Introduction
Osteoporosis is a major public health problem due to
its association with bone fragility and fracture, particularly as
the aging population has grown in recent years. Osteoporosis is
caused by an imbalance between bone resorption and formation, which
leads to a reduction in osteogenic ability as well as to excessive
bone resorption. These adverse processes may result in delayed bone
healing or nonunion of fractures (1–3). The
identification of osteopromotive and inductive agents with the
potential to stimulate bone formation has raised hope for the
augmentation of osteoporotic fracture healing (4,5). Local
delivery of recombinant human bone morphogenetic protein (rhBMP-2)
has been approved by the U.S. Food and Drug Administration for the
promotion of spinal fusion and fracture healing (6). In in vivo studies, local
application of BMP-2 in animal models of osteoporotic bone fracture
was demonstrated to be a promising therapeutic approach (7–9). In
spite of the success of its clinical application (10,11),
administration of BMP-2 has a number of drawbacks. The optimum
release pattern of rhBMP-2 has remained to be established, and
excessive dosages of BMP may be dangerous, as they have other
effects in processes including organogenesis, cell differentiation,
cell proliferation and apoptosis. Furthermore, the production and
purification of the recombinant protein is costly, making this
therapy expensive (12–14).
Considering that the side effects of rhBMP-2 are
dose-dependent, it may be reasonable to reduce the large doses of
BMP-2 that are currently used. One possible alternative to overcome
this problem is synchronous drug combinations to enhance the
potency of the BMP-2 used. The combination of systemic or local
anti-osteoporosis treatments with local delivery of BMP-2 has also
been studied for enhancing bone formation (15,16). As
osteoporosis is a systemic skeletal disease, appropriate
simultaneous management of the osteoporotic fracture and
osteoporosis is adequate and essential.
The current clinical treatment regimens for
osteoporosis are anti-resorptive drugs, including estrogen,
estrogen receptor analogues, calcitonin and bisphosphates, which
maintain the bone mass by inhibiting osteoclast function. However,
studies on the negative effects of fracture healing treatments and
potential complications, including breast cancer, uterine bleeding
and cardiovascular events, have raised concerns regarding their
long-term use (17,18).
Chinese herbal medicines have been widely used to
prevent and treat diseases for thousands of years. Psoralen
(molecular structure displayed in Fig.
1A) is a coumarin derivative extracted from the dried fruit of
Psoralen Corylifolia L., which is a well-known medicine with
ascribed properties of ‘bone strengthening’ based on The
Traditional Chinese Medicine theory. In an ex vivo study,
psoralen increased the osteogenic potential of bone marrow
mesenchymal stem cells (bMSCs), and stimulated the differentiation
of bMSCs to osteoblasts, with enhanced alkaline phosphatase (ALP)
activity (19). It has been reported
that psoralen promotes bone mass formation, increases bone strength
and improves the trabecular bone microstructure in ovariectomized
(OVX) mice, suggesting that it may be used as a natural compound
for the treatment of osteoporosis (20).
The aim of the present study was to evaluate the
effects of combination therapy with BMP-2 and psoralen on fracture
repair in OVX mice. Serum analyses of substances including
bone-specific ALP (BALP) and C-terminal telopeptide of type-1
collagen (CTX-1) were also performed to study bone metabolism.
Materials and methods
Animals
In total, 40 female C57BL/6 mice (body weight,
20.7±1.3 g; age, 12 weeks) were purchased from the Hubei Research
Center of Laboratory Animal (Wuhan, China). They were housed and
acclimatized at the experimental animal laboratory of Yangtze
University (Jingzhou, China) under controlled temperature (25°C)
and humidity (55%) with a 12-h light/dark cycle, with free access
to food and water.
Establishment of osteoporotic
model
Female mice (n=35) were subjected to OVX and those
in the sham group (n=5) received a sham surgery. After 6 weeks, the
establishment of the standard osteoporotic animal models was
confirmed prior to bone fracture surgery. To verify the success of
OVX, 5 randomly selected OVX mice and the 5 sham-operated mice were
euthanized to measure the estrogen levels, and the femoral
metaphysis was harvested for histological evaluation.
Study design
All OVX animals were randomly stratified into one of
the following groups: Model group, rhBMP-2 group and Psoralen +
rhBMP-2 group (Table I). According
to previous studies, fracture bridging appeared to occur at 21 days
after the fracture (21). Therefore,
the mice of the present study were sacrificed on day 21 after
injury and their limbs and blood were harvested for further
analysis [micro computed tomography (CT), histological, mechanical
and serum analysis].
 | Table I.Animal groups and interventions. |
Table I.
Animal groups and interventions.
| Group | N | Intervention |
|---|
| Model | 10 | Fracture, local
implant of ACS + oral gavages of saline |
| rhBMP-2 | 10 | Fracture, local
implant of ACS loaded with 2.5 µg rhBMP-2 and oral gavages of
saline |
| Psoralen +
rhBMP-2 | 10 | Fracture, local
implant of ACS loaded with 2.5 µg rhBMP-2 and oral gavages of
psoralen |
Drug treatments
The doses of psoralen and rhBMP-2 were selected
based on previous studies (20,22).
Psoralen and rhBMP-2 were purchased from Yongjian Pharmaceutical
Co. (Taizhou, China) and Amylet Scientific (PeproTech, Inc., Rocky
Hill, NJ, USA), respectively. Psoralen powder was ground and
suspended in physiological saline to achieve a final concentration
of 4 mg/ml. rhBMP-2 protein (2.5 µg) was reconstituted with 50 µl
PBS to produce a 50 µg/ml solution. The rhBMP-2 solution was soaked
onto a 4×4×5 mm-sized absorbable collagen sponge (ACS; type-I
collagen derived from bovine tendon; MEDECHI Medical Group,
Shanghai, China) for a minimum of 15 min prior to implantation. A
single implant was placed through the soft-tissue wound as an onlay
bridging the fracture site. The mice were administered psoralen or
physiological saline by oral gavage at a dose of 20 mg/kg per day
from the first post-operative day (n=10 in each group).
