Introduction
Depression is a mental disorder that poses a serious
threat to the physical and mental health of affected patients.
However, its etiology and pathogenesis remain to be fully
elucidated (1). Based on previous
research, certain hypotheses regarding the neurobiological
mechanisms associated with depression have been proposed, including
the central monoamine neurotransmitter dysfunction hypothesis, the
neurotransmitter receptor hypothesis and the neurokinin hypothesis
(2–4). The occurrence of clinical depression
has been reported to be associated with neurobiological defects in
affected patients (5–7). Certain aspects, including whether
various cognitive impairment states may be caused by abnormalities
in localized brain function, and whether these abnormalities differ
between these states, are questions that may now be addressed using
functional molecular imaging techniques.
18F-fluorodeoxyglucose
(18F-FDG) positron emission tomography (PET) is a
commonly used molecular imaging method for studying brain
metabolism (8). 18F-FDG
may be transported into the human brain through the blood-brain
barrier, and subsequently participates in the steps of glucose
metabolism within brain cells (9).
Therefore, 18F-FDG PET imaging may be used analyze the
regional cerebral metabolic rate of glucose uptake (rCMRglc) to
evaluate the activity of neurons (10,11).
Blood oxygen level-dependent functional magnetic resonance imaging
(BOLD-fMRI) has been used to study brain functional disease for
several years (12). Resting-state
BOLD-fMRI is a valuable means of analyzing human brain function
(13). Methods for processing large
quantities of data obtained by BOLD-fMRI have arisen at an
opportune moment (14). Two types,
which are relatively simple and are used most frequently in
clinical applications, are the regional activity characteristic
analysis method and the linear correlation analysis method
(15,16). The first of these methods is
associated with regional homogeneity (ReHo) (16) and amplitude of low-frequency
fluctuation (ALFF) (17), whereas
the latter method is associated with functional connection (FC)
(18). The present study focused on
investigating the regional activity in the brains of patients with
MDD. Since ALFF directly analyzes the oscillation amplitude of the
BOLD signal, its advantage is that it uses all frequency domain
information, but the disadvantage is that it only focuses on
frequency domain information; at the same time, oscillations of
cooperation between different areas in the human brain are always
dependent on time synchronization. By contrast, ReHo reflects each
individual voxel of the whole brain and its 26 neighboring
individual time series of synchronicity, and thus represents a
complete analysis of brain function activities. Therefore, the ReHo
method was used to analyze BOLD data in the present study.
ReHo analysis is one way to analyze BOLD-fMRI data.
It mainly evaluates the level of similarity in changes in the
intensity of BOLD signals in the same time series and consequently,
it indirectly reflects the temporal consistency of regional
neuronal activities. Changes in the ReHo value in a cerebral region
indicate that the regional neuronal activities are not synchronized
with the surrounding cerebral regions in the same time series
(16). Thus, abnormal ReHo values
are associated with abnormality in cerebral regional neural
activity, and ReHo analysis may detect such brain regions with
abnormal activity. In recent years, rCMRglc and ReHo analyses have
been applied with an increasing frequency in studies on various
mental diseases, including MDD (19–21).
Previous studies on patients with depression using
18F-FDG PET or BOLD-fMRI have indicated metabolic and
ReHo abnormalities in certain specific cerebral regions, including
the prefrontal, temporal, cingulate cortex, corpus striatum and
hippocampus (22–24), and consequently, various aspects of
the pathogenesis and neurobiological mechanisms of depression have
been elucidated. A hypothesis that proposed
limbic-cortical-striatal-pallidal-thalamic (LSCPT) neurological
circuits of the brain has also been put forward to uncover the
neuropathological mechanisms of depression (23,25,26). In
previous PET or fMRI studies (27–29),
although cerebral glucose hypometabolism in the prefrontal cortex
has been generally considered to be an important change, the
results remain conflicting concerning certain parts of the brain,
including the anterior cingulate gyrus and the corpus striatum,
which has hindered the attempts to interpret the results with the
aim of providing neurobiological mechanism(s) of depression. Since
the results of different studies may not be directly comparable due
to differences in subjects, devices used and/or the research
conditions, it is important to investigate the brain glucose
metabolism and ReHo in the same group of patients with depression.
Based on the abovementioned hypothesis, the present multimodal
neuroimaging study was performed on a group of untreated patients
with MDD using brain 18F-FDG PET and resting-state
BOLD-fMRI techniques to investigate changes in the cerebral glucose
uptake and ReHo values, and to determine whether any association
existed between the two types of changes, with a group of healthy
control subjects included as a reference. To the best of our
knowledge, no similar studies have been reported previously.
Materials and methods
Participants
Imaging protocols were used to obtain
18F-FDG PET and resting-state BOLD-fMRI scans with a
maximum interval of 3 days between the two scans for 23 untreated
patients with MDD and 18 age- and sex-matched healthy control
subjects. A total of five MDD patients and one healthy subject were
excluded due to excessive movement during BOLD-fMRI scanning;
ultimately, 18F-FDG-PET and resting-state BOLD-fMRI
images from 18 patients with MDD and 17 controls were included in
the quantitative analyses.
MDD patients were recruited from the Zhengzhou
University People's Hospital (Zhengzhou, China) between November
2012 and December 2013. The healthy control subjects were recruited
via advertisements and received reimbursements.
