Introduction
Sevoflurane is one of the most prominent
inhalational anesthetics used in cesarean delivery and pediatric
clinical application, for its rapid induction and recovery
properties. The critical vulnerable period of anesthetic
neurotoxicity depends on rapid synaptogenesis, and lasts from
mid-gestation to several years after birth (1,2). Recent
retrospective cohort studies demonstrated that anesthesia
application in young children younger than 3 years old could lead
to behavioral and developmental cognitive disorders (3–5).
Sevoflurane neurotoxicity in immature brain is associated with
alterations in behavior, spatial learning, memory and reading,
writing, math learning disabilities, even effects later in
adulthood (6–10). More evidence suggested that
sevoflurane could induce neuronal apoptosis of developing brain
(11,12). For the frequent application of
sevoflurane in anesthetics exposure for childbirth and surgeries to
prevent pain, it is urgent to find effective remedy to prevent
anesthetic neurotoxicity.
Lycium barbarum, fruit of wolfberry, is
commonly applied as food addictive and traditional medicinal herb
in many countries, especially China and other Asian countries
(13). Lycium barbarum
polysaccharides (LBPs), the main effective component of Lycium
barbarum, possess a variety of beneficial effects in
anti-aging, anti-cancer, immune modulation and neuroprotection
(14–17). LBP is found to decrease oxidative
stress in hippocampus, promote cell proliferation and neurons
differentiation (18,19). It is also reported to enhance
neurogenesis in hippocampus and subventricular zone, to improve
cognition via apoptosis regulation in hippocampal neurons under
stress, and to prevent cognitive and memory deficits in
scopolamin-treated rats (20–24).
However, whether and how LBP protects neurons from injury of
sevoflurane is still unclear.
Apoptosis, also called programmed cell death,
eliminates dysfunctional or superfluous cells to maintain normal
tissue functions. However, abnormal apoptosis could induce cell
injury or death. Sevoflurane has been reported to promote the
activation of cysteine aspartate-specific protease (Caspase) and
apoptosis pathway (11,25). Extracellular signal-regulated kinase
1/2 (ERK1/2), a critical member of mitogen-activated protein kinase
(MAPK) cascades, plays a prominent role in cell survival and death.
Recent researches have demonstrated that ERK1/2 plays positive
roles in neuroprotective processes. Sevoflurane neurotoxicity in
immature brain is related to ERK1/2 signaling pathway and apoptosis
related factors such as Caspase-3, B-cell lymphoma/leukemia-2
(Bcl-2) and Bcl-2 associated X (Bax) (6–8). It
would be interesting to know whether LBP effects on
sevoflurane-injured neurons through ERK1/2 pathway.
In conclusion, we constructed sevoflurane-injured
rat hippocampal neuron model to validate possible function of LBP
on cell apoptosis induced by sevoflurane. Furthermore, we also
measured the role of ERK1/2 MAPK signaling pathway on it. It may
provide novel light for clinical application of LBP as remedy for
anesthetic neurotoxicity.
Materials and methods
Primary nerve cells cultures
Adult female Sprague-Dawley (SD) rats, weighing
200–300 g, were purchased from Beijing Vital River Company
(Beijing, China) and raised for 14 days under normal environment
(22–24°C, 55–65% humidity, 12-h light/dark cycle) after mating
between female and male rats. Then the embryos were isolated for
following experiments. The present study was approved by
Institutional Animal Care and Use Committee of Qinghai Red Cross
Hospital (Xining, China). After being sacrificed by cervical
dislocation, the rats were immersed and disinfected in alcohol. The
pregnant uterus were exposed and cut off under aseptic conditions,
the fetus was removed, and the hippocampus tissues were isolated
and digested by 0.125% trypsin for 30 min, and nerve cells were
collected and cultured in Neuralbasal media (MTI-GlobalStem,
Gaithersburg, MD, USA) supplemented with 2% B27, 10 mmol/l HEPES,
and 0.5 mmol/l L-glutamine, in 5% CO2-containg incubator
at 37°C. Cell culture media was changed every 3 days. Cell
morphology was observed by DMi8 optical microscope (Leica
Microsystems GmbH, Wetzlar, Germany) after being cultured for 3 and
7 days, respectively.
Primary hippocampal neurons cultured for 7 days were
pre-treated with LBPs with different concentrations (100, 200, 400
and 600 µg/ml) for 6 h, and injured by 3% sevoflurane [Baxter
Healthcare (Shanghai) Co., Ltd., Shanghai, China] gas mixture of
95% O2 and 5% CO2 subsequently, the flow rate
of which was delivered at 2 l/min. 3% seveflurane was used to
construct the injury model, the concentration of which was just
higher than that commonly used in clinical application and was
commonly used in researches too (6,26), The
groups were named LBP100+SEV, LBP200+SEV, LBP400+SEV, LBP600+SEV
groups, respectively, Cells treated with 3% sevoflurane gas alone
were named SEV group, Cells treated with 400 µg/ml LBPs were named
LBP400 group, and cells without any treatment were considered as
Control group.