Surgical protocol
All surgical equipment was sterilized in an
autoclave. Sterile gowns, gloves, surgical masks and theater caps
were used. Mice were anesthetized by intraperitoneal injection of
ketamine hydrochloride (100 mg/kg body weight) and xylazine (4
mg/kg body weight). A standardized fracture was created on the
mid-diaphysis of the left femur in each mouse and stabilized with
marrow-nailing. Next, an ACS carrier saturated with 2.5 µg rhBMP-2
or PBS alone was inserted into the fracture. The muscles were
subsequently repositioned and the skin was closed with suture. All
of the animals received an intramuscular antibiotic and analgesic
injection for 3 post-operative days. Unrestricted activity was
allowed after the mice woke up from anesthesia.
Micro-CT examination
On day 21, five specimens per group were scanned by
a Scanco MicroCT 60 system (45 kVp; 114 mA; Scanco Medical,
Basserdorf, Switzerland) using a 12-µm voxel size. The
intramedullary pin was removed prior to scanning. In the callus
analysis, the original cortical bone was excluded, and region of
interest contained only new callus tissue. Mineralized tissue was
distinguished from air or soft tissue at a fixed, global threshold
with the lower limit corresponding to a mineral density of 421 mg
hydroxyapatite/cm3 and the upper limit corresponding to
3,000 mg hydroxyapatite/cm3 (23). Total callus volume (TV), mineralized
callus volume (BV), and tissue mineral density (TMD) were measured,
and fraction of mineralized callus (BV/TV) was calculated as
previously described (24).
Histology
For histological assessments, specimens from 5
animals per group were harvested on day 21 after fracture and fixed
in 10% formalin for 24 h, followed by decalcification in 10% EDTA
for 2 weeks at room temperature. The specimens were then embedded
in paraffin and cut longitudinally. Sagittal sections (5 µm) were
stained with H&E for assessment of basic morphology and with
Masson's trichrome for analysis of bone and cartilage using a
standard histological staining protocol (25,26).
Mechanical testing
The mechanical properties of the femurs with
fracture harvested from 5 animals per group on day 21 post-surgery
were assessed using a three-point bending test. Prior to mechanical
testing, the femurs that had been preserved in a fridge (4°C) for
12 h were warmed overnight at 22°C. A material test machine
(Instron-8841; Instron Corp., Norwood, MA, USA) with a 25 N load
cell was used to test the femur to failure. The femurs were
positioned horizontally with the anterior surface facing upwards,
centered on the supports 10 mm apart. A constant load was applied
at the fracture center with a displacement rate of 5 mm/min and
directed vertically towards the mid-shaft with the anterior surface
facing upward. After failure, the load vs. displacement curves were
recorded. The maximum load and stiffness were calculated from the
load-deflection curve recorded by a connected computer.
Serum analysis
On day 21 after fracture, blood (1 ml) was drawn
from each of 5 animals per group by cardiac puncture under
isoflurane anesthesia. Following standing in 2 ml centrifuge tubes
on ice for 10 min, samples were centrifuged to separate the serum
(10,000 × g for 10 min at 4°C), which was stored at −80°C. The
serum samples were analyzed for BALP (cat. no. SEB091Mu), CTX-1
(cat. no. CEA665Mu) using ELISA kits from Cloud-Clone Co., Ltd.
(Wuhan, China). The serum samples were also analyzed for estrogen
using Cobas 6000 e601 Immunology analyzer (Roche Diagnostics GmbH,
Mannheim, Germany).
Statistical analyses
Values are expressed as the mean ± standard
deviation. All statistical comparisons were performed using SPSS
17.0 software for Windows (SPSS, Inc., Chicago, IL, USA). Data were
analyzed by one-way analysis of variance with a least-significant
difference post-hoc test. Differences between means were considered
statistically significant at the 5% confidence level
(P<0.05).
Results
Confirmation of osteoporosis
The bilateral OVX led to a significant decrease in
estrogen levels compared with those measured in sham mice
(P<0.05; Fig. 1B). The effects of
decreased estrogen levels were also observed to result in gonadal
hypotrophy in OVX mice (Fig. 1C).
Compared with sham mice, the bone trabeculae of mice in the OVX
group were arranged sparsely or had disappeared and indicated
significant resorption (Fig. 1D and
E). These results confirmed the successful establishment of
osteoporosis in the OVX mice.
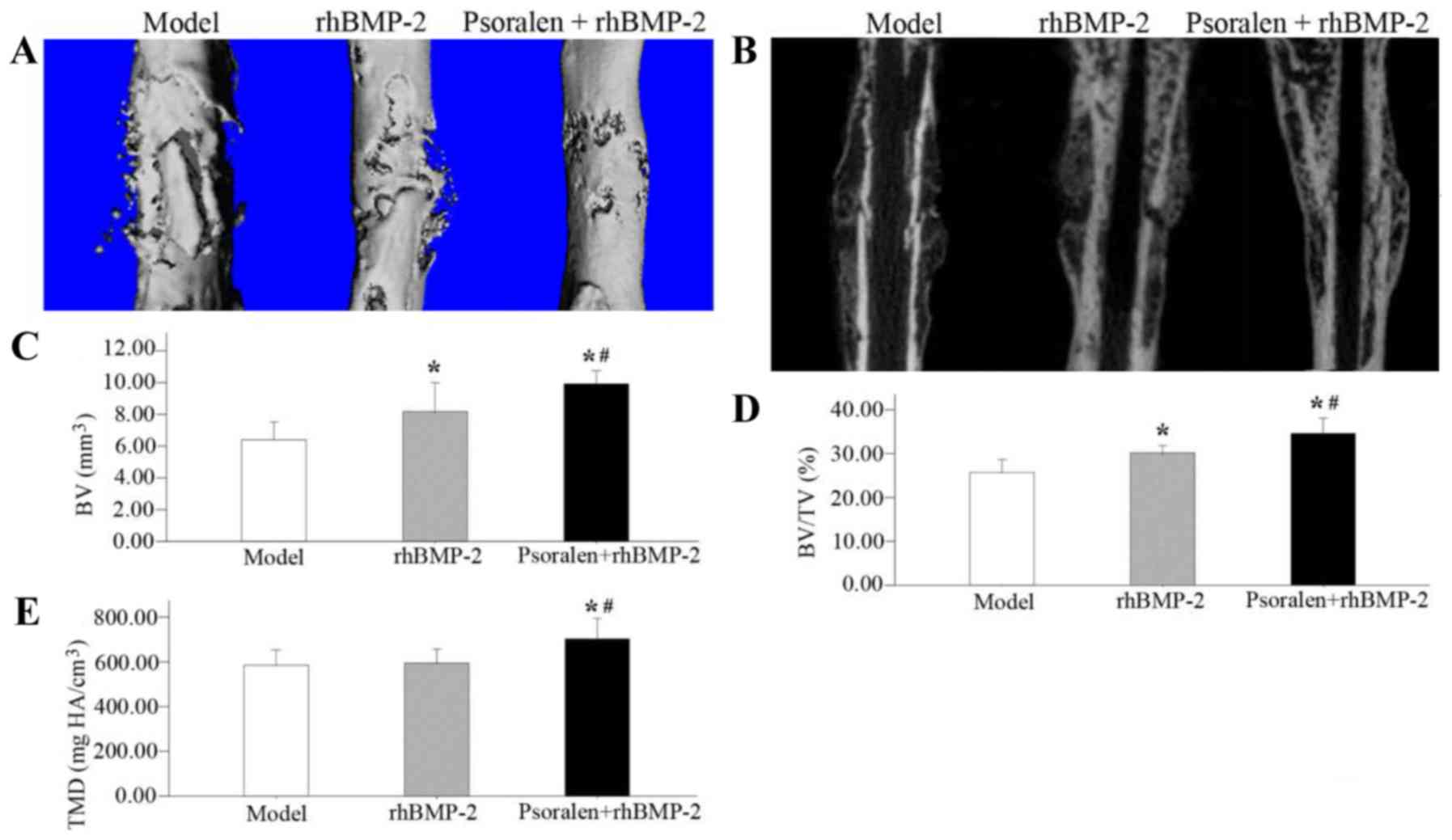
Micro-CT examination
On day 21, the Micro-CT surface analyses indicated
that fracture bridging occurred in the rhBMP-2 group and the
Psoralen + rhBMP-2 group. Compared with the rhBMP-2 group, the
healed bones in the Psoralen + rhBMP-2 group exhibited a
near-physiological shape. In the model group, the fracture healed
inadequately and at the same time, a large periosteal callus
remained (Fig. 2A and B).
The quantitative results revealed that compared with
the model group, the Psoralen + rhBMP-2 group had a higher BV
(P=0.001), BV/TV (P=0.001) and TMD (P=0.010), while the rhBMP-2
group exhibited an increase in BV (P=0.022) and BV/TV (P=0.008),
but no difference in TMD (P=0.814). Comparing the two treatment
groups, the Psoralen + rhBMP-2 group had a significantly greater
BV(P=0.024), BV/TV (P=0.009) and TMD (P=0.015) than the rhBMP-2
group (Fig. 2C-E).
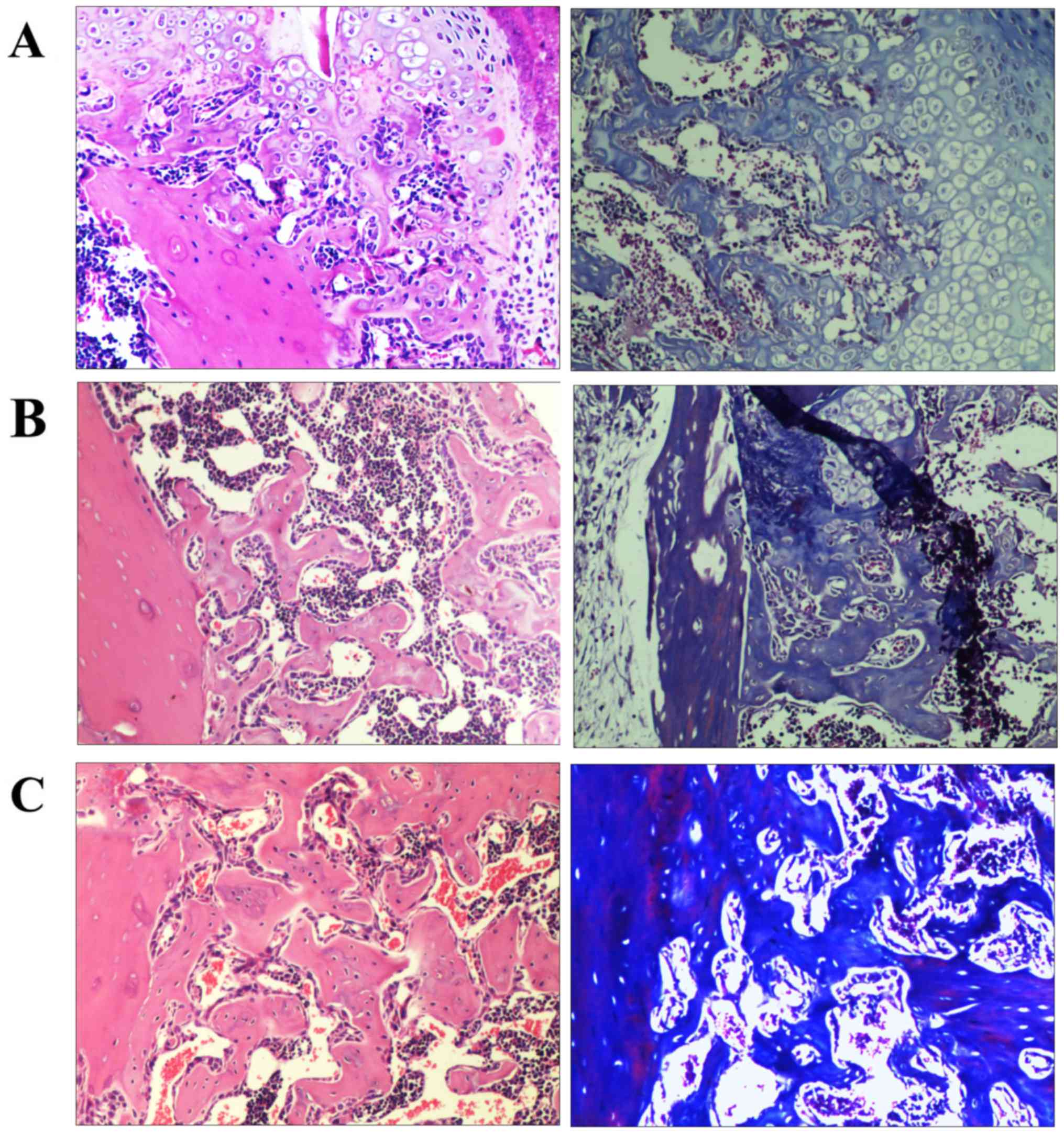
Histology
Representative histological images of fractures on
day 21 are presented in Fig. 3. The
calluses of all groups were composed of fibroblasts, cartilage and
newly formed trabecular bone. Histological analysis of the model
group samples indicated abundant amounts of chondroid callus
fracture area and the formation of immature bone (Fig. 3A). The rhBMP-2 and Psoralen + rhBMP-2
groups exhibited progressive mineralized callus formation compared
with the model group. However, the Psoralen + rhBMP-2 group
displayed significantly more mineralized and mature callus compared
with the rhBMP-2 group (Fig. 3B and
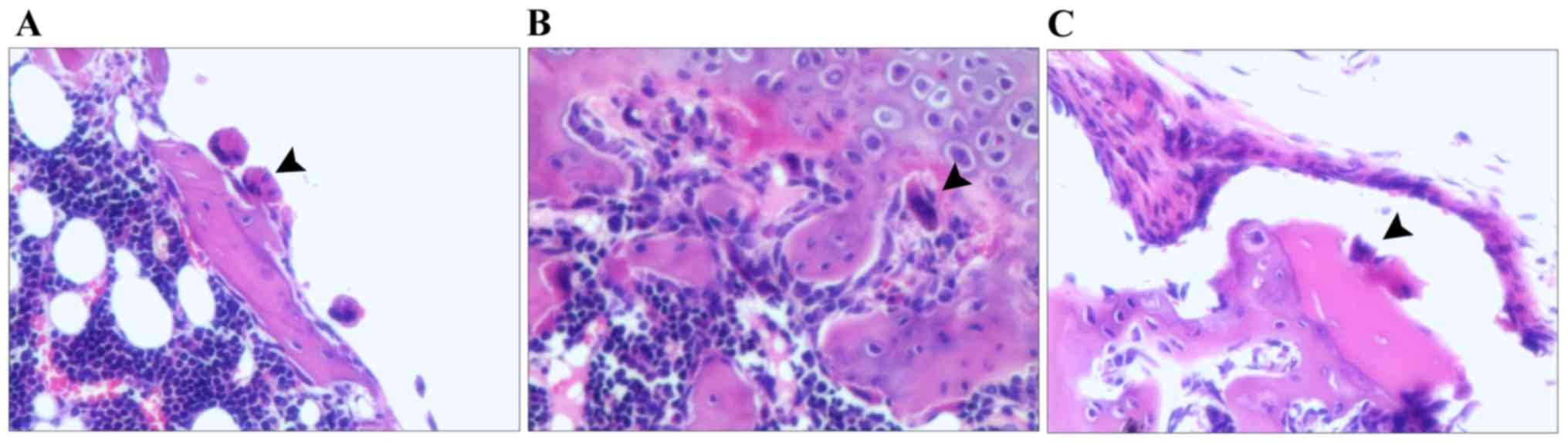
C). Fig. 4A-C displays the
osteoclasts in the model group, rhBMP-2 group and Psoralen +
rhBMP-2 group, respectively, which were located in close
association with the bone surface and appeared to be actively
absorbing bone. In the Psoralen + rhBMP-2 group, osteoclasts were
comparatively more sparse, but osteoclasts had a similar shape in
all groups.
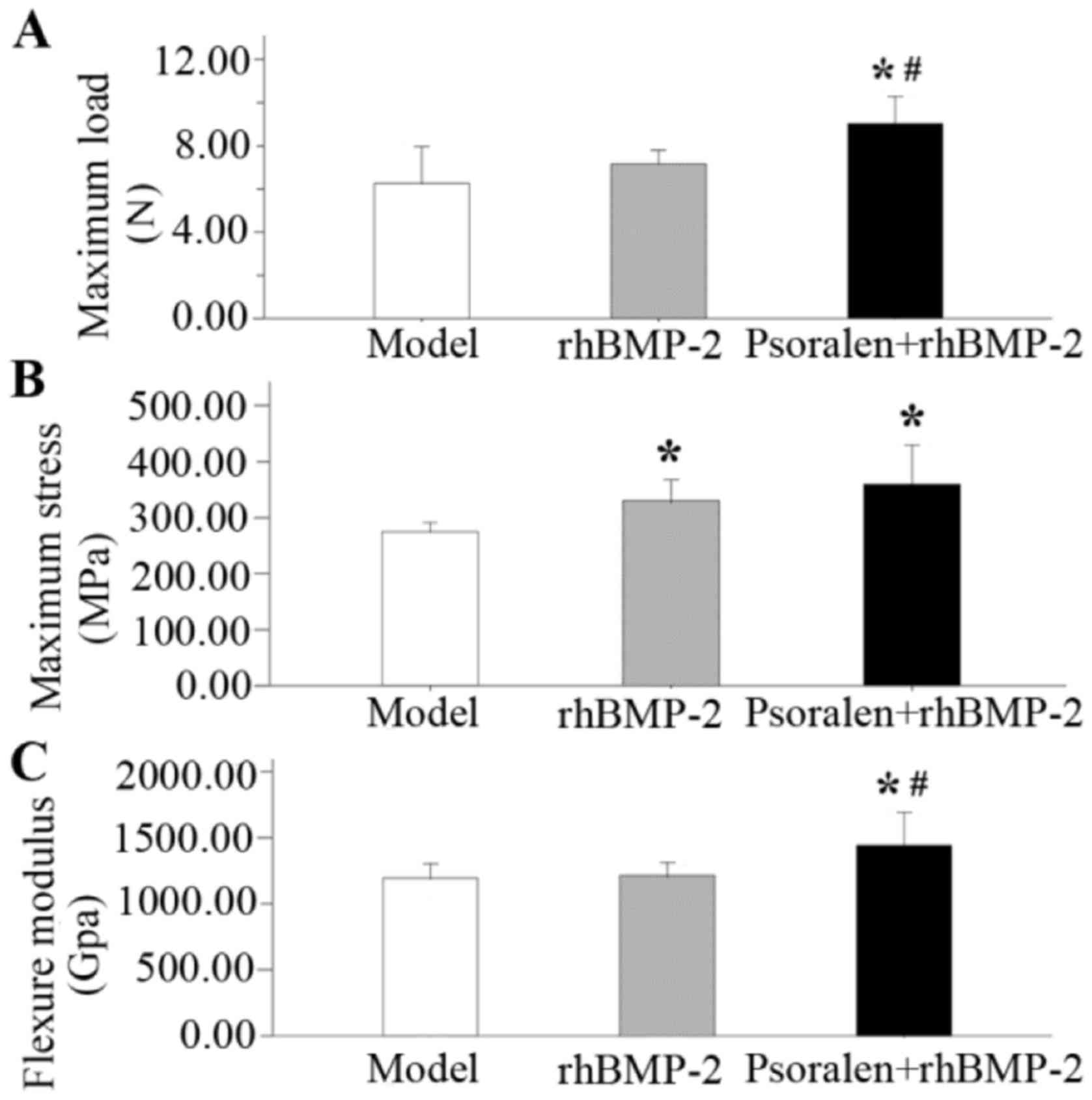
Mechanical testing
At 21 days after fracture, the data from the
different treatment groups were compared with those of the model
group. Averaged data regarding the biomechanical properties of the
fractured femurs in each group are presented in Fig. 5. The highest values for maximum load,
maximum stress and the flexure modulus were seen in the Psoralen +
rhBMP-2 group. Compared with the model group, the psoralen +
rhBMP-2 group had a significantly higher maximum load (P=0.001;
Fig. 5A), maximum stress (P=0.004;
Fig. 5B) and the flexure modulus
(P=0.014; Fig. 5C), while the
rhBMP-2 group only exhibited improved in maximum stress (P=0.036).
The maximum load (P=0.014) and flexure modulus (P=0.021) in the
Psoralen + rhBMP-2 group were significantly higher compared with
those in the rhBMP-2 group.
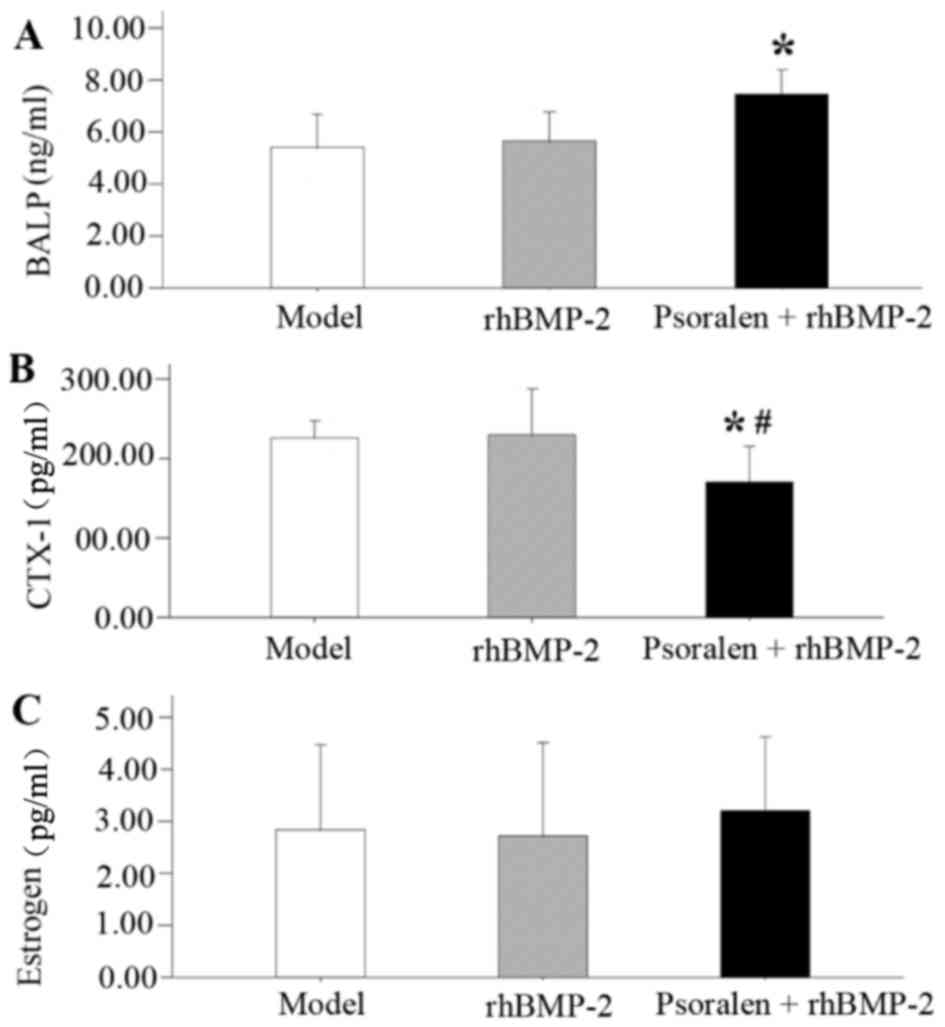
Serum analysis
On day 21, the serum levels of BALP and CTX-1 were
measured to provide an evaluation of bone formation and resorption
activity after fracture under psoralen and/or rhBMP-2 treatment
(Fig. 6). BALP, a bone formation
marker, was significantly increased in the Psoralen + rhBMP-2 group
compared with that in the model group (P=0.004; Fig. 6A). BALP was also slightly increased
in the rhBMP-2 group, but the difference was not statistically
significant compared with the model group (P=0.667). Compared with
that in the model group and the rhBMP-2 group, the serum levels of
CTX-1, a bone resorption marker, were significantly decreased in
the Psoralen + rhBMP-2 group (P=0.030 and 0.021, respectively;
Fig. 6B). Regarding estrogen levels,
no differences were detected among the groups (Fig. 6C).
Discussion
The present study demonstrated that in OVX mice,
combined treatment with psoralen + rhBMP-2 had more potent effects
on fracture healing than rhBMP-2 alone, as demonstrated by
radiological, histological and mechanical analyses, with the
highest values in micro-CT and mechanical parameters. These results
indicate that orally administered psoralen and locally supplied
rhBMP-2 had an additive effect on fracture healing in osteoporotic
mice.
In the present study, at 6 weeks after the bilateral
OVX, significant decreases in estrogen levels and uterine size were
identified. Histological analysis of the distal femurs indicated
that the trabecular bone almost disappeared near the growth plate
in the OVX model, making it feasible to examine fracture healing in
osteoporotic mice. Fracture healing is a multi-stage repair process
that involves complex yet well-orchestrated steps that are
initiated in response to injury, eventually resulting in the repair
and restoration of function. Although the exact mechanism of
impaired bone healing in post-menopausal osteoporosis remains to be
fully elucidated, clinical studies and animal experiments have
consistently indicated that fracture healing is impaired and
delayed in osteoporotic bone (27,28). As
the aging population is expected to double by 2050 and the
occurrence of osteoporotic fractures is to rise in the near future,
impairment in osteoporotic fracture healing is becoming an emerging
public health concern.
In contrast to conventional anti-resorptive drugs,
bone-anabolic drugs build up new bone, which results in a faster
increase of bone mass and strength. There is a great requirement
for additional and affordable anabolic treatments to remedy
impaired fracture healing. The evidence supporting the close
association of BMPs with bone metabolism and the identification of
the role of BMPs in the process of fracture healing and the
pathogenesis of osteoporosis makes them attractive target molecules
for the development of anabolic therapies for the prevention as
well as for the treatment of osteoporotic fractures (29,30).
Consistent with other studies, which have reported enhanced
fracture repair and stimulated early new bone formation with
rhBMP-2 treatment in ovariectomized rats (7,8), the
present study indicated that rhBMP-2 has a positive effect on
fracture healing in estrogen-deficient mice.
Certain medications are approved for the treatment
of osteoporosis, including bisphosphonates, denosumab and estrogen
replacement therapy; however, their effects on fracture healing in
osteoporosis are limited and controversial. For instance,
bisphosphonates, a major type of anti-osteoporosis medication used
in the clinic, have negative effects of excessive suppression of
physiological bone turnover, which results in inhibition of the
bone remodeling process and impair of fracture healing (31). Certain studies even suggest that
suspension of bisphosphonate use should be considered during the
fracture healing period (32,33). As
an alternative treatment for osteoporosis and numerous other
diseases, the therapeutic effects of natural products derived from
plants have become an attractive research topic (34,35).
Previous study has indicated that psoralen acts via
activating BMP signaling to promote osteoblast differentiation
(36). In the present study,
psoralen enhanced anabolic bone formation caused by rhBMP-2. The
micro CT imaging and histological analysis demonstrated that the
group treated with a combination of oral psoralen and local rhBMP-2
produced more mature mineralized callus than local therapy with
rhBMP-2 alone in OVX mice. Furthermore, the biomechanical
evaluation supported the results of the radiological and
histological analyses. The highest biomechanical stability was
measured in the group treated with psoralen + rhBMP-2. One reason
for this may be that the systemic administration of psoralen and
the local application of rhBMP-2 increased the local BMP levels to
thereby have an additive effect on fracture healing in OVX
mice.
In the present study, systemic administration of
psoralen in the Psoralen + rhBMP-2 group exerted a dual regulatory
effect to mediate osteoblast-osteoclast interactions. The serum
analysis revealed that co-administration of psoralen not only
increased the BALP levels, but also decreased the OVX-induced CTX-1
levels in the Psoralen + rhBMP-2 group. The histological analysis
also revealed a decreased amount of osteoclasts in the Psoralen +
rhBMP-2 group. It has been reported that BMPs indirectly stimulate
osteoclastogenesis through osteoblasts via the receptor activator
of nuclear factor κ-Β (RANK)/RANK ligand (RANKL) pathway, which may
lead to the premature resorption of BMP-induced bone (37,38). A
preclinical trial reported that BMP-2 caused initial resorption
when placed in the metaphysis in a non-human primate core defect
model in which bisphosphonates were successfully used to prevent
the unwanted catabolic phase induced by BMP-2 (39). Zhang et al (40) have demonstrated that psoralen
markedly inhibits the differentiation of osteoclasts by enhancing
the expression of estrogen receptor and suppressing that of IL-17R.
In another in vivo study, psoralen was reported to prevent
osteoporosis via increasing osteoporogeterin expression and
reducing RANKL levels to suppress osteoclast differentiation and
maturity (41). This may provide an
explanation for the results obtained in the present study,
indicating that daily systemic administration of psoralen may
reduce bone resorption produced by increased activity of
osteoclasts caused by rhBMP-2.
No matter how complex the mechanisms of action may
be, the phytoestrogens in psoralen may have an important role in
the amelioration of post-menopausal bone loss. To determine whether
psoralen behaved in a similar manner to estradiol in OVX mice, the
estrogen levels in the serum were determined. The results indicated
that treatment of OVX mice with psoralen did not significantly
improve the estrogen levels compared with those in the model group,
therefore further studies are needed to investigate the effect of
psoralen on the uterus.
Of note, the present study had several limitations.
First, the animals were euthanized on day 21, and therefore it was
not possible to determine the long-term influence of the treatments
on bone remodeling. Second, given the saturation of psoralene in
water, some of the suspended powder may have stuck to the syringe.
The effective dose of gavage may be lower than that expected in the
experimental design. Furthermore, only one dose of psoralen was
assessed in the present study, and the observation of the healing
in the psoralen group may have been better if a range of psoralen
doses had been tested. In addition, further studies are required to
confirm whether there is indeed a difference in the local BMPs
levels among the groups. Finally, the specific molecular mechanisms
of the synergistic effects of psoralen and rhBMP-2 in promoting
osteogenesis are elusive and require further study.
In conclusion, psoralen + rhBMP-2 in combination
produced better fracture healing than rhBMP-2 in mice with
OVX-induced osteoporosis. While rhBMP-2 is a costly substance,
psoralen, as an active component from natural herbs, is
inexpensive, has been approved worldwide, is well-tolerated and has
few side effects. Combining a local osteo-inductive agent with a
systemic anabolic agent may be a novel paradigm in the treatment of
osteoporotic fracture.
Acknowledgements
We acknowledge the support received from Professor
Kebin Liu (Department of Orthopaedics, The First Affiliated
Hospital of Yangtze University, Jingzhou, China) for proofreading
the manuscript.
Funding
The present study was financially supported by the
Natural Science Foundation of Hubei Province (grant no.
2012FFB03601) and the Science and Technology Development Plan
Project of Jingzhou City (grant no. 2017041).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KH contributed to the conception of the study and
wrote the manuscript. JZ contributed significantly to the
statistical analyses and the writing of the manuscript. GW and KH
performed the experiments and data analyses. SP helped to design
the experiments, interpret the data and perform the analysis with
constructive discussions. The final version of the manuscript has
been read and approved by all authors, and each author believes
that the manuscript represents honest work.
Ethics approval and consent to
participate
The Animal experimentation ethics committee of
Yangtze University (Jingzhou, China) approved the experimental
animal protocol of the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Broderick JM, Bruce-Brand R, Stanley E and
Mulhall KJ: Osteoporotic hip fractures: The burden of fixation
failure. ScientificWorldJournal. 2013:5151972013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singer A, Exuzides A, Spangler L, O'Malley
C, Colby C, Johnston K, Agodoa I, Baker J and Kagan R: Burden of
illness for osteoporotic fractures compared with other serious
diseases among postmenopausal women in the United States. Mayo Clin
Proc. 90:53–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCann RM, Colleary G, Geddis C, Clarke
SA, Jordan GR, Dickson GR and Marsh D: Effect of osteoporosis on
bone mineral density and fracture repair in a rat femoral fracture
model. J Orthop Res. 26:384–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldhahn J, Féron JM, Kanis J, Papapoulos
S, Reginster JY, Rizzoli R, Dere W, Mitlak B, Tsouderos Y and
Boonen S: Implications for fracture healing of current and new
osteoporosis treatments: An ESCEO consensus paper. Calcif Tissue
Int. 90:343–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watson JT and Nicolaou DA: Orthobiologics
in the augmentation of osteoporotic fractures. Curr Osteoporos Rep.
13:22–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
U.S. Food and Drug Administration, .
InFuse TM Bone Graft/LT-CAGETM lumbar tapered fusion
device-P000058. 2002.https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P000058January
23–2014
|
|
7
|
Sarban S, Senkoylu A, Isikan UE, Korkusuz
P and Korkusuz F: Can rhBMP-2 containing collagen sponges enhance
bone repair in ovariectomized rats? A preliminary study. Clin
Orthop Relat Res. 467:3113–3120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park SB, Park SH, Kim NH and Chung CK:
BMP-2 induced early bone formation in spine fusion using rat
ovariectomy osteoporosis model. Spine J. 13:1273–1280. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zarrinkalam MR, Schultz CG, Ardern DW,
Vernon-Roberts B and Moore RJ: Recombinant human bone morphogenetic
protein-type 2 (rhBMP-2) enhances local bone formation in the
lumbar spine of osteoporotic sheep. J Orthop Res. 31:1390–1397.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boden SD, Kang J, Sandhu H and Heller JG:
Use of recombinant human bone morphogenetic protein-2 to achieve
posterolateral lumbar spine fusion in humans: A prospective,
randomized clinical pilot trial: 2002 volvo award in clinical
studies. Spine (Phila Pa 1976). 27:2662–2673. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hurlbert RJ, Alexander D, Bailey S, Mahood
J, Abraham E, McBroom R, Jodoin A and Fisher C: Rhbmp-2 for
posterolateral instrumented lumbar fusion: A multicenter
prospective randomized controlled trial. Spine (Phila Pa 1976).
38:2139–2148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bess S, Line BG, Lafage V, Schwab F,
Shaffrey CI, Hart RA, Boachie-Adjei O, Akbarnia BA, Ames CP, Burton
DC, et al: Does recombinant human bone morphogenetic protein-2 use
in adult spinal deformity increase complications and are
complications associated with location of rhBMP-2 use? A
prospective, multicenter study of 279 consecutive patients. Spine
(Phila Pa 1976). 39:233–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simmonds MC, Brown JV, Heirs MK, Higgins
JP, Mannion RJ, Rodgers MA and Stewart LA: Safety and effectiveness
of recombinant human bone morphogenetic protein-2 for spinal
fusion: A meta-analysis of individual-participant data. Ann Intern
Med. 158:877–889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faundez A, Tournier C, Garcia M, Aunoble S
and Le Huec JC: Bone morphogenetic protein use in spine
surgery-complications and outcomes: A systematic review. Int
Orthop. 40:1309–1319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kyllönen L, D'Este M, Alini M and Eglin D:
Local drug delivery for enhancing fracture healing in osteoporotic
bone. Acta Biomater. 11:412–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu NY, Gdalevitch M, Murphy CM, Mikulec K,
Peacock L, Fitzpatrick J, Cantrill LC, Ruys AJ, Cooper-White JJ,
Little DG and Schindeler A: Spatial control of bone formation using
a porous polymer scaffold co-delivering anabolic rhBMP-2 and
anti-resorptive agents. Eur Cell Mater. 27:98–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Larsson S and Fazzalari NL:
Anti-osteoporosis therapy and fracture healing. Arch Orthop Trauma
Surg. 134:291–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hegde V, Jo JE, Andreopoulou P and Lane
JM: Effect of osteoporosis medications on fracture healing.
Osteoporos Int. 27:861–871. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Huang JH, Liu SF, Zhao YJ, Shen
ZY, Wang YJ and Bian Q: The osteoprotective effect of psoralen in
ovariectomy-induced osteoporotic rats via stimulating the
osteoblastic differentiation from bone mesenchymal stem cells.
Menopause. 19:1156–1164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan X, Bi Y, Yan Z, Pu Q, Li Y and Zhou
K: Psoralen and isopsoralen ameliorate sex hormone
deficiency-induced osteoporosis in female and male mice. Biomed Res
Int. 2016:68694522016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manigrasso MB and O'Connor JP: Comparison
of fracture healing among different inbred mouse strains. Calcif
Tissue Int. 82:465–474. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murnaghan M, Mcllmurray L, Mushipe MT and
Li G: Time for treating bone fracture using rhBMP-2: A randomised
placebo controlled mouse fracture trial. J Orthop Res. 23:625–631.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toupadakis CA, Granick JL, Sagy M, Wong A,
Ghassemi E, Chung DJ, Borjesson DL and Yellowley CE: mobilization
of endogenous stem cell populations enhances fracture healing in a
murine femoral fracture model. Cytotherapy. 15:1136–1147. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bouxsein ML, Boyd SK, Christiansen BA,
Guldberg RE, Jepsen KJ and Müller R: Guidelines for assessment of
bone microstructure in rodents using micro-computed tomography. J
Bone Miner Res. 25:1468–1486. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Toğral G, Arikan M, Korkusuz P, Hesar RH
and Ekşioğlu MF: Positive effect of tadalafil, a
phosphodiesterase-5 inhibitor, on fracture healing in rat femur.
Eklem Hastalik Cerrahisi. 26:137–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manigrasso MB and O'Connor JP:
Characterization of a closed femur fracture model in mice. J Orthop
Trauma. 18:687–695. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheung WH, Miclau T, Chow SK, Yang FF and
Alt V: Fracture healing in osteoporotic bone. Injury. 47
Suppl:S21–S26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tarantino U, Cerocchi I, Scialdoni A,
Saturnino L, Feola M, Celi M, Liuni FM, Iolascon G and Gasbarra E:
Bone healing and osteoporosis. Aging Clin Exp Res. 23:62–64.
2011.PubMed/NCBI
|
|
29
|
Kanakaris NK, Petsatodis G, Tagil M and
Giannoudis PV: Is there a role for bone morphogenetic proteins in
osteoporotic fractures? Injury. 40 Suppl:S21–S26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Lieshout EM and Alt V: Bone graft
substitutes and bone morphogenetic proteins for osteoporotic
fractures: What is the evidence? Injury. 47 Suppl:S43–S46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Girgis CM, Sher D and Seibel MJ: Atypical
femoral fractures and bisphosphonate use. N Engl J Med.
362:1848–1849. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Savaridas T, Wallace RJ, Salter DM and
Simpson AH: Do bisphosphonates inhibit direct fracture healing?: A
laboratory investigation using an animal model. Bone Joint J.
95:1263–1268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ha KY, Park KS, Kim SI and Kim YH: Does
bisphosphonate-based anti-osteoporosis medication affect
osteoporotic spinal fracture healing? Osteoporos Int. 27:483–488.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leung PC and Siu WS: Herbal treatment for
osteoporosis: A current review. J Tradit Complement Med. 3:82–87.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang DZ, Yang F, Yang Z, Huang J, Shi Q,
Chen D and Wang YJ: Psoralen stimulates osteoblast differentiation
through activation of bmp signaling. Biochem Biophys Res Commun.
405:256–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kanatani M, Sugimoto T, Kaji H, Kobayashi
T, Nishiyama K, Fukase M, Kumegawa M and Chihara K: Stimulatory
effect of bone morphogenetic protein-2 on osteoclast-like cell
formation and bone-resorbing activity. J Bone Miner Res.
10:1681–1690. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okamoto M, Murai J, Yoshikawa H and
Tsumaki N: Bone morphogenetic proteins in bone stimulate
osteoclasts and osteoblasts during bone development. J Bone Miner
Res. 21:1022–1033. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Seeherman HJ, Li XJ, Gavin D, Wozney JM
and Bouxsein ML: Bisphosphonate limits initial bone resorption
without decreasing bone induction in rhBMP-2/ACS treated nonhuman
primate core defects. Bone. 30 Suppl:44S. 2002.
|
|
40
|
Zhang WJ, Xie BP, Li WJ, Li JP, Gan GX and
Zhang J: Inhibitory effect of psoralen on osteoclast formation and
its underling mechanism in vitro. J Third Milit Med Univ.
39:641–645. 2017.(In Chinese).
|
|
41
|
Wang JH, Guo M, Zheng L and Wang Z:
Effects of psoralen on osteoporogeterin and receptor activator
nuclear factor kappa B ligand mRNA expression in rat osteoblasts.
Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 14:6927–6930.
2010.(In Chinese).
|