All patients were diagnosed according to the
Diagnostic and Statistical Manual of Mental Disorders IV criteria
(30) by two experienced
psychiatrists with a specialization in MDD. The Hamilton Depression
Rating Scale (HAM-D) (31) was used
to rate the severity of depression and the Hamilton Anxiety Rating
Scale (HAM-A) (32) was used to rate
the severity of anxiety. All patients were diagnosed with MDD for
the first time, and none of them had received any anti-depressant
treatment prior to undergoing the imaging examinations. The
participants were selected using the following criteria: i)
Right-handedness; ii) aged between 18 and 50 years; iii) no history
of neurological illnesses or other serious physical disease; iv) no
history of alcohol or drug dependence; v) an ability and
willingness to cooperate with the experimental procedures; and vi)
written informed consent.
The clinical characteristics of the patients and of
the healthy control subjects are presented in Table I.
 | Table I.Clinical and demographic
characteristics of MDD patients (n=18) and healthy controls
(n=17). |
Table I.
Clinical and demographic
characteristics of MDD patients (n=18) and healthy controls
(n=17).
| Characteristic | MDD group | Healthy
controls | P-value |
|---|
| Age (years) | 32 | 33 | 0.57a |
| Education
(years) | 17 | 18 | 0.53a |
| Sex (male/female,
n) | 6/12 | 6/11 | 0.59b |
| HAM-D | 19 | 5 |
<0.05a |
| HAM-A | 13 | 3 |
<0.05a |
Resting-state BOLD-fMRI
The resting state was defined as no performance of
any prescribed cognitive task during the BOLD-fMRI scan (33). Participants were instructed to simply
remain motionless, keep their eyes closed and not to think of
anything in particular.
Image acquisition
MRIs were acquired with a GE Discovery MR 750
scanner (GE Healthcare, Little Chalfont, UK). A three-dimensional
fast spoiled gradient-echo sequence was employed [repetition time
(TR), 8.2 msec; echo time (TE), 3.2 msec; inversion time (TI), 450
msec; slice thickness, 1 mm; number of slices, 156; image matrix,
256×256; field of view (FOV), 24 × 24 cm] and a resting BOLD
sequence (TR, 2,000 msec; TI, 450 msec; TE, 30 msec; slice
thickness, 4 mm; number of slices, 32; image matrix, 64×64; FOV,
24×24 cm).
18F-FDG PET images were obtained using a
GE Discovery VCT PET-CT set (GE Healthcare). 18F-FDG was
performed using a GE MINI trace medical cyclotron (GE Healthcare)
and an FDG automatic synthesis device. Subsequently, quality
assurance tests were performed. Prior to the examination, all
patients were required to fast for at least 6 h, and the fasting
blood glucose levels of the patients were >6.1 mmol/l.
18F-FDG was injected in an intravenous bolus; the dose
was 5.55 MBq/kg. Following the injection, each subject remained in
a resting state in a quiet environment for a 50-min uptake period.
The brain acquisition time of each patient was 40 min. Brain PET-CT
scanning parameters were as follows: Voltage, 120 kV; current, 240
mA; and thickness, 5 mm. An acquisition counter using an iterative
method was used to reconstruct the transverse, sagittal and coronal
images.
Image pre-processing
Statistical Parametric Mapping (SPM8; Wellcome
Department of Imaging Neuroscience, London, UK) was used to
complete the image pre-processing. Due to the instability of the
initial MRI signals, the first 10 volumes of each functional time
series were rejected, leaving 200 volumes. The remaining fMRI
images were converted into the Analyze7 format. Subsequently, the
head motion and slice acquisition of the converted images were
corrected. All images exhibited a maximum displacement of <2 mm
in the x, y or z direction and <1° of angular motion during the
whole fMRI scan. The fMRI images were subsequently normalized to
the standard SPM8 template of echo planar imaging, and then
spatially smoothed with a Gaussian kernel of 2×2×2 mm3
[full-width half maximum (FWHM)].
ReHo analysis
ReHo analysis (10)
was performed by computing Kendall's coefficient of concordance
(KCC) of the time series of a given voxel with those of its nearest
neighbors (26 voxels) on a voxel-wise basis. The KCC was calculated
according to the following formula:
W={Ʃ(Ri)2-n(R)2}/(1/12)K2(n3-n)
and R=[(n+1)K]/2, with W as the KCC among given voxels, ranging
from 0 to 1; Ri as the sum rank of the ith time-point; R as the
mean of Ri values; K as the number of the time series within a
measured cluster (K=27, one given voxel plus the number of its
neighbors); and n as the number of ranks (n=200). The program for
computing the KCC was coded in MATLAB 8.0 (MathWorks Inc., Natick,
MA, USA).
PET data analysis
Brain PET images were converted into the Analyze7
format using SPM8 (5). Motion
correction was applied to the converted images. Subsequently, the
images were spatially normalized to standard anatomical space
(again conforming to the standard Talairach template used in SPM8)
to allow for inter-participant averaging and comparison.
Transformed images were then smoothed with a Gaussian kernel of
4×4×4 mm3 (FWHM) to eliminate the influence of
physiological noise.
Statistical analysis
Two-sample t-test of voxel-based statistical
analyses on the cerebral 18F-FDG PET images was
performed for comparing between the patient and control group. The
resulting statistical map was set at a combined threshold of
corrected P<0.05 and a minimum cluster size of 10 voxels.
To explore the ReHo difference between MDD patients
and controls, a second-level two-sample t-test was performed for
the individual ReHo maps in a voxel-by-voxel manner comparing
patients with MDD with the control group. The resulting statistical
map was set at a combined threshold of a corrected P<0.05 and a
minimum cluster size of 10 voxels (34).
The comparison of ReHo and FDG uptake was made using
SPSS v18.0 (SPSS Inc., Chicago, IL, USA). Chi-square analysis was
used to assess the association of activated brain regions in MDD
patients determined by PET and fMRI. Pearson correlation analysis
was applied to analyze the correlation between the standardized
uptake value (SUV) and the ReHo of the abnormal regions of the
patients with MDD. P<0.05 was considered to indicate a
statistically significant difference.