In addition, for the best repairing effect,
LBP400+SEV cells were chosen to be treated with 5 µmol/l ERK1/2
inhibitor PD98059 (Merck, Germany) 1 h before 400 µg/ml LBP
treatment (ERK1/2 inhibitor+LBP400+SEV group). The changes were
compared to that in Control, SEV, LBP400+SEV groups.
Cell Counting Kit-8 (CCK-8) assay
Cell viabilities of primary hippocampal neurons in
different groups (Control, SEV, LBP100+SEV, LBP200+SEV, LBP400+SEV,
LBP600+SEV groups) were determined by CCK-8 assay (Beyotime
Institute of Biotechnology, Haimen, China) in dedicated times (2, 4
and 6 h), according to the manufacturer's protocols. Briefly
speaking, following the determined treatment, cells were seeded in
96-well plates and reacted with 20 µl CCK-8 reagent for 1 h in 5%
CO2-containing incubator at 37°C. CCK-8 is a
yellow-colored dye, which can be reduced by succinate dehydrogenase
in mitochondria of living cells. forming soluble blue-purple
formazan and depositing in cells, whereas dead cells do not have
this function. The optical density (OD) values were measured by a
microplate reader (Omega Bio-Tek, Inc., Norcross, GA, USA) at 450
nm.
Carboxyfluorescein diacetate
succinimidyl ester (CFSE) assay
Mature neurons are terminal differentiation cells.
However, the embryo hippocampus neurons have the ability of
proliferation and differentiation (25,27–29).
CFSE cell proliferation kit (Invitrogen, USA) was used to analyze
cell proliferation abilities of different groups (Control, SEV,
LBP100+SEV, LBP200+SEV, LBP400+SEV groups), which detected both
living and dead cells, according to the manufacturer's protocols.
Cells were resuspended to 1×106/ml in 1 ml preheated
phosphate buffer solution (PBS) in sterile centrifuge tubes. 2 µl
CFSE (5 mmol/l) stocking reagent was added into cell suspension to
the final concentration of 10 µmol/l, then cells were incubated at
37°C for 10 min and then in 10 ml icy culture media for 5 min in
dark. Finally, cells were inoculated in 24-well plates
(1×105/well) and incubated with 5% CO2 at
37°C, then cells were digested, collected and detected by FACS
Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA),
with the whole process avoiding light.
Apoptosis detection
The apoptosis rates of hippocampal neurons in
different groups (Control, SEV, LBP100+SEV, LBP200+SEV, LBP400+SEV
groups) were determined by Annexin V/PI (propidine iodide)
double-stain assay, according to the manufacturer's instructions
(BioVision, Inc., Milpitas, CA, USA). Phosphatidylserine exposed to
the outside of cell membrane in early stage of apoptosis could be
detected by Annexin V/FITC (fluorescein isothiocyanate), and DNA
within membrane-injured cells could be detected by PI in the
late-stage of apoptosis. The combination of them could effectively
detect apoptotic cells. In the experiment, cells were resuspended
by 100 µl binding buffer to the final concentration of
1×106 cells/ml. Then 5 µl Annexin V/FITC and 10 µl PI
(20 µg/ml) were added in. After the incubation for 15 min away from
light at room temperature, cells were analyzed by a flow cytometer
(BD Biosciences), with 488 nm as exciting light wavelength, and 515
nm (FITC), 560 nm (PI) as detecting light wavelengths.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to analyze the mRNA expression
levels of apoptosis-related factors such as Caspase-3, Bax, Bcl-2
in different groups, including Control, SEV, LBP100+SEV,
LBP200+SEV, LBP400+SEV and ERK1/2 inhibitor+LBP400+SEV groups.
GAPDH was considered as internal reference. Firstly, total RNA was
extracted by TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and reversely transcribed to cDNA with a first
strand cDNA kit (Takara Bio, Inc., Otsu, Japan), according to the
protocols provided by manufacturer. PCR amplification was conducted
using the SYBR Premix Ex Taq kit (Takara Bio, Inc.). Briefly, after
pre-denaturation at 95°C for 30 sec, amplification of 40 cycles
were conducted: denaturation at 95°C for 5 sec, annealing/extension
at 60°C for 30 sec, which was performed in ABI 7300 Thermocycler
(Applied Biosystems; Thermo Fisher Scientific, Inc.) The primer
sequences were as follows: Caspase-3 (sense:
5′TGGAATGTCAGCTCGCAATG3′ and antisense: 5′CAGGTCCGTTCGTTCCAAAA3′);
Bax, sense: 5′AGATCATGAAGACAGGGGCC3′ and antisense:
5′ATCCTCTGCAGCTCCATGTT3′; Bcl-2, sense: 5′AACTCTTCAGGGATGGGGTG3′
and antisense: 5′GCTGGGGCCATATAGTTCCA3′; GAPDH, sense:
5′AGTCTACTGGCGTCTTCACC3′ and antisense:
5′CCACGATGCCAAAGTTGTCA3′).
Western blot analysis
Western blotting was conducted to measure protein
levels, including apoptosis related factors (active-Caspase-3, Bax,
Bcl-2) and p-ERK1/2 (phosphorylated ERK1/2), t-ERK1/2
(total-ERK1/2), in different groups including Control, SEV,
LBP100+SEV, LBP200+SEV, LBP400+SEV and RK1/2 inhibitor+LBP400+SEV
groups. Briefly speaking, proteins were firstly extracted and the
quantities were determined by BCA assay (Beyotime Institute of
Biotechnology,). Then they were concentrated and separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE), and electrotransfered onto a polyvinylidene fluoride
(PVDF) membrane (EMD Millipore, Billerica, MA, USA). After the
blockage with 5% nonfat dry milk for 1 h, the blotting membranes
were incubated with specific primary antibodies overnight at 4°C,
respectively. GAPDH was used as loading control. The primary
antibodies were as follows: rabbit anti-active-Caspase-3 (Abcam,
Cambridge, UK; ab2302, 1:200), anti-Bax (Abcam; ab32503, 1:2,000),
anti-Bcl-2 (Abcam; ab59348, 1:1,000), anti-ERK1/2 (Abcam; ab17942,
1:1,000), anti-p-ERK1/2 (Cell Signaling Technology, Inc., Danvers,
MA, USA; 4370, 1:2,000), anti-GAPDH (Abcam; ab9485, 1:2,500). After
that, membranes were incubated with the appropriate secondary
antibody goat anti-rabbit HRP-conjugated IgG H&L (Abcam;
ab6721, 1:5,000) for 2 h. The PVDF membrane were exposed to and
detected by X-ray film with enhanced chemiluminescense (ECL)
detection system reagents (Amersham; GE Healthcare, Chalfont,
UK).
Statistical analysis
All results were presented as mean ± standard
deviation of three independent experiments. Statistical analysis
was performed using a SPSS 22.0 statistical package (IBM Corp.,
Armonk, NY, USA), then differences among multi-groups were analyzed
by Welch's one-way analysis of variance (ANOVA), following by
Games-Howell test. P<0.05 was considered significant, P<0.01
was considered especially significant.
Results
LBP promoted cell viability of
hippocampal neurons injured by sevoflurane
Hippocampal primary neurons were isolated from SD
embryonic rats and cell morphologies were found in good growing
conditions after 3 and 7 days culture, observed by light
microscopy. On the 3rd day, most of cells displayed classical
morphologies of neural cells, with round, oval or shuttle shapes,
smooth surface, longer neurites with obvious branches connected to
be nets. On the 7th day, neurons aggregated with shuttle or
polygonal shapes, with more neurites tightly connected to be nets,
indicating the rapid brain development period which was vulnerable
to anesthesia-induced neuronal injury (Fig. 1A).
 | Figure 1.LBP promoted cell viability of
hippocampal neurons injured by sevoflurane. (A) Cell morphology of
hippocampus primary neurons separated from SD embryonic rats was
observed under light microscopy, after 3 and 7 days culture. (B)
CCK-8 assay was used to detect function of LBP with different
concentrations (100, 200, 400 and 600 µg/ml) on cell viability of
3% sevoflurane treated neurons in different groups (Control,
LBP400, SEV, LBP100+SEV, LBP200+SEV, LBP400+SEV, LBP600+SEV), at
different times (2, 4 and 6 h). (C and D) CFSE assay was conducted
to verify the promotion function of LBP on cell differentiation of
neurons, after 6 h treatment of sevoflurane. ##P<0.01
vs. Control group, *P<0.05, **P<0.01 vs. SEV group. LBP,
Lycium barbarum polysaccharides; SD, Sprague-Dawley; CCK-8,
Cell Counting Kit-8; CFSE, carboxyfluorescein diacetate
succinimidyl ester. |
CCK-8 assay was used to detect function of LBP with
different concentrations (100, 200, 400 and 600 µg/ml) on cell
viability of hippocampal primary neurons treated with 3%
sevoflurane, at determined times (2, 4 and 6 h). Function of 400
µg/ml LBP alone was also detected. LBP alone had no effect on
neurons viability. Cell viability of neurons decreased
significantly with sevoflurane treatment, compared with control
group (P<0.01). The inhibition rate of 3% sevoflurane on neurons
viability was 53% at 6 h, so 3% sevoflurane treatment for 6 h was
chosen for following experiments. Meanwhile cell viability of
sevoflurane-injured neurons increased notably by LBP in dose (100,
200, 400 and 600 µg/ml) and time dependent (2, 4, 6 h) manners,
compared with SEV group (P<0.05) (Fig. 1B). For the effect of 600 µg/ml was
similar to 400 µg/ml LBP, we just studied function of LBP (100, 200
and 400 µg/ml) in the following experiments.
CFSE assay was conducted to verify the promotion
function of LBP with different concentrations (100, 200 and 400
µg/ml) on cell proliferation abilities of hippocampal primary
neurons treated by 3% sevoflurane for 6 h. The flow cytometry (FCM)
detection showed that the cell proliferation ability of neurons was
inhibited significantly, with decreased M1 values, by sevoflurane
in SEV group, compared with control group (P<0.01), which was
promoted significantly, with increased M1 values, by LBP in
dose-dependent manners (100, 200 and 400 µg/ml) (P<0.05;
Fig. 1C and D).
