor β/δ in ultraviolet
radiation-induced skin cancer. EMBO Mol Med. 6:80–98. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang CH, Hsu CJ, Yang WH and Fong YC:
Lipoteichoic acid enhances IL-6 production in human synovial
fibroblasts via TLR2 receptor, PKCdelta and c-Src dependent
pathways. Biochem Picalharmacol. 79:1648–1657. 2010. View Article : Google Scholar
|
|
24
|
Boyer B, Bourgeois Y and Poupon MF: Src
kinase contributes to the metastatic spread of carcinoma cells.
Oncogene. 21:2347–2356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giehl K and Menke A: Microenvironmental
regulation of E-cadherin-mediated adherens junctions. Front Biosci.
13:3975–3985. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Humar B, Fukuzawa R, Blair V, Dunbier A,
More H, Charlton A, Yang HK, Kim WH, Reeve AE, Martin I and
Guilford P: Destabilized adhesion in the gastric proliferative zone
and c-Src kinase activation mark the development of early diffuse
gastric cancer. Cancer Res. 67:2480–2489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lawler K, O'Sullivan G, Long A and Kenny
D: Shear stress induces internalization of E-cadherin and
invasiveness in metastatic oesophageal cancer cells by a
Src-dependent pathway. Cancer Sci. 100:1082–1087. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Summy JM and Gallick GE: Src family
kinases in tumor progression and metastasis. Cancer Metastasis Rev.
22:337–358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Elsberger B: Translational evidence on the
role of Src kinase and activated Src kinase in invasive breast
cancer. Crit Rev Oncol Hematol. 89:343–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou J and Menko AS: The role of Src
family kinases in cortical cataract formation. Invest Ophthalmol
Vis Sci. 43:2293–2300. 2002.PubMed/NCBI
|
|
31
|
Zhou J and Menko AS: Coordinate signaling
by Src and p38 kinases in the induction of cortical cataract.
Invest Ophthalmol Vis Sci. 45:2314–2323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walker JL, Wolff IM, Zhang L and Menko AS:
Activation of SRC kinases signals induction of posterior capsule
opacification. Invest Ophthalmol Vis Sci. 48:2214–2223. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peng L, Yang J, Ning C, Zhang J, Xiao X,
He D, Wang X, Li Z, Fu S and Ning J: Rhein inhibits integrin-linked
kinase expression and regulates matrix metalloproteinase-9/tissue
inhibitor of metalloproteinase-1 ratio in high glucose-induced
epithelial-mesenchymal transition of renal tubular cell. Biol Pharm
Bull. 35:1676–1685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gu L, Gao Q, Ni L, Wang M and Shen F:
Fasudil inhibits epithelial-myofibroblast transdifferentiation of
human renal tubular epithelial HK-2 cells induced by high glucose.
Chem Pharm Bull (Tokyo). 61:688–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sutariya B, Jhonsa D and Saraf MN: TGF-β:
The connecting link between nephropathy and fibrosis.
Immunopharmacol Immunotoxicol. 38:39–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang JY, Gao YB, Zhang N, Zou DW, Wang P,
Zhu ZY, Li JY, Zhou SN, Wang SC, Wang YY and Yang JK: miR-21
overexpression enhances TGF-β1-induced epithelial-to-mesenchymal
transition by target smad7 and aggravates renal damage in diabetic
nephropathy. Mol Cell Endocrinol. 392:163–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang N, Gao Y, Zou D, Wang J, Li J, Zhou
S, Zhu Z, Zhao X, Xu L and Zhang H: Effects of Chinese Medicine
Tong xinluo on Diabetic nephropathy via inhibiting TGF- β 1-induced
epithelial-to-mesenchymal transition. Evid Based Complent Alternat
Med. 2014:1234972014.
|
|
38
|
Lu Y, Tang L, Li Y and He Q: High
glucose-induced fibronectin upregulation in cultured mesangial
cells involves caveolin-1-dependent RhoA-GTP activation via Src
kinase. Mol Med Rep. 14:963–968. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sayin N, Kara N and Pekel G: Ocular
complications of diabetes mellitus. World J Diabetes. 6:92–108.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie X, Lan T, Chang X, Huang K, Huang J,
Wang S, Chen C, Shen X, Liu P and Huang H: Connexin43 mediates
NF-κB signalling activation induced by high glucose in GMCs:
Involvement of c-Src. Cell Commun Signal. 11:382013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alisson-Silva F, Freire-de-Lima L, Donadio
JL, Lucena MC, Penha L, Sá-Diniz JN, Dias WB and Todeschini AR:
Increase of O-glycosylated oncofetal fibronectin in high
glucose-induced epithelial-mesenchymal transition of cultured human
epithelial cells. PLoS One. 8:e604712013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
DeMaio L, Buckley ST, Krishnaveni MS,
Flodby P, Dubourd M, Banfalvi A, Xing Y, Ehrhardt C, Minoo P, Zhou
B, et al: Ligand-independent transforming growth factor-β type I
receptor signalling mediates type I collagen-induced
epithelial-mesenchymal transition. J Pathol. 226:633–644. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wilson C, Nicholes K, Bustos D, Lin E,
Song Q, Stephan JP, Kirkpatrick DS and Settleman J: Overcoming
EMT-associated resistance to anti-cancer drugs via Src/FAK pathway
inhibition. Oncotarget. 5:7328–7341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee JY, Chang JW, Yang WS, Kim SB, Park
SK, Park JS and Lee SK: Albumin-induced epithelial-mesenchymal
transition and ER stress are regulated through a common ROS-c-Src
kinase-mTOR pathway: effect of imatinib mesylate. Am J Physiol
Renal Physiol. 300:F1214–F1222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pang L, Li Q, Wei C, Zou H, Li S, Cao W,
He J, Zhou Y, Ju X, Lan J, et al: TGF-β1/Smad signaling pathway
regulates epithelial-to-mesenchymal transition in esophageal
squamous cell carcinoma: In vitro and clinical analyses of cell
lines and nomadic Kazakh patients from northwest Xinjiang, China.
PLoS One. 9:e1123002014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Maeda M, Shintani Y, Wheelock MJ and
Johnson KR: Src activation is not necessary for transforming growth
factor (TGF)-beta-mediated epithelial to mesenchymal transitions
(EMT) in mammary epithelial cells. PP1 directly inhibits TGF-beta
receptors I and II. J Biol Chem. 281:59–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bartscht T, Lehnert H, Gieseler F and
Ungefroren H: The Src family kinase inhibitors PP2 and PP1
effectively block TGF-beta1-induced cell migration and invasion in
both established and primary carcinoma cells. Cancer Chemother
Pharmacol. 70:221–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hoshino Y, Katsuno Y, Ehata S and Miyazono
K: Autocrine TGF-β protects breast cancer cells from apoptosis
through reduction of BH3-only protein, Bim. J Biochem. 149:55–65.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Itoh S and Itoh F: Implication of TGF-β as
a survival factor during tumour development. J Biochem.
151:559–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hovater MB and Sanders PW: Effect of
dietary salt on regulation of TGF-β in the kindney. Semin Nephrol.
32:269–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Deharvengt S, Marmarelis M and Korc M:
Concomitant targeting of EGF receptor, TGF-beta and SRC points to a
novel therapeutic approach in pancreatic cancer. PLoS One.
7:e396842012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Samarakoon R, Chitnis SS, Higgins SP,
Higgins CE, Krepinsky JC and Higgins PJ: Redox-induced Src kinase
and caveolin-1 signaling in TGF-β1-initiated SMAD2/3 activation and
PAI-1 expression. PLoS One. 6:e228962011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dong S, Khoo A, Wei J, Bowser RK,
Weathington NM, Xiao S, Zhang L, Ma H, Zhao Y and Zhao J: Serum
starvation regulates E-cadherin upregulation via activation of
c-Src in non-small-cell lung cancer A549 cells. Am J Physiol Cell
Physiol. 307:C893–C899. 2014. View Article : Google Scholar : PubMed/NCBI
|