Results
Result of HAM-D and HAM-A
The HAM-D and HAM-A scores of the 18 patients with
MDD were all >17 and >7, respectively, while, at the same
time, the two types of score were <7 in all of the control
subjects (Table I). The inter-group
differences in the HAM-D and HAM-A scores were statistically
significant.
Result of 18F-FDG PET
Compared with the control subjects, the 18 MDD
patients had a decreased glucose uptake on brain 18F-FDG
PET (determined as rCMRglc values) in the bilateral superior, the
middle and the inferior frontal gyrus, in the bilateral superior
and middle temporal gyrus, in the bilateral anterior cingulate
cortex, in the bilateral putamen and caudate, and in the left
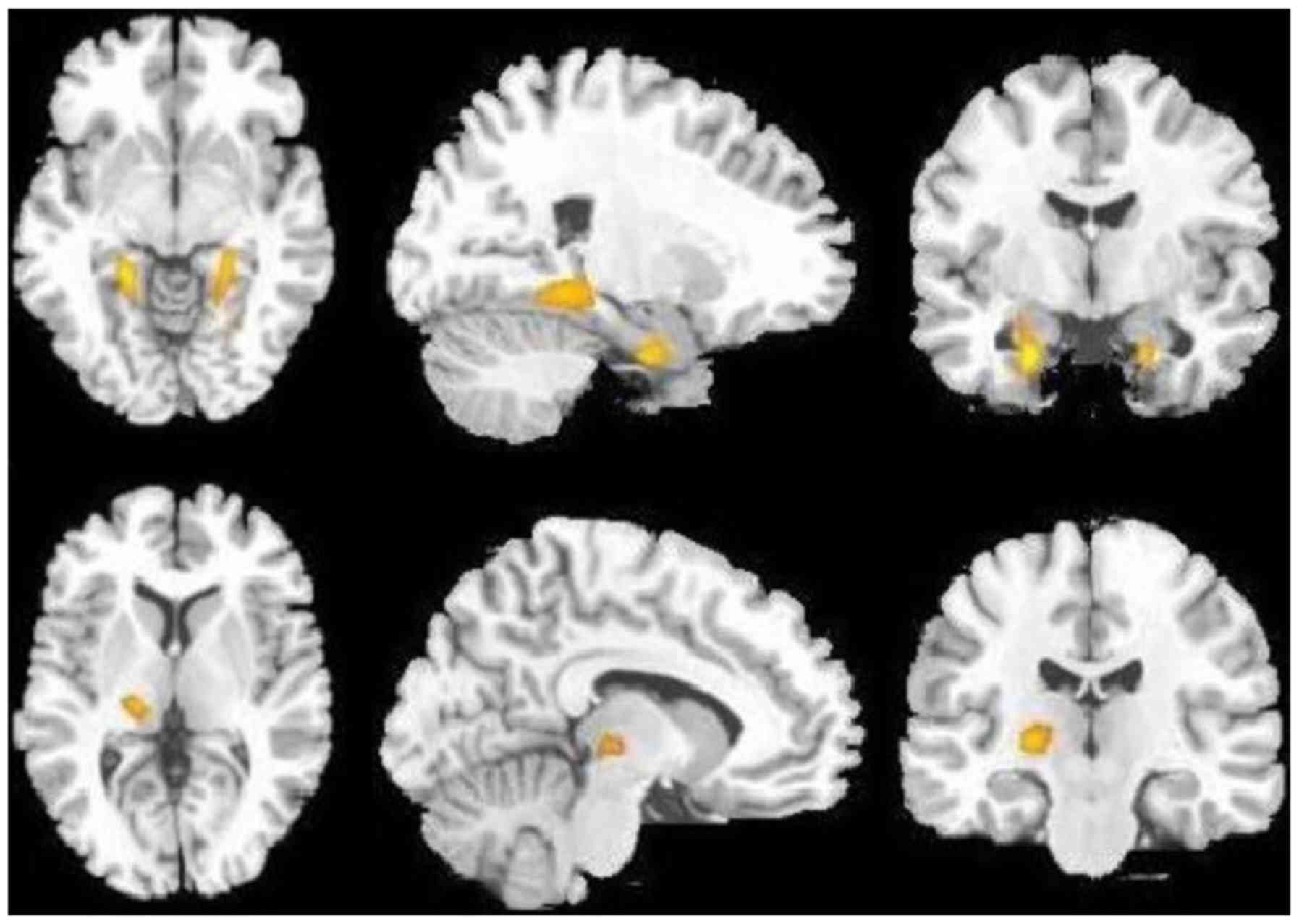
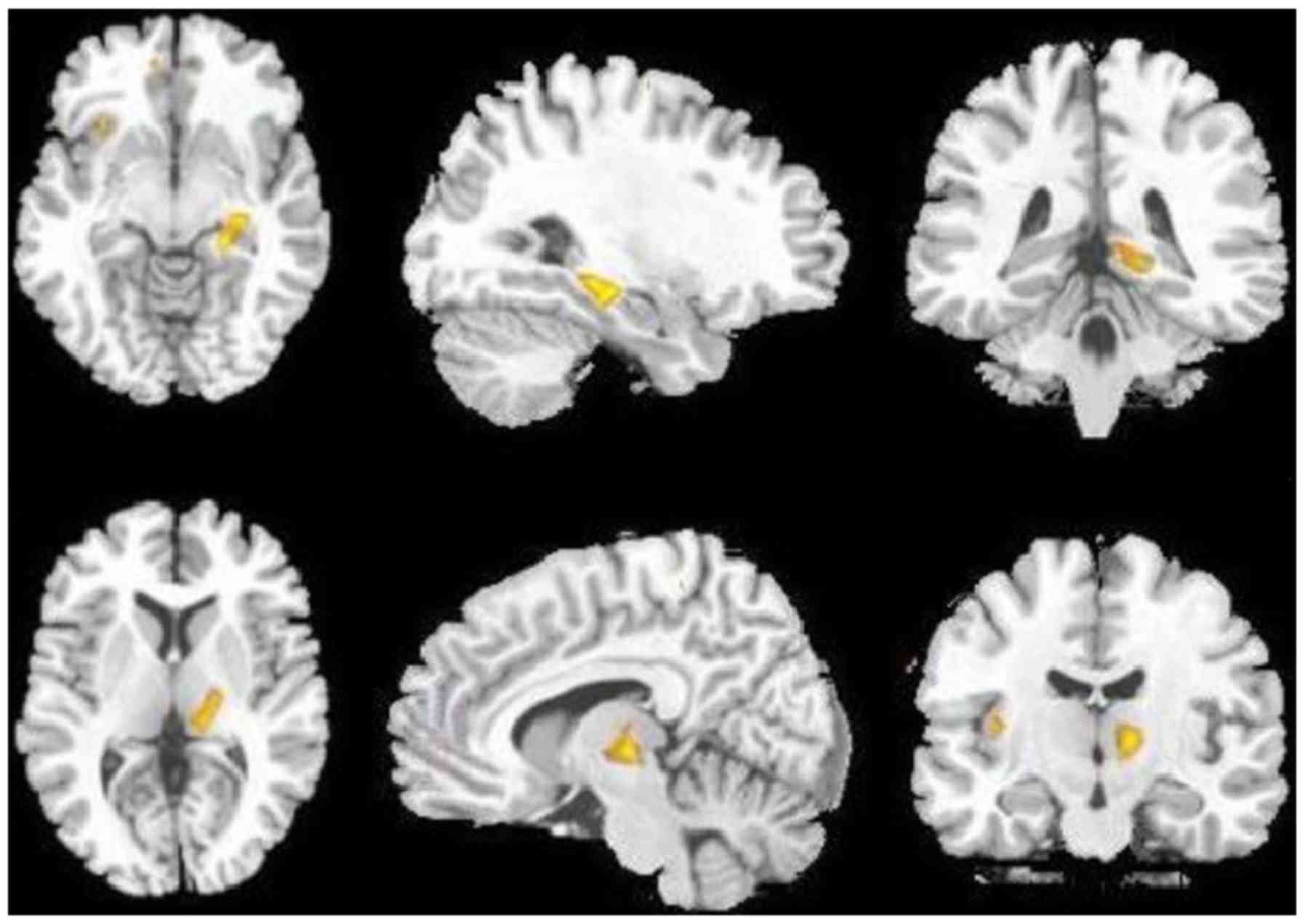
globus pallidus (Fig. 1; Table II), but an increased glucose uptake
in the bilateral hippocampus and left thalamus (Fig. 2; Table
II).
 | Table II.Cerebral regions with abnormal
changes and correlation analysis of abnormal changes between
positron emission tomography and functional magnetic resonance
imaging in patients with major depressive disorder. |
Table II.
Cerebral regions with abnormal
changes and correlation analysis of abnormal changes between
positron emission tomography and functional magnetic resonance
imaging in patients with major depressive disorder.
| Anatomical
area | rCMRglc
changes | ReHo changes | r | P-value |
|---|
| Frontal gyrus |
|
|
|
|
| Left
superior frontal gyrus | Decreased | Decreased | 0.52 | 0.04 |
| Left
middle frontal gyrus | Decreased | Decreased | 0.64 | <0.01 |
| Left
inferior frontal gyrus | Decreased | None | 0.51 | 0.04 |
| Right
superior frontal gyrus | Decreased | Decreased | 0.63 | 0.01 |
| Right
middle frontal gyrus | Decreased | Decreased | 0.57 | 0.03 |
| Right
inferior frontal gyrus | Decreased | None | 0.59 | 0.02 |
| Temporal gyrus |
|
|
|
|
| Left
superior temporal gyrus | Decreased | None | 0.52 | 0.04 |
| Left
middle temporal gyrus | Decreased | None | 0.57 | 0.03 |
| Right
superior temporal gyrus | Decreased | None | 0.62 | 0.01 |
| Right
middle temporal gyrus | Decreased | None | 0.59 | 0.02 |
| Basal ganglia |
|
|
|
|
| Left
putamen | Decreased | Decreased | 0.63 | 0.01 |
| Right
putamen | Decreased | Decreased | 0.68 | <0.01 |
| Left
caudate | Decreased | None | 0.61 | 0.01 |
| Right
caudate | Decreased | None | 0.41 | 0.12 |
| Left
globus pallidus | Decreased | Decreased | 0.83 | <0.01 |
| Cingulate
cortex |
|
|
|
|
| Left
anterior cingulate cortex | Decreased | Decreased | 0.78 | <0.01 |
| Right
anterior cingulate cortex | Decreased | None | 0.37 | 0.16 |
| Hippocampus |
|
|
|
|
| Left
hippocampus | Increased | None | 0.71 | <0.01 |
| Right
hippocampus | Increased | Increased | 0.74 | <0.01 |
| Thalamus |
|
|
|
|
| Left
thalamus | Increased | None | 0.64 | <0.01 |
| Right
thalamus | None | Increased | 0.62 | 0.01 |
Result of fMRI
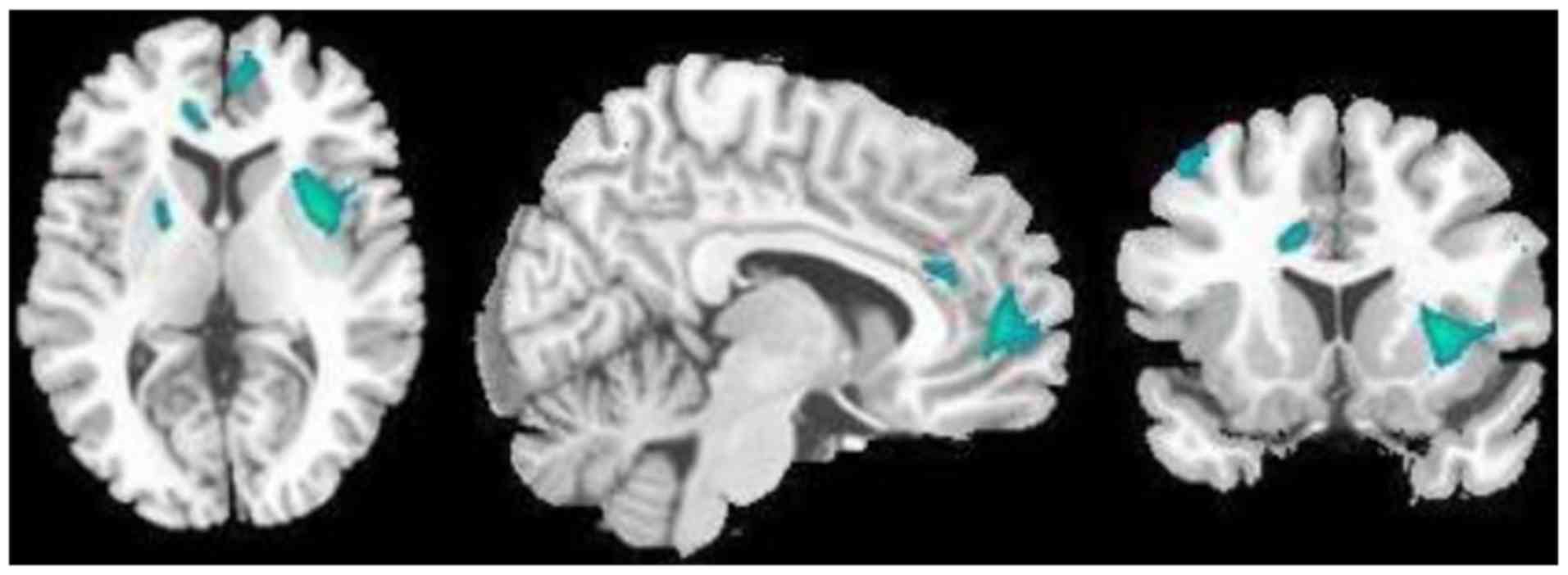
Furthermore, in the 18 patients with MDD, the ReHo
values from the resting-state BOLD-fMRI were decreased in the
bilateral superior and middle frontal gyrus, in the left globus
pallidus, the bilateral putamen and the left anterior cingulate
cortex (Fig. 3; Table II), but increased in the right
hippocampus and thalamus (Fig. 4;
Table II).
Relation between 18F-FDG
PET and fMRI
No obvious statistically significant differences
were identified between the reduced metabolism and ReHo brain
regions of MDD patients (χ2=9.16; P=0.90) and between
the increased metabolism and ReHo brain regions
(χ2=3.96; P=0.27), when comparing the activated brain
regions of PET and MRI. The SUV of the bilateral superior, middle
and inferior frontal gyrus, bilateral superior and middle temporal
gyrus, bilateral putamen, the left caudate and pallidum, the left
anterior cingulate cortex and the bilateral hippocampus and
thalamus were correlated with the ReHo values (r=0.51–0.83;
P<0.05); however, no correlation was detected between the SUV
and ReHo values in the right caudate and anterior cingulate cortex
(r=0.41 and 0.37, respectively; P>0.05; Table II).
Discussion
18F-FDG PET images are able to reflect
cerebral activity by providing information on the uptake of
metabolites. When the cerebral activity becomes weak, the uptake
decreases, and hypometabolism is revealed in the PET images.
However, when the cerebral activity is stronger, hypermetabolism
may be observed using PET. fMRI revealed a consistency of regional
neuronal activities, as indicated by the ReHo of the BOLD signal.
It evaluated the level of similarity in intensity changes of BOLD
signals and indirectly reflected regional neuronal activities. A
decreased ReHo indicates poor consistency of neuronal activities,
which describes regional neuronal activities not synchronizing with
the surrounding cerebral regions and indirectly reflecting
decreased cerebral function. Conversely, an increased ReHo
indicates good consistency of neuronal activities, describing
synchronized regional neuronal activities with the surrounding
cerebral regions and indirectly reflecting hyperfunction. The two
types of imaging methods employed in the present study are able to
reflect the cerebral function and activity from different aspects.
It is of great benefit for the advancement of research into the
pathogenesis of MDD to study changes of cerebral metabolic activity
and function in affected patients. Certain previous studies
indicated that MDD patients have metabolic or functional
abnormalities in part of the cerebral cortex and the limbic system,
and they may exhibit a reasonably characteristic pattern of
cerebral damage (25,35). However, the results derived from
those studies were almost entirely based on PET or fMRI scans of
separate subjects, thereby making it difficult to elucidate the
pathogenesis of MDD from them. The present study was therefore
performed with the reasoning that if PET and fMRI were to be used
in combination to scan the same group of untreated MDD patients,
more effective image data may be acquired in order to explore the
etiology and pathological mechanisms of MDD. The present study
demonstrated that several cerebral regions exhibited abnormal
glucose metabolism and ReHo, and the abnormal cerebral regions were
mainly distributed in parts including the cerebral cortex, limbic
system and the thalamus. The results of the present study supported
the hypothesis of abnormal LSCPT neurological circuits in patients
with depression (36,37). The current study demonstrated that
there are abnormal changes of PET and fMRI in some cerebral regions
of LSCPT in patients with MDD, including in the temporal lobe,
cingulate gyrus and hippocampus. The abnormal changes in the
cerebral cortex of patients with MDD included hypometabolism and
hypofunction in certain regions of the frontal and temporal lobe,
while hypometabolism was also identified in the bilateral superior,
the middle and inferior frontal gyrus, and decreased ReHo values
were observed in the bilateral superior and middle frontal gyrus,
with a high correlation existing between them. The frontal lobe
serves an important role in attention, perception, planning
ability, sustainable behavior, working memory and executive
function. Abnormalities in this area may be the most important
results with respect to depression in patients. Changes in frontal
lobe function are likely to provide the basis of depression, and
also to be closely associated with the symptoms of clinical MDD
(38). The present study confirmed
the above point of view based on the analysis of glucose metabolism
and ReHo. Previously published studies that respectively used
BOLD-fMRI or FDG-PET in depressed patients identified decreases in
ReHo values and glucose uptake in the temporal lobe (39,40). In
the present study, it was revealed that glucose uptake in the
bilateral middle and anterior temporal gyrus was decreased,
although no reduction in the ReHo value was observed in this
region; however, a correlation did exist between them. The anterior
cingulate cortex and corpus striatum are the major components of
the limbic system that exert an important role in encoding episodic
memory, emotional processing and cognizance. In theory, the
cerebral function of MDD patients in the striatum and the anterior
cingulate gyrus is expected to decline; however, in previously
published studies, numerous inconsistencies and uncertainties have
arisen. Kennedy et al (38)
identified that the glucose metabolism decreased in the ventral
striatum (caudate nucleus and putamen), but increased in the right
pregenual anterior cingulate cortex in a group of depressed
patients. However, a study by Mayberg et al (41) also demonstrated hypermetabolism in
the putamen-pallidum in a subset of depressed patients. Kimbrell
et al (42) reported that
glucose metabolism in the subgenual anterior cingulate cortex was
reduced, and the extent of reduction was positively correlated with
the severity of depression. A study by De Asis et al
(36) indicated an increased level
of metabolism in the anterior cingulate cortex. Another fMRI study
reported decreased ReHo values of the striatum and cingulate
cortex, and a good correlation was identified between them
(35). The rCMRglc and ReHo values
of the striatum and cingulate cortex were all decreased in the
present study. The striatum regions where hypometabolism was
identified were distributed in the bilateral lenticular nucleus,
the caudate nucleus and the left pallidum, with a decreased ReHo
value identified in the bilateral lenticular nucleus. This result
further supports the hypothesis that the limbic system, including
the striatum and cingulate gyrus, becomes dysfunctional under
conditions of depression. In the present study on patients with
MDD, thalamic and hippocampal metabolism and the ReHo values were
increased, which is consistent with the results of a previous study
(39), and is also consistent with
the clinical manifestations of increased cerebral function. Other
studies indicated that the cerebral function was abnormal in the
amygdale of MDD patients (43,44), but
in the present study, no abnormal changes in the bilateral amygdale
were identified in MDD patients. Furthermore, certain abnormalities
of the limbic system, including the temporal lobe, hippocampus and
cingulated gyrus were identified, which may support that depression
is associated with the impairment of nerve cell function in the
limbic system.
In the present study, the results regarding abnormal
cerebrum were largely consistent between the two methods. The
characteristics of these changes in MDD patients were not only
consistent with the hypothesis of LSCPT neurological circuits but
also clarify that brain activities in the anterior cingulated
cortex and corpus striatum are reduced. These results suggest that
the two imaging techniques are reliable and the evidence from PET
and fMRI should be convincing. Another possibly important result
was the difference in the extent of the abnormal cerebral regions
identified by PET and fMRI: The abnormal cerebral regions on brain
PET were obviously larger than those on fMRI in the MDD patients of
the present study. As mentioned above, in certain regions,
including the bilateral inferior frontal and anterior medial
temporal gyrus, the bilateral caudate nucleus, the left pallidus
and the right anterior cingulate gyrus, only a decrease in glucose
metabolism was demonstrated with no reduced ReHo. These results
also indicate that cerebral metabolic abnormalities in MDD patients
may occur earlier than ReHo abnormalities, or that
18F-FDG PET may be more sensitive in detecting brain
lesions of MDD patients than BOLD-fMRI. These differences were not
only observed in MDD patients, but also in patients with numerous
other neurological diseases, including dementia, Alzheimer's
disease and seizures (11,45,46).
Although the results of the present study are
relatively solid due to the use of a multi-mode imaging method,
age- and sex-matched control subjects as a reference, normalized
data processing regarding regional metabolism and regional
homogeneity, the present study has several limitations. First,
there was a lack of images for patients with MDD before and after
treatment to perform a comparative study. The collection of the
post-medication data of these patients is underway. Furthermore,
the present study did not perform any comparison between the
imaging and clinical results of the patients. Finally, the age and
sex of the patients were not fully considered in the present study.
These are all areas of future study.
In conclusion, the multimode imaging technique using
18F-FDG PET and resting-state BOLD-fMRI is valuable for
investigating brain lesions in MDD patients. MDD patients have
relatively characteristic modes of abnormal brain glucose
metabolism and regional neuronal activity, which supports the
theory of LSCPT neurological circuits. Furthermore,
18F-FDG PET may be more sensitive in detecting brain
lesions of MDD patients than BOLD-fMRI.
Acknowledgements
The content of this study has been previously
presented as a poster (47; abstract poster no. 2241) at a Society
of Nuclear Medicine and Molecular Imaging conference in June 2017
held in Denver, CO, USA.
Funding
The present study was supported by the Henan
Provincial Medical Science and Technology Planning Project (grant
no. 201701016).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
CF conceived and designed the experiments, and wrote
the manuscript. HZ contributed to collecting clinical samples and
analysis. AX and YG performed the acquisition and analysis of data.
JX contributed to the analysis of the data. DS contributed to
interpreting the results and revising the manuscript. All authors
read and approved the manuscript.
Ethical approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhengzhou University People's Hospital (Zhengzhou,
China). All subjects provided informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Knutson B, Bhanji JP, Cooney RE, Atlas LY
and Gotlib IH: Neural responses to monetary incentives in major
depression. Biol Psychiatry. 63:686–692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maes M, Leonard BE, Myint AM, Kubera M and
Verkerk R: The new ‘5-HT’ hypothesis of depression: Cell-mediated
immune activation induces indoleamine 2, 3-dioxygenase, which leads
to lower plasma tryptophan and an increased synthesis of
detrimental tryptophan catabolites (TRYCATs), both of which
contribute to the onset of depression. Prog Neuropsychopharmacol
Biol Psychiatr. 35:702–721. 2011. View Article : Google Scholar
|
|
3
|
López-Figueroa AL, Norton CS,
López-Figueroa MO, Armellini-Dodel D, Burke S, Akil H, López JF and
Watson SJ: Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA
expression in subjects with major depression, bipolar disorder, and
schizophrenia. Biol Psychiatry. 55:225–233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Binder EB, Salyakina D, Lichtner P,
Wochnik GM, Ising M, Pütz B, Papiol S, Seaman S, Lucae S, Kohli M,
et al: Polymorphisms in FKBP5 are associated with increased
recurrence of depressive episodes and rapid response to
antidepressant treatment. Nat Genet. 36:13192004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lorenzetti V, Allen NB, Fornito A and
Yücel M: Structural brain abnormalities in major depressive
disorder: A selective review of recent MRI studies. J Affect
Disord. 117:1–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lui S, Parkes LM, Huang X, Zou K, Chan RC,
Yang H, Zou L, Li D, Tang H, Zhang T, et al: Depressive disorders:
Focally altered cerebral perfusion measured with arterial
spin-labeling MR imaging. Radiology. 251:476–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim MJ, Hamilton JP and Gotlib LH: Reduced
caudate gray matter volume in women with major depressive disorder.
Psychiatry Res. 164:114–122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Videbech P: PET measurements of brain
glucose metabolism and blood flow in major depressive disorder: A
critical review. Acta Psychiatr Scand. 101:11–20. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mankoff DA, Shields AF and Krohn KA: PET
imaging of cellular proliferation. Radiol Clin North Am.
43:153–167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verger A, Roman S, Chaudat RM, Felician O,
Ceccaldi M, Didic M and Guedj E: Changes of metabolism and
functional connectivity in late-onset deafness: Evidence from
cerebral 18F-FDG-PET. Hear Res. 353:8–16. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Staffaroni AM, Melrose RJ, Leskin LP,
Riskin-Jones H, Harwood D, Mandelkern M and Sultzer DL: The
functional neuroanatomy of verbal memory in Alzheimer's disease:
[18F]-Fluoro-2-deoxy-D-glucose positron emission
tomography (FDG-PET) correlates of recency and recognition memory.
J Clin Exp Neuropsychol. 39:682–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ogawa S, Lee TM, Nayak AS and Glynn P:
Oxygenation-sensitive contrast in magnetic resonance image of
rodent brain at high magnetic fields. Magn Reson Med. 14:68–78.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biswal B, Yetkin FZ, Haughton VM and Hyde
JS: Functional connectivity in the motor cortex of resting human
brain using echo-planar MRI. Magn Reson Med. 34:537–541. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Damoiseaux JS, Rombouts SA, Barkhof F,
Scheltens P, Stam CJ, Smith SM and Beckmann CF: Consistent
resting-state networks across healthy subjects. Proc Natl Acad Sci
USA. 103:13848–13853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fox MD and Raichle ME: Spontaneous
fluctuations in brain activity observed with functional magnetic
resonance imaging. Nat Rev Neurosci. 8:700–711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zang Y, Jiang T, Lu Y, He Y and Tian L:
Regional homogeneity approach to fMRI data analysis. Neuroimage.
22:394–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu R, Chien YL, Wang HL, Liu CM, Liu CC,
Hsieh MH, Hwu HG and Tseng WY: Frequency-specific alternations in
the amplitude of low-frequency fluctuations in schizophrenia. Hum
Brain Mapp. 35:627–637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Kwaasteniet B, Ruhe E, Caan M, Rive M,
Olabarriaga S, Groefsema M, Heesink L, van Wingen G and Denys D:
Relation between structural and functional connectivity in major
depressive disorder. Biol Psychiatry. 74:40–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Wang K, Yu C, He Y, Zhou Y, Liang
M, Wang L and Jiang T: Regional homogeneity, functional
connectivity and imaging markers of Alzheimer's disease: A review
of resting-state fMRI studies. Neuropsychologia. 46:1648–1656.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoptman MJ, Zuo XN, Butler PD, Javitt DC,
D'Angelo D, Mauro CJ and Milham MP: Amplitude of low-frequency
oscillations in schizophrenia: A resting state fMRI study.
Schizophr Res. 117:13–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Towgood KJ, Pitkanen M, Kulasegaram R,
Fradera A, Soni S, Sibtain N, Reed LJ, Bradbeer C, Barker GJ, Dunn
JT, et al: Regional cerebral blood flow and FDG uptake in
asymptomatic HIV-1 men. Hum Brain Mapp. 34:2484–2493. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Xu C, Xu Y, Wang Y, Zhao B, Lv Y,
Cao X, Zhang K and Du C: Decreased regional homogeneity in insula
and cerebellum: A resting-state fMRI study in patients with major
depression and subjects at high risk for major depression.
Psychiatry Res. 182:211–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fitzgerald PB, Laird AR, Maller J and
Daskalakis ZJ: A meta-analytic study of changes in brain activation
in depression. Hum Brain Mapp. 29:683–695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo WB, Sun XL, Liu L, Xu Q, Wu RR, Liu
ZN, Tan CL, Chen HF and Zhao JP: Disrupted regional homogeneity in
treatment-resistant depression: A resting-state fMRI study. Prog
Neuropsychopharmacol Biol Psychiatry. 35:1297–1302. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujimoto T, Takeuchi K, Matsumoto T,
Fujita S, Honda K, Higashi Y and Kato N: Metabolic changes in the
brain of patients with late-onset major depression. Psychiatry Res.
164:48–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee HS, Choo IH, Lee DY, Kim JW, Seo EH,
Kim SG, Park SY, Shin JH, Kim KW and Woo JI: Frontal dysfunction
underlies depression in mild cognitive impairment: A FDG-PET study.
Psychiatry Investig. 7:208–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamilton JP, Etkin A, Furman DJ, Lemus MG,
Johnson RF and Gotlib IH: Functional neuroimaging of major
depressive disorder: A meta-analysis and new integration of base
line activation and neural response data. Am J Psychiatry.
169:693–703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen S, Wu X, Lui S, Wu Q, Yao Z, Li Q,
Liang D, An D, Zhang X, Fang J, et al: Resting-state fMRI study of
treatment-naive temporal lobe epilepsy patients with depressive
symptoms. Neuroimage. 60:299–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hamilton JP, Chen G, Thomason ME, Schwartz
ME and Gotlib IH: Investigating neural primacy in major depressive
disorder: Multivariate Granger causality analysis of resting-state
fMRI time-series data. Mol Psychiatry. 16:763–772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kendler KS and Gardner CO Jr: Boundaries
of major depression: An evaluation of DSM-IV criteria. Am J
Psychiatry. 155:172–177. 1998.PubMed/NCBI
|
|
31
|
Zimmerman M, Martinez JH, Young D,
Chelminski I and Dalrymple K: Severity classification on the
Hamilton depression rating scale. J Affect Disord. 150:384–388.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kummer A, Cardoso F and Teixeira AL:
Generalized anxiety disorder and the Hamilton Anxiety Rating Scale
in Parkinson's disease. Arq Neuropsiquiatr. 68:495–501. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fox MD and Greicius M: Clinical
applications of resting state functional connectivity. Front Syst
Neurosci. 4:192010.PubMed/NCBI
|
|
34
|
Wu T, Long X, Zang Y, Wang L, Hallett M,
Li K and Chan P: Regional homogeneity changes in patients with
Parkinson's disease. Human brain mapping. 30:1502–1510. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yao Z, Wang L, Lu Q, Liu H and Teng G:
Regional homogeneity in depression and its relationship with
separate depressive symptom clusters: A resting-state fMRI study.
Journal of Affective Disorders. 115:430–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Asis JM, Silbersweig DA, Pan H, Young
RC and Stern E: Neuroimaging studies of fronto-limbic dysfunction
in geriatric depression. Clin Neurosci Res. 2:324–330. 2003.
View Article : Google Scholar
|
|
37
|
Ketter TA, George MS, Kimbrell TA, Benson
BE and Post RM: Functional brain imaging, limbic function, and
affective disorders. Neuroscientist. 2:55–65. 1996. View Article : Google Scholar
|
|
38
|
Kennedy SH, Evans KR, Krüger S, Mayberg
HS, Meyer JH, McCann S, Arifuzzman AI, Houle S and Vaccarino FJ:
Changes in regional brain glucose metabolism measured with positron
emission tomography after paroxetine treatment of major depression.
Am J Psychiatry. 158:899–905. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu JC, Gillin JC, Buchsbaum MS, Schachat
C, Darnall LA, Keator DB, Fallon JH and Bunney WE: Sleep
deprivation PET correlations of Hamilton symptom improvement
ratings with changes in relative glucose metabolism in patients
with depression. J Affect Disord. 107:181–186. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Germain A, Nofzinger EA, Meltzer CC, Wood
A, Kupfer DJ, Moore RY and Buysse DJ: Diurnal variation in regional
brain glucose metabolism in depression. Biol Psychiatry.
62:438–445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mayberg HS, Brannan SK, Tekell JL, Silva
JA, Mahurin RK, McGinnis S and Jerabek PA: Regional metabolic
effects of fluoxetine in major depression: Serial changes and
relationship to clinical response. Biol Psychiatry. 48:830–843.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kimbrell TA, Ketter TA, George MS, Little
JT, Benson BE, Willis MW, Herscovitch P and Post RM: Regional
cerebral glucose utilization in patients with a range of severities
of unipolar depression. Biol Psychiatry. 51:237–252. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Drevets WC: Neuroimaging abnormalities in
the amygdala in mood disorders. Ann N Y Acad Sci. 985:420–444.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abercrombie HC, Schaefer SM, