Introduction
Obstructive jaundice (OJ) is a common clinical
symptom in cholangiocarcinoma and pancreatic cancer, which usually
impairs liver function and reduces the regenerative capacity of
liver (1). Pathological changes
resulting from OJ, including cholangitis, cholestatic liver injury
and digestive disorders, may exhibit adverse effects on surgical
outcomes (2). However, major
hepatectomy is essential and inevitable in a number of patients,
particularly in those with hilar cholangiocarcinoma and OJ. The
degree of ischemia-reperfusion (I/R) injury and the function of the
remnant liver have been key focuses during hepatectomy and are
associated with post-operative morbidity and mortality (3). I/R injury induces formation of reactive
oxygen species that cause oxidative stress and cell death,
ultimately leading to a sterile inflammatory response that causes
hepatocellular damage and liver dysfunction that can result in
acute liver failure in most severe cases (4). The current treatment strategy is to
perform biliary drainage prior to surgery and to minimize the scale
of the surgical procedure (1).
However, the outcome is not favorable. Systematic review and
meta-analysis have revealed that preoperative biliary drainage was
associated with increased postoperative morbidity and wound
infection (5). As previously
established, using a suitable method for the occlusion of hepatic
blood inflow has a marked effect in reducing blood loss and
alleviating liver damage following liver resection (4). The current study hypothesized that the
method of occlusion of hepatic blood inflow affects the outcomes of
hepatectomy in patients with OJ.
The Pringle maneuver, occlusion of the portal triad
(OPT), has been acknowledged for its ability in reducing blood loss
during liver resection, and severe I/R injury is inevitable if the
operation is complicated and prolonged occlusion is required
(6,7). In addition, it has been demonstrated
that the icteric liver exhibits reduced tolerance for I/R injury
(8,9). However, the majority of
cholangiocarcinoma cases coexist with OJ (10). At present, numerous occlusion
strategies, including selective hemihepatic vascular occlusion,
step-by-step vascular control and ischemic preconditioning have
been designed to control the liver portal blood and reduce I/R
injury (11–13). A method for occlusion of the portal
vein (OPV), while preserving hepatic artery blood flow during liver
surgery was initially proposed and was demonstrated to be effective
in a rat model of normothermic liver I/R (14). Although partial hepatectomy is a good
model for liver regeneration, the liver injury induced by
predisposed OJ and operation-related I/R may lead to suppressed
liver regeneration (7–9). Proliferating cell nuclear antigen
(PCNA) and Ki-67 labeling indexes are two nuclear antigens
associated with hepatocyte proliferation, which are often used to
characterize the hepatic regenerative response (15). The current study was designed to
evaluate the protective degree of OPV on the liver regeneration of
OJ rats following partial hepatectomy.
Materials and methods
Animals and experimental model of
OJ
A total of 216 male Sprague-Dawley rats (age, 6–8
weeks; weight, 200–220 g), were obtained from the Experimental
Animal Center of the Academy of Military Medical Science (Beijing,
China). Animals were fed standard chow with water ad libitum and
kept at 24°C and 40% humidity with a 12 h light/dark cycle. OJ was
induced by ligation of the common bile duct (CBD). To ensure the
accuracy of the procedure, an operating microscope for small animal
surgery was used (GX.SS.22-3, Binocular Operation Microscope;
Shanghai Medical Optical Instruments Factory Co., Ltd, Shanghai,
China). The current study was conducted in accordance with the
guidelines set by the Guide for the Care and Use of Laboratory
Animals (16) and was approved by
the Ethical and Research Committee of the Chinese PLA Medical
School (Beijing, China).
Prior to surgery, rats were fasted overnight with
free access to water. Anesthesia was performed by isoflurane/oxygen
inhalation (4% isoflurane for induction, 1.5% for maintenance of
anesthesia). Hair on the abdomen of the rats was removed and an
upper midline abdominal incision of ~3 cm was made following
sterilization. The CBD was exposed and two 8-0 monofilament,
non-absorbable nylon sutures (Shanghai Pudong Jinhuan Medical
Products Co., Ltd, Shanghai, China) were placed under the bile duct
between branches and anastomoses of CBD and pancreas. The sutures
were ligated and the CBD between the two sutures was dissected. The
abdominal wall was closed with a continuous suture. A second
surgery was performed on rats with OJ following 7 days (17).
Experimental groups
Rats with OJ were randomly assigned into three
groups: C group, sham operation/control group; OPT group, rats with
OPT, including portal vein and hepatic artery; and OPV group, rats
with OPV and preservation of the hepatic artery. The duration of
occlusion was set to 15, 20, 30, 40, 50 and 60 min, and the 7-day
survival rate following the second surgery was investigated in the
OPT and OPV group (n=10 for each time point in each group, total
n=120). The duration of occlusion was set to 30 min for liver
injury and liver regeneration studies, and the sample time points
were at 6, 24, 72 h and 7 days post hepatectomy (n=24 for C group,
n=24 for OPV group and n=36 for OPT group). A total of 12 rats were
used for the measurement of liver blood flow.
Surgical procedures for partial liver
ischemia and hepatectomy in rats with OJ
After a total of 7 days following the surgery
inducing OJ in rats, a second surgery was performed. The procedure
combined biliary recanalization and partial liver ischemia under
portal blood bypass through the caudate lobe (14) and a partial hepatectomy was performed
in all rats. Rats with OJ were anesthetized as described above. The
abdomen was entered through the incision of the previous procedure
and dilated CBDs were isolated. A cannula (8×0.8 mm) was inserted
into the duodenal side of the original bile duct and fixed. The
second tip of the cannula was inserted into the dilated CBD and
fixed.
When the recanalization of the bile duct was
completed, the left and median liver lobes, which were resected in
following steps, were ligated with 3-0 silk sutures. The pedicle,
for the OPT group, or the portal vein, for the OPV group, of the
right liver lobe was dissected and clamped with a microvascular
clamp. In the C group, the pedicle was dissected but not clamped.
The clamping was maintained for 15–60 min. During ischemia, the
abdomen was covered with plastic film to prevent evaporation of
body fluids. Following occlusion, clamps were removed and liver
reperfusion was initiated. The left, median and caudate lobes were
resected and the abdomen was closed with 3-0 sutures. Following
surgery, all rats were provided with rodent chow and water ad
libitum until 6, 24, 72 h and 7 days post hepatectomy.
Sample collection
Serum and liver tissue samples were collected at 6,
24, 72 h and 7 days following partial hepatectomy (n=6 for each
time point of each group). Serum samples from rats with OJ that did
not undergo the second surgery were also collected for comparison
(n=6). All rats were anesthetized as mentioned above and 5 ml blood
was obtained from the inferior vena cava for serum testing. Then ≥2
ml blood was drawn to sacrifice the rats weighing 200–220 g by
exsanguination. The liver were resected and weighted. The blood
samples were centrifuged at 2,500 × g at 4°C for 15 min. The
supernatant was stored at −80°C for serum tests. Tissue samples
that were taken from the remnant liver were cut into two pieces.
One piece was immediately frozen in liquid nitrogen and stored at
−80°C for detection of gene and protein expression, the other piece
was further sliced and fixed at 4°C for 24 h with 10% formaldehyde
in 0.1 M phosphate buffer (pH 7.4) for histopathological study.
Measurement of blood flow of the
hepatic artery and portal vein
A total of 6 normal and 6 rats with OJ were paired
according to body weight. Blood flow of the hepatic artery (HAF)
and portal vein (PVF) was measured using the Transonic T206 Flow
Meter (Transonic Systems Inc., Ithaca, NY, USA) during the second
surgery. Following anesthesia and laparotomy, the common portal
pedicle of the liver was dissected and a suitable probe was placed
around the hepatic artery and portal vein. Blood flow was monitored
for 1 min.
Measurement of microvascular liver
blood flow (LBF) using laser speckle contrast imaging
Microvascular LBF in rats with OJ was assessed using
laser speckle contrast imaging (moorFLPI-2 Full-Field Laser
Perfusion Imager; Moor Instruments, Axminster, UK) during the
second operation. Following PVF and HAF measurements, LBF of the
whole liver was measured as previously described (18). Following ligation of the left and
median liver lobes and clamping of the pedicle for the OPT group,
or the portal vein for the OPV group, of the right liver lobe, the
LBF of the right liver lobe was measured 5 min post-clamping. The
sampling frequency was 21 images/sec and the image acquisition rate
was 1 frame/sec in the normal resolution mode. The duration of
recording was 15 sec. MoorFLPI-2 Review (version 4.0; Moor
Instruments) was used to analyze the images and quantify the
perfusion in arbitrary laser speckle perfusion units (LSPU).
Liver function test
Serum levels of alanine transaminase (ALT) and
direct bilirubin (DBIL) were used as general markers of liver
injury, which was measured with a serum analyzer (Cobas-Mira Plus;
Roche Diagnostics GmbH, Mannheim, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from frozen liver tissues
using the RNA Simple Total RNA kit (Tiangen Biotech Co., Ltd.,
Beijing, China) according to the manufacturer's protocol. RNA (4
µg) was reverse transcribed using the RevertAid First Strand cDNA
Synthesis kit and Oligo-dT primers (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). qPCR was performed with the SYBR Premix Ex Taq
II (Takara Bio, Inc., Otsu, Japan) and the StepOnePlus Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Primers used were as follows: Tumor necrosis factor (TNF)-α
forward, 5′-AAATGGGCTCCCTCTCATCAGTTC-3′ and reverse,
5′-TCTGCTTGGTGGTTTGCTACGAC-3′; interleukin (IL)-1β forward,
5′-CACCTCTCAAGCAGAGCACAG-3′ and reverse,
5′-GGGTTCCATGGTGAAGTCAAC-3′; hypoxanthine phosphoribosyltransferase
(HPRT) forward, 5′-GCTGAAGATTTGGAAAAGGTG-3′ and reverse,
5′-AATCCAGCAGGTCAGCAAAG-3′. The relative gene expression was
normalized to HPRT. Values were calculated using the
2−ΔΔCq method as previously described and
expressed as fold change vs. normal rat (19).
Histological assessment and Ki-67
immunohistochemistry
Formalin-fixed tissues were embedded in paraffin and
5-µm sections were cut. Sections were stained for 3 min with
hematoxylin and 2 min with eosin at room temperature for
histological examination using a light microscope (magnification,
×400). For immunohistochemistry, sections were incubated with a
mouse anti-Ki-67 monoclonal antibody (1:50; cat. no. 550609; BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocol. Samples were counterstained with
hematoxylin for 2 min at room temperature. The percentage of
Ki-67-positive hepatocytes was determined in ten random visual
fields using a light microscope (Leica Microsystems GmbH, Wetzlar,
Germany) at magnification, ×400 and the Ki-67 labeling index. All
histological analyses were performed in a blinded manner with
respect to the experimental groups.
Evaluation of liver regeneration
following hepatectomy
Liver regeneration was evaluated using the rate of
regeneration following hepatectomy of rats with OJ. Rate of
regeneration=actual remnant liver weight at test point/W ×100%,
where W represents the weight of the whole liver prior to
hepatectomy. W was calculated as weight of the resected liver/76%
(the weight of the resected liver lobes account for 76% of the
whole liver) (20).
Western blot analysis
Frozen liver samples were homogenized in
radioimmunoprecipitation assay buffer (Cell Signaling Technology,
Inc., Danvers, MA, USA) supplemented with 1 mM phenyl methane
sulfonyl fluoride and 1× phosphatase inhibitor. Homogenates were
centrifuged at 10,000 × g for 10 min at 4°C. Supernatants were
collected and protein concentrations were determined using the
bicinchoninic acid method. Samples containing 50 µg of protein were
separated on 10% SDS-PAGE gels and transferred to polyvinylidene
difluoride membranes. Membranes were blocked with 5% skimmed milk
for 1 h at room temperature and incubated overnight at 4°C with
primary antibodies: A mouse monoclonal antibody of
anti-proliferating cell nuclear antigen (PCNA; 1:200; cat. no.
sc-56; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit
anti-caspase-3 (Asp175) (5A1E) monoclonal antibody (cat. no. 9664)
and rabbit anti-caspase-9 (Asp353) polyclonal antibody (cat. no.
9507) (both 1:1,000; Cell Signaling Technology, Inc.). Following,
membranes were incubated with horseradish peroxidase-conjugated
goat anti-mouse (sc-2005) or goat anti-rabbit (sc-2004) secondary
antibodies (1:2,000; Santa Cruz Biotechnology, Inc.) for 1 h at
room temperature. Blots were developed with Super-Signal
chemiluminescent substrate (cat. no. 34580; Thermo Fisher
Scientific, Inc.). Blots were analyzed against mouse anti-GAPDH
monoclonal antibodies (1:2,000; cat. no. KM9002T; Sungene Biotech
Co., Ltd., Tianjin, China) expression, which was used as loading
control.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Student's t-test was used for the comparison of two groups and
one-way ANOVA with Student-Newman-Keuls test was used for the
comparison of three groups. SPSS (version 17.0; SPSS, Inc.,
Chicago, IL, USA) was used for analyses. P<0.05 was considered
to indicate a statistically significant difference.
Results
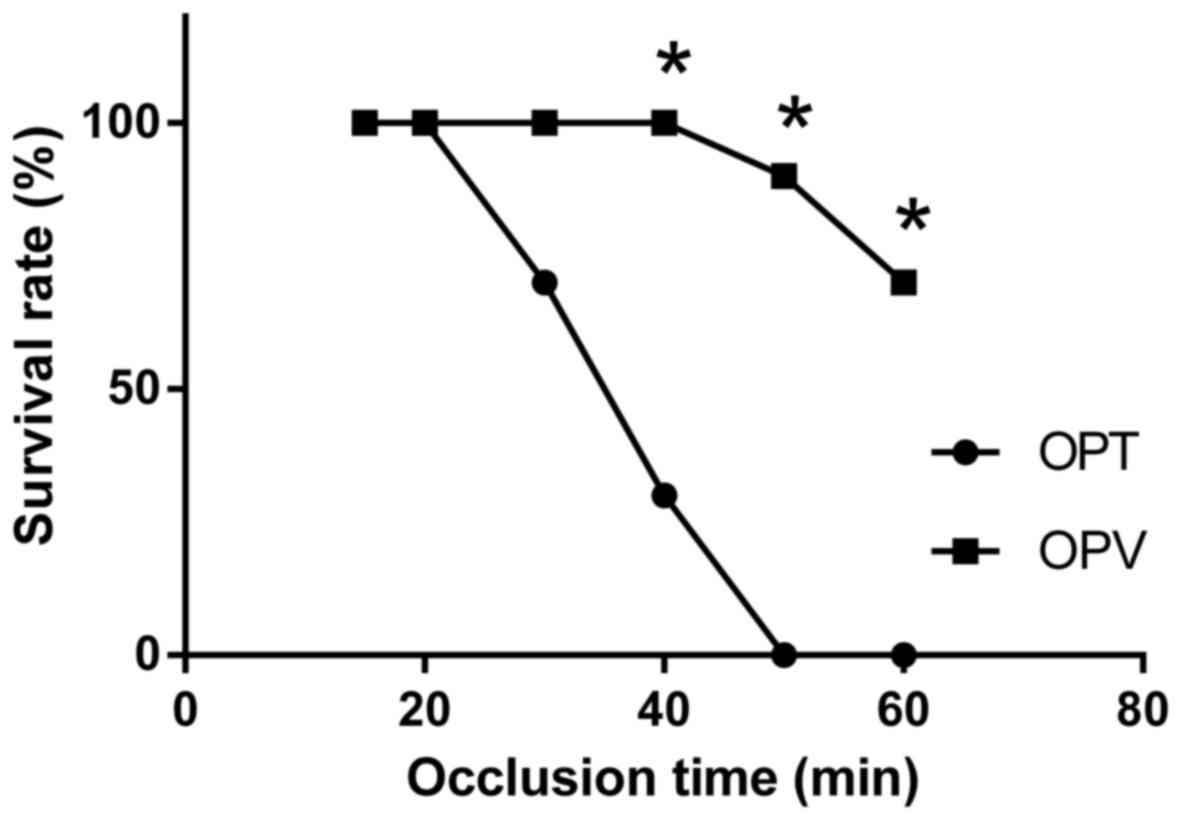
Preservation of hepatic artery flow
during liver blood inflow control extends the duration of liver
ischemia in rats with OJ
All rats with OJ survived until the second surgical
procedure. The dilated CBDs with a diameter of 8–10 mm were visible
during the second laparotomy. To assess the tolerance of rats with
OJ to liver ischemia and hepatectomy, the safe limit for the
duration of liver ischemia in rats with OJ that underwent OPT and
OPV was investigated. In the OPT group, the 7-day survival rate
decreased when the occlusion time was >20 min (Fig. 1). The safe limit for the duration of
liver ischemia for the OPV group was extended to 40 min without
affecting the survival rate. At an occlusion time of 50 min, the
7-day survival rate decreased to 90% (9/10) in the OPV group and 0%
(0/10) in the OPT group. The 7-day survival rate of the OPV group
was significantly increased compared with the OPT group at 40, 50
and 60 min of occlusion, respectively (P<0.05; Fig. 1). Determined safe limits for the
duration of liver ischemia were 20 and 40 min for the OPT and OPV
group, respectively. OPV, with preserved HAF in rats with OJ and
partial hepatectomy, markedly prolonged the duration of liver
ischemia during hepatectomy.
Hepatic hemodynamic changes in rats
with OJ and liver ischemia
Liver PVF and HAF in rats with OJ were measured with
an ultrasonic flow meter and compared with those of normal rats.
PVF decreased significantly from 13.83±1.60 to 8.17±0.75 ml/min in
rats with OJ compared to the control (P<0.05) and HAF increased
significantly from 1.10±0.14 to 1.98±0.38 ml/min in rats with OJ
compared with the control (P<0.05; Fig. 2A). As HAF was low compared with PVF,
the total liver blood inflow was decreased 7 days following
induction of OJ.
During the portal blood occlusion prior to
hepatectomy, LBF of the right liver lobe was measured with a laser
speckle contrast imager. LBF of the control group was 529.53±91.55
LSPU (Fig. 2B). Following portal
occlusion, LBF significantly decreased to 136.89±32.32 LSPU for the
OPT and 183.99±49.25 LSPU for the OPV group (P<0.05). OPT and
OPV significantly reduced the perfusion of liver microcirculation,
with OPT being more effective compared with OPV (P<0.05).
Effects of varying hepatic blood
inflow occlusions on hepatic biochemical markers and expression of
proinflammatory cytokine genes
ALT and DBIL levels of rats with OJ prior to the
second surgery were 112.6±16.78 IU/l and 158.2±11.38 µmol/l,
respectively. Following biliary drainage, portal occlusion and
hepatectomy, ALT levels in rats with OJ increased, with the value
for the OPT group significantly higher compared with the OPV and
control groups at 6 and 24 h of reperfusion (P<0.05; Fig. 3A). At 24 h of reperfusion, the ALT
level in the OPV group was significantly higher compared with the
control group (P<0.05). Due to the recovery of biliary drainage,
DBIL levels in all rats decreased following the second operation,
with the value for the OPT group significantly higher compared with
the OPV and control groups at 6 h of reperfusion (P<0.05;
Fig. 3B). At days 3 and 7 following
hepatectomy, ALT and DBIL levels of all rats had decreased and no
significant difference was observed between the groups. Rats in the
OPT group suffered the most severe liver I/R injury among the three
groups and OPV significantly reduced I/R-induced liver injury
compared with the OPT method.
The current study assessed mRNA expression of the
proinflammatory cytokines IL-1β and TNF-α (Fig. 3C and D) in the remnant liver tissue
using RT-qPCR. mRNA expression of IL-1β and TNF-α was significantly
upregulated in the OPT group compared with the control and OPV
groups at 6 and 24 h following I/R and hepatectomy (P<0.05). The
remnant liver in the control and OPV groups expressed similar
levels of IL-1β and TNF-α mRNA at all of the time points. The data
indicated that OPT induced a more severe inflammatory response and
damage compared with OPV.
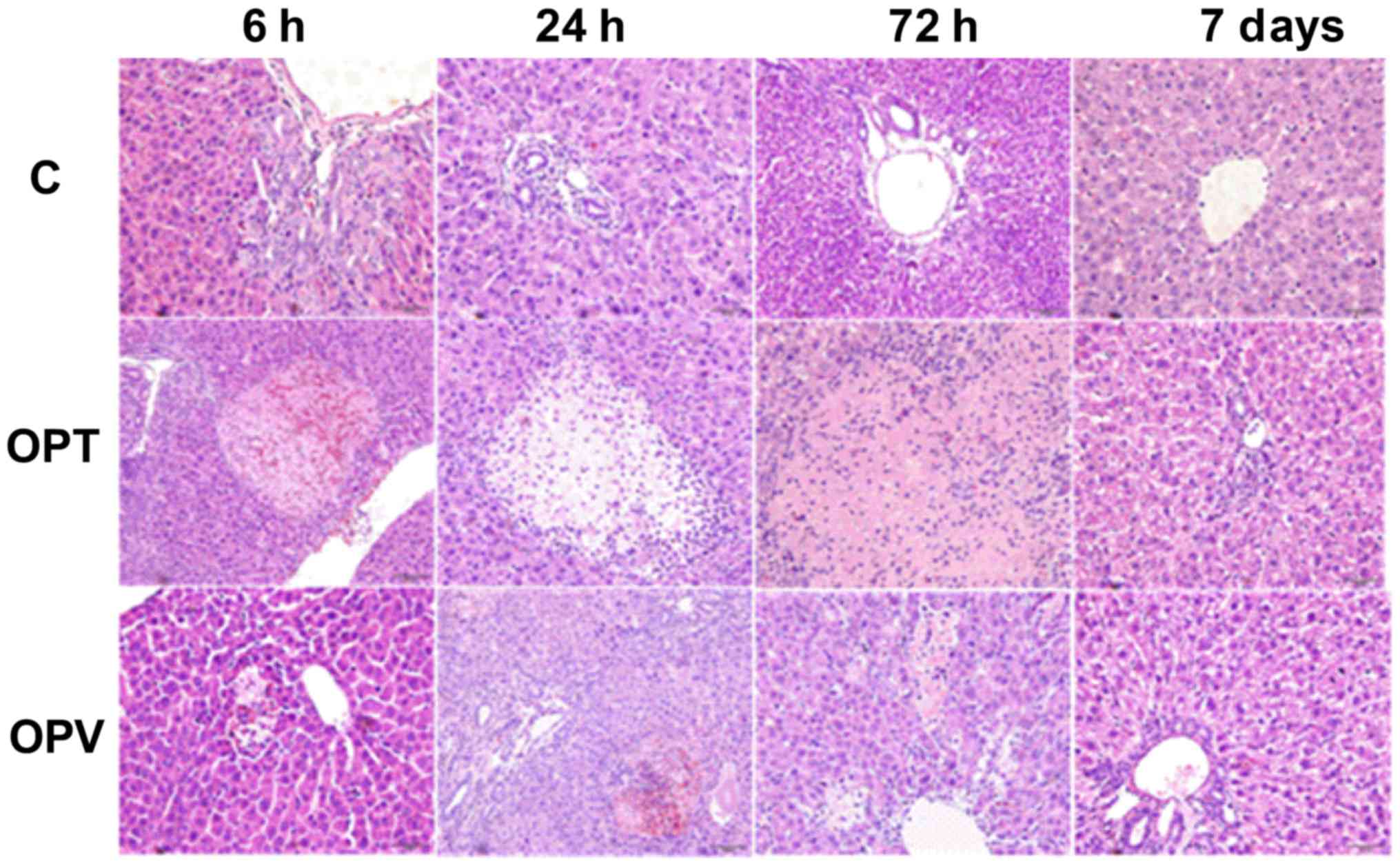
Pathological changes in the liver of
rats with OJ following varying methods of portal occlusion and
partial hepatectomy
Hepatic pathological changes in rats with OJ in the
C, OPT and OPV groups were assessed at 6, 24 and 72 h and 7 days
following different portal occlusion procedures and hepatectomy
(Fig. 4). Proliferative encysted
cholangioles were observed in all groups, with prevalence at 6 and
24 h following the second surgical procedure. In addition, marked
hepatocyte necrosis was detected in the OPT group with many
neutrophils invading into the necrotic area at 6 and 24 h post
hepatectomy. The necrotic area was enlarged at 72 h following the
second surgical procedure. No necrotic area was observed in the C
group and hepatocyte necrosis in the OPV group was minimal when
compared with the OPT group. OPV was effective in preventing
hepatocytic necrosis in rats with OJ, I/R and partial
hepatectomy.
Liver regeneration in rats with OJ
that underwent partial hepatectomy and varying portal occlusion
procedures
Regeneration of the remnant liver following
different intraoperative hepatic blood inflow occlusion procedures
and partial hepatectomy were evaluated on days 3 and 7 following
I/R and hepatectomy. Rates of liver regeneration on day 3
post-hepatectomy of C, OPV and OPT groups were 69.27±4.97,
66.18±4.43 and 59.70±5.03%, respectively (Fig. 5A). The rate of hepatic regeneration
of the OPT group was significantly lower compared with the C and
OPV groups (P<0.05), indicating that total hepatic pedicle
clamping for 30 min prior to hepatectomy inhibited liver
regeneration. Preservation of HAF during hepatic blood inflow
occlusion in the OPV group did not significantly reduce liver
regeneration compared with the control group. On day 7 following
the second surgical procedure, rates of liver regeneration of C,
OPV and OPT reached 96.17±3.84, 94.23±5.44 and 91.33±9.29%,
respectively. No statistical differences were detected among the
groups.
In order to further characterize the liver
regenerative response, expression of Ki-67 is indicated as a brown
nucleus in immunostained tissue sections, a nuclear antigen that is
associated with hepatocyte proliferation, was assessed in remnant
liver tissues. On day 3 post-operation, Ki-67 labeling indices of
the OPT group (26.68±3.27%) were significantly reduced compared
with the C group (38.96±4.14%) and the OPV group (31.94±4.38%)
(P<0.05; Fig. 5A) with the OPV
group also lower than the C group (P<0.05; Fig. 5B and C). On day 7 following
hepatectomy, Ki-67 expression was substantially decreased and no
significant differences were detected among the groups (Fig. 5B and C).
Effects of varying liver blood inflow
occlusion procedures on hepatocyte proliferation and apoptosis
To further assess the liver regenerative capacity of
rats with OJ following hepatectomy with varying liver blood inflow
occlusive methods, PCNA, caspase-3 and 9 expression was determined
by western blotting (Fig. 6). PCNA
is a marker that is associated with hepatocyte proliferation and
caspase-3 and −9 are associated with hepatocyte apoptosis (21,22).
PCNA expression increased at 24 h following I/R and
hepatectomy in all groups compared with the 6 h measurements. PCNA
expression at 24 h in group C was markedly higher compared with OPT
and OPV groups. PCNA expression in the OPV group was significantly
increased compared with the OPT group at 24 and 72 h following
hepatectomy (P<0.05; Fig. 6). The
opposite pattern was detected for caspase-3 and 9 expression, where
expression in the OPT group was higher compared with the OPV group
at 6 and 24 h following I/R and hepatectomy (P<0.05; Fig. 6). The results demonstrated that OPV
was effective in improving hepatocyte proliferation and reducing
hepatocyte apoptosis in rats with OJ following hepatectomy.
Discussion
The Pringle maneuver is frequently used in
hepatectomy due to its effectiveness in reducing blood loss during
operation (4,6,7).
However, I/R injury is inevitable in patients that require massive
hepatectomy or in those with poor liver function, including
cirrhosis and jaundice (5,7). Several strategies, including
intermittent Pringle maneuver, selective hemi-hepatic vascular
occlusion and ischemic preconditioning, have been proposed to
improve the tolerance of the remnant liver to I/R injury (11–13,23).
However, none of these methods has been accepted as an ideal
technique that results in reductions in liver injury and blood
loss. It has previously been reported that OPV and preservation of
HAF can significantly reduce hepatic I/R injury without increasing
the risk of blood loss (14). This
new surgical procedure provides better liver cytoprotection
compared with OPT. In a previous study with CCl4-induced
cirrhotic rats, OPV alone was also demonstrated to be effective in
decreasing liver injury (24).
OJ is frequently reported in hepatobiliary surgery.
The risk of liver failure is greater in individuals with jaundice
compared with those without, due to pathological changes in
jaundice, including cholangitis and cholestatic liver injury
(25,26). Therefore, the current study
investigated effects of the OPV method for controlling liver blood
inflow and reducing liver injury in rats with OJ and
hepatectomy.
As is demonstrated in the present study, total LBF
of rats with OJ was markedly decreased compared with the normal
rats. Although HAF of the OJ rats was increased, total LBF of the
hepatic artery and portal vein decreased due to a decrease in PVF.
This hemodynamic change in the liver of rats with OJ may result
from compressed and damaged terminal parts of the portal vein and
the hepatic sinusoid, which was due to the swelling of the OJ
(26). This may account for the
observation that rats with OJ have a decreased tolerance for
hepatic portal occlusion. Following portal occlusion, LBF decreased
significantly in both OPT and OPV groups. In a previous study it
was demonstrated that the preservation of the hepatic artery when
performing OPV may reduce I/R injury by supplying more oxygen to
the liver and without markedly increasing blood loss compared with
the Pringle maneuver in normal rats (14).
To the best of our knowledge, the present study is
the first to determine the safe limit for the duration of liver
ischemia as 20 min for the OPT and 40 min for the OPV method in
rats with OJ. In addition, prolonging the occlusion time to 50 min
significantly decreased the 7-day survival rate of the OPV group
compared with the OPT group. Following portal occlusion and
hepatectomy, ALT levels in the OPT group significantly increased
and peaked at 6 and 24 h post-operation. ALT levels in the OPV and
control groups were similar, which was consistent with the hepatic
histological changes that were observed.
In order to further investigate the molecular
changes in the liver following hepatectomy with OJ and portal blood
occlusion, TNF-α and IL-1β expression in the remnant liver were
analyzed. It was revealed that TNF-α and IL-1β mRNA expression in
the OPT group was significantly higher compared with the control
and OPV groups. The results demonstrated that preserving HAF may
significantly reduce the inflammatory response compared with the
OPT method, which is generally produced by I/R-activated
inflammatory cells (27). In a
previous study, it was demonstrated that liver tissue
malondialdehyde values were significantly lower and
Na+-K+-ATPase activity was significantly
higher in the OPV group compared with the OPT group (14). An imbalance between the expression of
genes that are involved in vasoconstriction and vasodilation was
also observed in the OPT group, but not in the OPV group (28). Preserving low liver blood perfusion
using the OPV method during liver surgery may be very effective for
preventing hepatic microcirculatory dysfunction and hepatocyte
injury.
When evaluating the advantages and disadvantages of
a method for portal occlusion, the effect on hepatic regeneration
is a vital factor. Hepatobiliary surgeons may select a method for
occlusion that improves the liver regenerative ability. Following
portal blood occlusion and hepatectomy of the same volumes of
tissues, hepatic regeneration of the OPV group was significantly
improved compared with the OPT group. At day 3 post-occlusion and
hepatectomy, rates of liver regeneration and Ki-67 labeling indices
of the OPV group were significantly higher compared with the OPT
group. The PCNA level in the OPV group was higher compared with the
OPT group according to western blot results. As indicated by the
results presented in the current study, OPV improved the time of
liver regeneration in rats with OJ compared with the OPT group.
Combined with findings from laser speckle contrast imaging, low
perfusion of oxygen in the hepatic artery blood and reduced damage
in the OPV group may be key factors for the observed improvements
in liver regeneration.
Overall, preserving HAF during portal triad blood
occlusion may significantly reduce I/R injury and improve liver
regeneration in rats with OJ that underwent partial hepatectomy
compared with the Pringle maneuver, which was performed in the OPT
group. The findings may provide guidance for future clinical
studies. It may decrease the risk of liver failure following major
hepatectomy, which is on occasion inevitable and potentially fatal
when the duration of hepatic inflow occlusion is prolonged and
resection of large volumes of liver tissue is necessary in order to
clear niduses, particularly in patients with jaundice that present
with hilar cholangiocarcinoma (29).
A limitation of the OPV method in clinical application is the need
to isolate the portal vein for occlusion by dissociating the
pedicle of Glisson's capsule. In addition, auxiliary measures,
including intraoperative low central venous pressure and appliance
of ligature, may be used at the same time to reduce the blood loss
in the application of OPV method in OJ patients who need a
hepatectomy.
In conclusion, to the best of our knowledge, this is
the first study on preserving HAF during portal triad blood
clamping in the liver of rats with OJ. The OPV method resulted in
less damage to the liver and it may facilitate improved liver
regeneration. It may increase the current limits on the duration of
portal blood occlusion. The OPV method may describe a novel
technique of choice for controlling hepatic blood inflow in liver
surgery, particularly in patients with complex jaundice cases, due
to its simplicity and as it may be safer compared with the Pringle
maneuver.
Acknowledgements
Not applicable.
Funding
This work was supported by the Project of the
National Natural Science Foundation of China (grant no. 81271738)
and the National Key Technology R&D Program of China (grant no.
2012BAI06B01).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contribution
ZK contributed to the animal experiments, data
collection and analysis. JJH contributed to the interpretation of
the data, writing and editing the article. XLG and KP contributed
to data analysis and histopathology examinations. JHD contributed
to conception and design. CHL contributed to conception, writing,
critical revision and editing of the article.
Ethics approval and consent to
participate
The current study was approved by the Ethical and
Research Committee of the Chinese PLA Medical School (Beijing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brandi G, Venturi M, Pantaleo MA and
Ercolani G: GICO: Cholangiocarcinoma: Current opinion on clinical
practice diagnostic and therapeutic algorithms: A review of the
literature and a long-standing experience of a referral center. Dig
Liver Dis. 48:231–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uppal DS and Wang AY: Advances in
endoscopic retrograde cholangiopancreatography for the treatment of
cholangiocarcinoma. World J Gastrointest Endosc. 7:675–687. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim YI: Ischemia-reperfusion injury of the
human liver during hepatic resection. J Hepatobiliary Pancreat
Surg. 10:195–199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Riel WG, van Golen RF, Reiniers MJ,
Heger M and van Gulik TM: How much ischemia can the liver tolerate
during resection? Hepatobiliary Surg Nutr. 5:58–71. 2016.PubMed/NCBI
|
|
5
|
Celotti A, Solaini L, Montori G, Coccolini
F, Tognali D and Baiocchi G: Preoperative biliary drainage in hilar
cholangiocarcinoma: Systematic review and meta-analysis. Eur J Surg
Oncol. 43:1628–1635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Man K, Fan ST, Ng IO, Lo CM, Liu CL and
Wong J: Prospective evaluation of Pringle maneuver in hepatectomy
for liver tumors by a randomized study. Ann Surg. 226:704–711.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Gulik TM, de Graaf W, Dinant S, Busch
OR and Gouma DJ: Vascular occlusion techniques during liver
resection. Dig Surg. 24:274–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hammond JS, Guha IN, Beckingham IJ and
Lobo DN: Prediction, prevention and management of postresection
liver failure. Br J Surg. 98:1188–1200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hameed A, Pang T, Chiou J, Pleass H, Lam
V, Hollands M, Johnston E, Richardson A and Yuen L: Percutaneous
vs. endoscopic pre-operative biliary drainage in hilar
cholangiocarcinoma-a systematic review and meta-analysis. HPB
(Oxford). 18:400–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blechacz B, Komuta M, Roskams T and Gores
GJ: Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev
Gastroenterol Hepatol. 8:512–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li M, Zhang C, Zhang T, Wang L, Ding Y,
Niu Z, He S and Yang Z: Outcome using selective hemihepatic
vascular occlusion and Pringle maneuver for hepatic resection of
liver cavernous hemangioma. World J Surg Oncol. 13:2672015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dou L, Meng WS, Su BD, Zhu P, Zhang W,
Liang HF, Chen YF and Chen XP: Step-by-step vascular control for
extracapsular resection of complex giant liver hemangioma involving
the inferior vena cava. Am Surg. 80:15–20. 2014.PubMed/NCBI
|
|
13
|
Gurusamy KS, Kumar Y, Pamecha V, Sharma D
and Davidson BR: Ischaemic pre-conditioning for elective liver
resections performed under vascular occlusion. Cochrane Database
Syst Rev: CD007629. 2009. View Article : Google Scholar
|
|
14
|
Chen YW, Li CH, Zhang AQ, Yang SZ, Zhang
WZ and Dong JH: Preserving hepatic artery flow during portal triad
blood inflow occlusion reduces liver ischemia-reperfusion injury in
rats. J Surg Res. 174:150–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakaguchi K, Takeuchi E, Suzuki M, Oda K,
Nagino M, Nimura Y and Yoshida S: DNA polymerases and Ki-67 nuclear
antigen are induced in correlation with the resected mass of rat
liver up to 90%. Langenbecks Arch Surg. 385:135–142. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Institute for Laboratory Animal Research:
Guide for the care and use of laboratory animals. 8th (ed.).
Washington (DC): National Academies Press (US); 2011
|
|
17
|
Huang X, Li CH, Zhang AQ, Kong Z, Gu WQ
and Dong JH: A simple rat model of in situ reversible obstructive
jaundice in situ reversible obstructive jaundice model. Ann Surg
Treat Res. 92:389–395. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li CH, Wang HD, Hu JJ, Ge XL, Pan K, Zhang
AQ and Dong JH: The monitoring of microvascular liver blood flow
changes during ischemia and reperfusion using laser speckle
contrast imaging. Microvasc Res. 94:28–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang PF, Li CH, Chen YW, Zhang AQ, Cai SW
and Dong JH: Preserving hepatic artery flow during portal triad
blood inflow occlusion improves remnant liver regeneration in rats
after partial hepatectomy. J Surg Res. 181:329–336. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paranjpe S, Bowen WC, Bell AW, Nejak-Bowen
K, Luo JH and Michalopoulos GK: Cell cycle effects resulting from
inhibition of hepatocyte growth factor and its receptor c-Met in
regenerating rat livers by RNA interference. Hepatology.
45:1471–1477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rehman H, Sun J, Shi Y, Ramshesh VK, Liu
Q, Currin RT, Lemasters JJ and Zhong Z: NIM811 prevents
mitochondrial dysfunction, attenuates liver injury, and stimulates
liver regeneration after massive hepatectomy. Transplantation.
91:406–412. 2011.PubMed/NCBI
|
|
23
|
Fu SY, Lau WY, Li GG, Tang QH, Li AJ, Pan
ZY, Huang G, Yin L, Wu MC, Lai EC and Zhou WP: A prospective
randomized controlled trial to compare Pringle maneuver,
hemihepatic vascular inflow occlusion, and main portal vein inflow
occlusion in partial hepatectomy. Am J Surg. 201:62–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu JJ, Li CH, Wang HD, Xu WL, Zhang AQ and
Dong JH: Portal vein clamping alone confers protection against
hepatic ischemia-reperfusion injury via preserving hepatocyte
function in cirrhotic rats. J Surg Res. 194:139–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakanishi Y, Tsuchikawa T, Okamura K,
Nakamura T, Tamoto E, Noji T, Asano T, Amano T, Shichinohe T and
Hirano S: Risk factors for a high Comprehensive Complication Index
score after major hepatectomy for biliary cancer: A study of 229
patients at a single institution. HPB (Oxford). 18:735–741. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xin KY, Yee LS, Yong TT and Fui AC:
Obstructive jaundice due to intraductal tumour thrombus in
recurrent hepatocellular carcinoma: What is the optimal therapeutic
approach? Hepatogastroenterology. 61:1863–1866. 2014.PubMed/NCBI
|
|
27
|
Wanner GA, Ertel W, Müller P, Höfer Y,
Leiderer R, Menger MD and Messmer K: Liver ischemia and reperfusion
induces a systemic inflammatory response through Kupffer cell
activation. Shock. 5:34–40. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li CH, Chen YW, Chen YL, Yao LB, Ge XL,
Pan K, Zhang AQ and Dong JH: Preserving low perfusion during
surgical liver blood inflow control prevents hepatic
microcirculatory dysfunction and irreversible hepatocyte injury in
rats. Sci Rep. 5:144062015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wiggers JK, Koerkamp Groot B, Cieslak KP,
Doussot A, van Klaveren D, Allen PJ, Besselink MG, Busch OR,
D'Angelica MI, DeMatteo RP, et al: Postoperative mortality after
liver resection for perihilar cholangiocarcinoma: Development of a
risk score and importance of biliary drainage of the future liver
remnant. J Am Coll Surg. 223(321–331): e12016.PubMed/NCBI
|