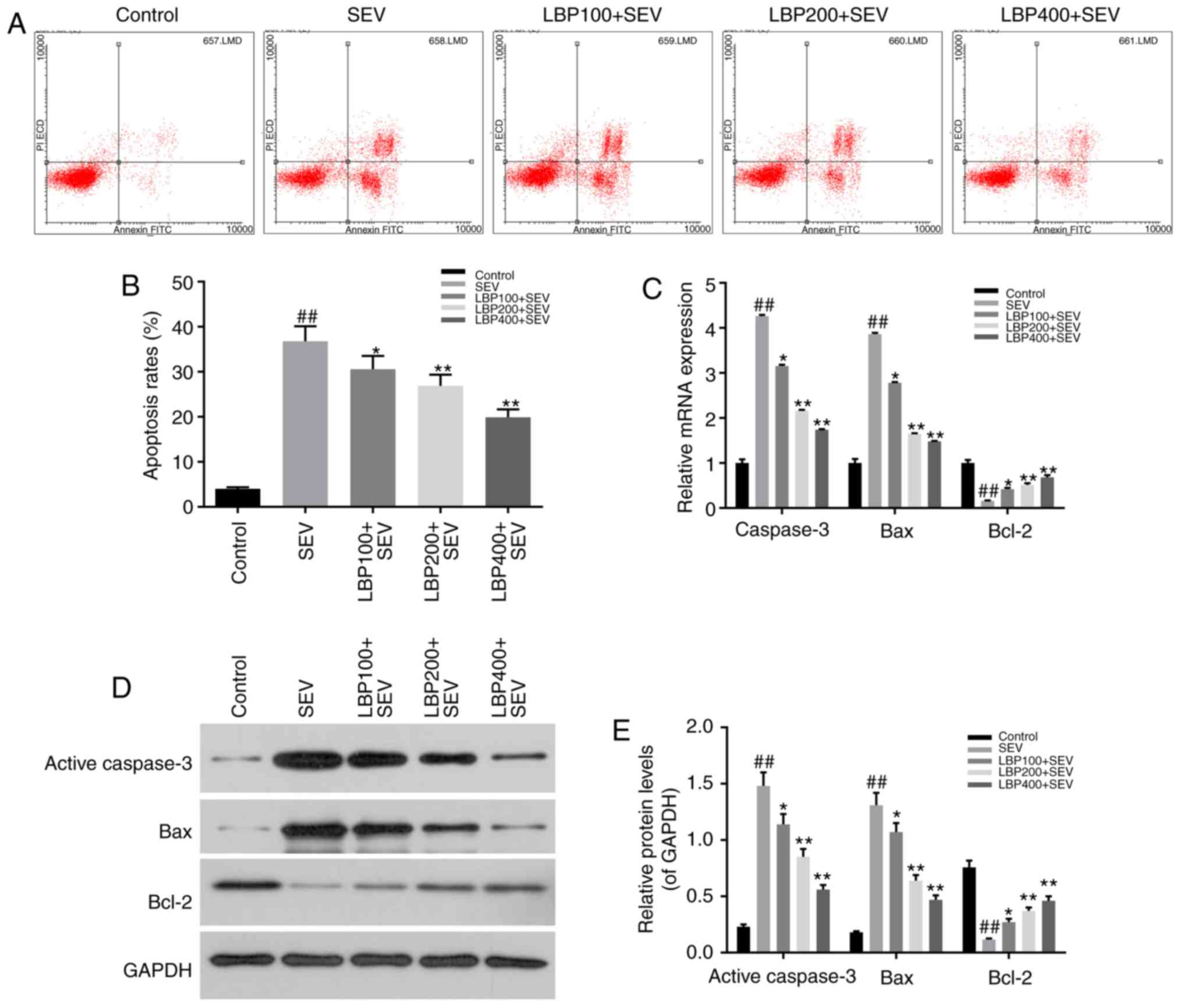
LBP inhibited cell apoptosis of
hippocampal neurons injured by sevoflurane
Annexin V/PI double-stain and FCM assay were
performed to evaluate the effect of LBP with different
concentrations (100, 200 and 400 µg/ml) on cell apoptosis status of
hipocampal neurons injured by 3% sevoflurane for 6 h. The results
suggested that sevoflurane remarkably facilitated cell apoptosis of
neurons in SEV group, compared with control group (P<0.01),
which was reduced notably by LBP in dose-dependent manners (100,
200 and 400 µg/ml) (P<0.05; Fig. 2A
and B).
The expression levels of apoptosis related factors,
such as active-Caspase-3, Bax, Bcl-2, were determined by RT-qPCR
and western blot assay in above groups. The results demonstrated
that sevoflurane dramatically up-regulated the expression levels of
pro-apoptosis factors active=Caspase-3 and Bax (P<0.01), and LBP
effectively down-regulated them dose-dependently (100, 200 and 400
µg/ml), both in mRNA and protein manners (P<0.05). Meanwhile,
sevoflurane notably down-regulated the expression of apoptosis
inhibitor Bcl-2 (P<0.01), and LBP effectively up-regulated them
dose-dependently (100, 200 and 400 µg/ml), both in mRNA and protein
manners (P<0.05; Fig. 2C-E).
LBP activated ERK1/2 pathway in
hippocampal neurons injured by sevoflurane
The phosphorylation levels of ERK1/2 in above groups
were assessed by western blot. The assay illustrated that, protein
levels of p-ERK1/2 decreased significantly in SEV group compared
with control group (P<0.01), which increased notably
dose-dependently (100, 200 and 400 µg/ml) when treated with LBP,
compared with SEV group (P<0.05). Meanwhile, t-ERK1/2 levels
were of no significant differences among different groups (Fig. 3A and B).
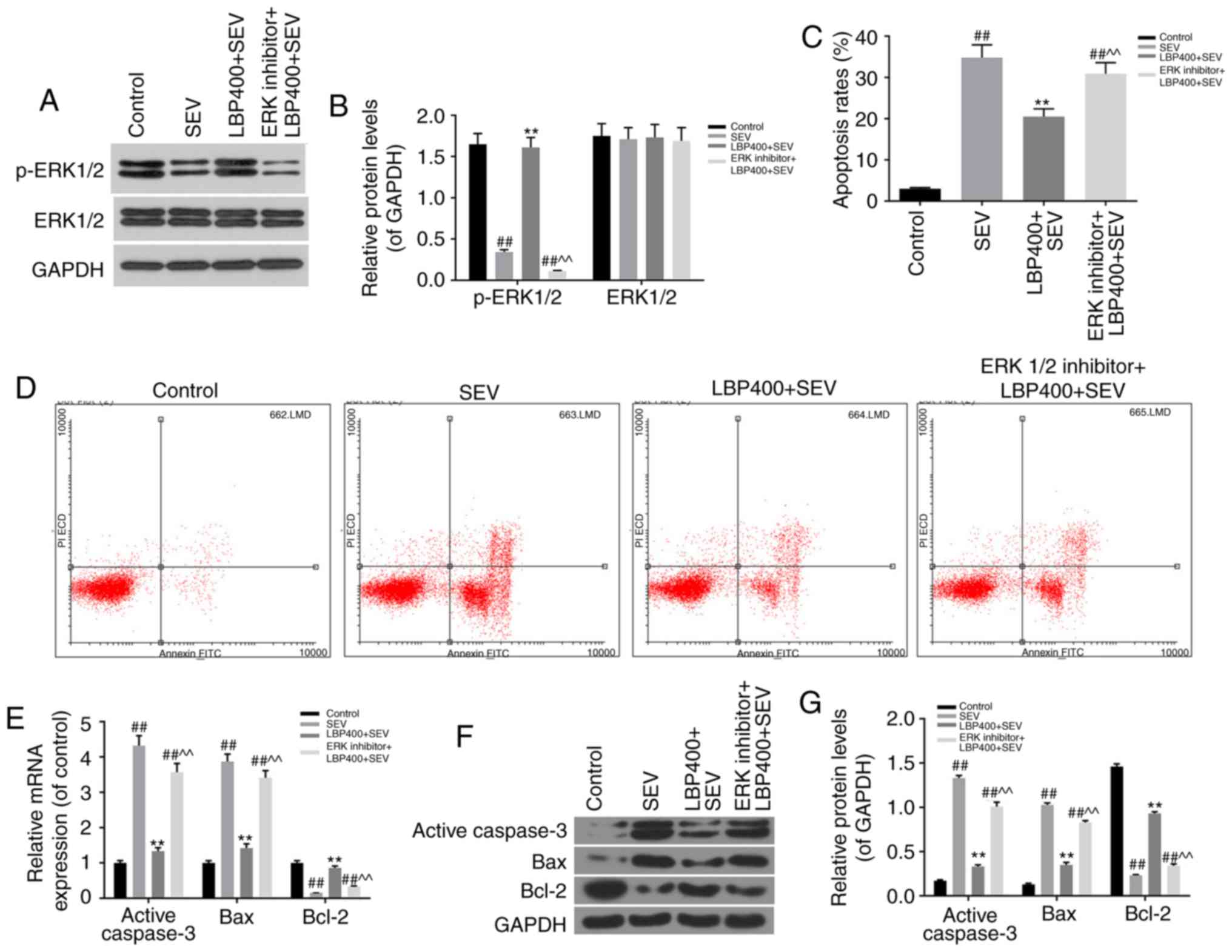
ERK1/2 inhibitor blocked function of
LBP on sevoflurane injured hippocampal neurons
Western blot assay on ERK1/2 protein levels in
Control, SEV, LBP400+SEV, ERK1/2 inhibitor+LBP400+SEV groups
revealed that, the increased protein levels of p-ERK1/2 in
LBP400+SEV group was blocked when pre-treated with ERK1/2 inhibitor
(P<0.05) (Fig. 4A and B). The FCM
analysis on apoptosis rates verified that, LBP declined the
apoptosis rates of hippocampal neurons treated with sevoflurane in
LBP400+SEV group, which was attenuated by ERK1/2 inhibitor
(P<0.05) (Fig. 4C and D). RT-qPCR
and western blot assays showed that levels of active=Caspase-3 and
Bax were promoted, Bcl-2 was inhibited by ERK1/2 inhibitor in
ERK1/2 inhibitor+LBP400+SEV group, compared with LBP400+SEV group
(P<0.05) (Fig. 4E-G).
Discussion
Sevoflurane, commonly used as inhalational
anesthetics in cesarean delivery and pediatric surgeries, is
reported to frequently induce behavioral and developmental
cognitive disorders in children. It is necessary to find possible
remedy to prevent developmental anesthetic neurotoxicity. LBPs are
effective to enhance neurogenesis in hippocampus and to improve
cognition via apoptosis regulation in hippocampal neurons under
stress. It is exciting to verify the possible molecular mechanism
of LBP protecting neurons from sevoflurane injury.
The hippocampus is the critical tissue for spatial
navigation and long-term memory in brain. In the present study, the
primary hippocampal neurons isolated from SD embryonic rats were
observed by light microscopy after culture for 3 and 7 days. Then
neurons cultured for 7 days were chosen for the following research,
for the best developing morphologies with much more tightly
connected neurites. LBP alone didn't affect cell viabilities of
hippocampal neurons. 3% seveflurane was used to construct the
injury model in the studies before (6,26),
Moreover, the inhibition rate of 3% sevoflurane on neurons
viability at 6 h was 53%, so 3% sevoflurane treatment for 6 h was
chosen for following experiments. Cell differentiation happens
along with the whole process of neurons development. As a
spontaneous cell-suicide process, apoptosis of neurons is needed
for regular brain development. However, abnormal apoptosis could
inhibit cell viability too. In our study, LBP was found promoting
cell viability, proliferation, and inhibiting cell apoptosis of
neurons injured by 3% seveflurane dose-dependently (100, 200 and
400 µg/ml).
Sevoflurane neurotoxicity in immature brain is
related to excessive apoptosis, including abnormal expression of
Bcl-2 family members and caspases (25). The members of Bcl-2 family, including
Bax, Bcl-2 and Bcl-XL, are the main regulators of apoptosis
(30,31). As a critical anti-apoptosis factor,
Bcl-2 inhibits apoptosis predominantly by inhibiting caspase
pathway. On the other hand, Bax conjugates with Bcl-2, forming
heterodimer, to induce the release of cytochrome c, activate
caspase pathway and promote apoptosis (32,33).
Caspases are a family of cysteine-aspartic proteases. Being the
final executor of caspase pathway, caspase-3 plays an important
role to degrade cellular components and induce apoptosis (34). Consistent with previous researches,
our study showed that sevoflurane-treatment on hippocampal neurons
significantly increased the expression levels of pro-apoptosis
factors like Bax and active-Caspase-3, and decreased the expression
levels of anti-apoptosis factors such as Bcl-2. It showed that, LBP
promoted cell viability and differentiation, inhibited apoptosis
dose-dependently by declining Bcl-2 and elevating Bax and
active-Caspase-3 expression, in hippocampal neurons injured by
sevoflurane in our study, as expected.
Recently, studies have provided evidence that
numerous pathways were involved in sevoflurane induced injury on
neurons (35). ERK1/2 MAPK pathway
is activated by different stimulus including growing factors,
mechanical stress and so on (36).
The activation of ERK1/2 is necessary for signal transduction, from
cell membrane surface receptors to nucleus. ERK1/2 MAPK signaling
pathway has been reported to play essential roles in developmental
neuronal survival and sevoflurane neurotoxicity (6–8), to
increase dendritic spine density during synaptogenesis in
hippocampal neurons, and to help improving learning and memory
abilities (37,38). MEK (ERK1/2 kinase) inhibitor PD98059
is specific for ERK1/2 pathway, by noncompetitively binding to MEK,
it is commonly used to block ERK1/2 pathway. In our study, LBP was
found effectively promoting ERK1/2 activation by increasing ERK1/2
phosphorylation levels, which was decreased remarkably by
sevoflurane in hippocampal neurons. The phosphorylation levels of
ERK1/2 were attenuated when additional ERK1/2 inhibitor PD98059 was
applied. Furthermore, ERK1/2 inhibitor could attenuate function of
LBP on apoptosis inhibition, in hippocampal neurons treated with
sevoflurane. Consistent with the promoted apoptosis, the expression
levels of Bax and active-Caspase increased, and Bcl-2 decreased
when ERK1/2 inhibitor existed, Though it would be better to
investigate effect of ERK1/2 activation on it at the same time, the
results have indicated that LBP protected neurons from sevoflurane
induced apoptosis through ERK1/2 pathway, which might attenuate
cell viability and proliferation abilities of primary hippocampal
neurons.
Based on the whole study, LBP could promote cell
viability, cell proliferation and inhibit apoptosis of primary
hippocampal neurons injured by sevoflurane, through activating
ERK1/2 MAPK pathway. Moreover additional ERK1/2 inhibitor could
attenuate function of LBP by promoting cell apoptosis. It provided
novel light to attenuate neurotoxicity of inhalational anesthetics
in clinic. Function of LBP on sevoflurane-induced behavioral
changes, cognitive and memorial disorders in vivo might be
further studied in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XW designed the study, conducted the cell culture
and wrote the manuscript. YX performed the CFSE, apoptosis
analysis, and detected apoptosis-associated factors and ERK1/2
phosphorylation.
Ethics approval and consent to
participate
The present study was approved by Institutional
Animal Care and Use Committee of Qinghai Red Cross Hospital
(Qinghai, China; Approval No. Z20160612).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dobbing J and Sands J: Comparative aspects
of the brain growth spurt. Early Hum Dev. 3:79–83. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yon JH, Daniel-Johnson J, Carter LB and
Jevtovic-Todorovic V: Anesthesia induces neuronal cell death in the
developing rat brain via the intrinsic and extrinsic apoptotic
pathways. Neuroscience. 135:815–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DiMaggio C, Sun LS, Kakavouli A, Byrne MW
and Li G: A retrospective cohort study of the association of
anesthesia and hernia repair surgery with behavioral and
developmental disorders in young children. J Neurosurg Anesthesiol.
21:286–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilder RT, Flick RP, Sprung J, Katusic SK,
Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL and
Warner DO: Early exposure to anesthesia and learning disabilities
in a population-based birth cohort. Anesthesiology. 110:796–804.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Servick K: Biomedical research.
Researchers struggle to gauge risks of childhood anesthesia.
Science. 346:1161–1162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang WY, Wang H, Luo Y, Jia LJ, Zhao JN,
Zhang HH, Ma ZW, Xue QS and Yu BW: The effects of metabotropic
glutamate receptor 7 allosteric agonist
N,N'-dibenzhydrylethane-1,2-diamine dihydrochloride on
developmental sevoflurane neurotoxicity: Role of extracellular
signal-regulated kinase 1 and 2 mitogen-activated protein kinase
signaling pathway. Neuroscience. 205:167–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang WY, Yang R, Hu SF, Wang H, Ma ZW and
Lu Y: N-stearoyl-L-tyrosine ameliorates sevoflurane induced
neuroapoptosis via MEK/ERK1/2 MAPK signaling pathway in the
developing brain. Neurosci Lett. 541:167–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang WY, Jia LJ, Luo Y, Zhang HH, Cai F,
Mao H, Xu WC, Fang JB, Peng ZY, Ma ZW, et al: Location- and
subunit-specific NMDA receptors determine the developmental
sevoflurane neurotoxicity through ERK1/2 signaling. Mol Neurobiol.
53:216–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edwards DA, Shah HP, Cao W, Gravenstein N,
Seubert CN and Martynyuk AE: Bumetanide alleviates epileptogenic
and neurotoxic effects of sevoflurane in neonatal rat brain.
Anesthesiology. 112:567–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shih J, May LD, Gonzalez HE, Lee EW, Alvi
RS, Sall JW, Rau V, Bickler PE, Lalchandani GR, Yusupova M, et al:
Delayed environmental enrichment reverses sevoflurane-induced
memory impairment in rats. Anesthesiology. 116:586–602. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu Y, Wu X, Dong Y, Xu Z, Zhang Y and Xie
Z: Anesthetic sevoflurane causes neurotoxicity differently in
neonatal naive and Alzheimer disease transgenic mice.
Anesthesiology. 112:1404–1416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Satomoto M, Satoh Y, Terui K, Miyao H,
Takishima K, Ito M and Imaki J: Neonatal exposure to sevoflurane
induces abnormal social behaviors and deficits in fear conditioning
in mice. Anesthesiology. 110:628–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang M, Chen H, Huang J, Li Z, Zhu C and
Zhang S: Effect of Lycium barbarum polysaccharide on human
hepatoma QGY7703 cells: Inhibition of proliferation and induction
of apoptosis. Life Sci. 76:2115–2124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang RC and So KF: Use of anti-aging
herbal medicine, Lycium barbarum, against aging-associated
diseases. What do we know so far? Cell Mol Neurobiol. 28:643–652.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li XM, Ma YL and Liu XJ: Effect of the
Lycium barbarum polysaccharides on age-related oxidative
stress in aged mice. J Ethnopharmacol. 111:504–511. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng D and Kong H: The effect of
Lycium barbarum polysaccharide on alcohol-induced oxidative
stress in rats. Molecules. 16:2542–2550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang WM, Chan E, Kwok CY, Lee YK, Wu JH,
Wan CW, Chan RY, Yu PH and Chan SW: A review of the anticancer and
immunomodulatory effects of Lycium barbarum fruit.
Inflammopharmacology. 20:307–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li SY, Yang D, Yeung CM, Yu WY, Chang RC,
So KF, Wong D and Lo AC: Lycium barbarum polysaccharides
reduce neuronal damage, blood-retinal barrier disruption and
oxidative stress in retinal ischemia/reperfusion injury. PLoS One.
6:e163802011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo Q, Cai Y, Yan J, Sun M and Corke H:
Hypoglycemic and hypolipidemic effects and antioxidant activity of
fruit extracts from Lycium barbarum. Life Sci. 76:137–149.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Liang Y, Chiu K, Yuan Q, Lin B,
Chang RC and So KF: Lycium barbarum (wolfberry) reduces
secondary degeneration and oxidative stress, and inhibits JNK
pathway in retina after partial optic nerve transection. PLoS One.
8:e688812013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lau BW, Lee JC, Li Y, Fung SM, Sang YH,
Shen J, Chang RC and So KF: Polysaccharides from wolfberry prevents
corticosterone-induced inhibition of sexual behavior and increases
neurogenesis. PLoS One. 7:e333742012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, Cheng X, Chen J, Yi X, Nie D, Sun
X, Qin J, Tian M, Jin G and Zhang X: Lycium barbarum
polysaccharides prevent memory and neurogenesis impairments in
scopolamine-treated rats. PLoS One. 9:e880762014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao J, Chen C, Liu Y, Li Y, Long Z, Wang
H, Zhang Y, Sui J, Wu Y, Liu L and Yang C: Lycium barbarum
polysaccharide improves traumatic cognition via reversing imbalance
of apoptosis/regeneration in hippocampal neurons after stress. Life
Sci. 121:124–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao P, Ma NT, Chang RY, Li YX, Hao YJ,
Yang WL, Zheng J, Niu Y, Sun T and Yu JQ: Mechanism of Lycium
barbarum polysaccharides on primary cultured rat hippocampal
neurons. Cell Tissue Res. 369:455–465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Xue Z and Sun A: Subclinical
concentration of sevoflurane potentiates neuronal apoptosis in the
developing C57BL/6 mouse brain. Neurosci Lett. 447:109–114. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ling Y, Li X, Yu L, Liang Q, Lin X, Yang
X, Wang H and Zhang Y: Sevoflurane exposure in postnatal rats
induced long-term cognitive impairment through upregulating
caspase-3/cleaved-poly (ADP-ribose) polymerase pathway. Exp Ther
Med. 14:3824–3830. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beccari S, Valero J, Maletic-Savatic M and
Sierra A: A simulation model of neuroprogenitor proliferation
dynamics predicts age-related loss of hippocampal neurogenesis but
not astrogenesis. Sci Rep. 7:165282017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu BW, Wu MS and Guo JD: Effects of
microRNA-10a on synapse remodeling in hippocampal neurons and
neuronal cell proliferation and apoptosis through the BDNF-TrkB
signaling pathway in a rat model of Alzheimer's disease. J Cell
Physiol. Dec 7–2017.(Epub ahead of print).
|
|
29
|
Yan BC, Jiang D, Wang J, Zhang Y, Zhu X,
Xu P, Yu X, Won MH and Su PQ: Both decreased Akt expression and
mTOR phosphorylation are related to decreased neuronal
differentiation in the hippocampal alveus of aged mice. Aging Clin
Exp Res. Oct 13–2017.(Epub ahead of print). PubMed/NCBI
|
|
30
|
Park JW, Lim MS, Ji SY, Cho MS, Park SJ,
Han SH and Kim JH: Effects of short-term exposure to sevoflurane on
the survival, proliferation, apoptosis, and differentiation of
neural precursor cells derived from human embryonic stem cells. J
Anesth. 31:821–828. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ozer AB, Ceribasi S, Ceribasi AO, Demirel
I, Bayar MK, Ustundag B, Ileri A and Erhan OL: Effects of
sevoflurane on apoptosis, BDNF and cognitive functions in neonatal
rats. Bratisl Lek Listy. 118:80–84. 2017.PubMed/NCBI
|
|
32
|
Yang X, Yang S, Hong C, Yu W and Guonian
W: Panax Notoginseng Saponins attenuates sevoflurane-induced nerve
cell injury by modulating AKT signaling pathway. Mol Med Rep.
16:7829–7834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ding ML, Ma H, Man YG and Lv HY:
Protective effects of a green tea polyphenol,
epigallocatechin-3-gallate, against sevoflurane-induced neuronal
apoptosis involve regulation of CREB/BDNF/TrkB and PI3K/Akt/mTOR
signalling pathways in neonatal mice. Can J Physiol Pharmacol.
95:1396–1405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang H, Liu CM, Sun J, Jin WJ, Wu YQ and
Chen J: Repeated 2% sevoflurane administration in 7 and 60-day-old
rats: Neurotoxicity and neurocognitive dysfunction. Anaesthesist.
66:850–857. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seutin VM: Mechanisms of actions of
inhaled anesthetics. N Engl J Med. 349:909–910. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geng Y, Zhou Y, Wu S, Hu Y, Lin K, Wang Y,
Zheng Z and Wu W: Sulforaphane induced apoptosis via promotion of
mitochondrial fusion and ERK1/2-mediated 26S proteasome degradation
of novel pro-survival bim and upregulation of bax in human
non-small cell lung cancer cells. J Cancer. 8:2456–2470. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alonso M, Medina JH and Pozzo-Miller L:
ERK1/2 activation is necessary for BDNF to increase dendritic spine
density in hippocampal CA1 pyramidal neurons. Learn Mem.
11:172–178. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adams JP and Sweatt JD: Molecular
psychology: Roles for the ERK MAP kinase cascade in memory. Annu
Rev Pharmacol Toxicol. 42:135–163. 2002. View Article : Google Scholar : PubMed/NCBI
|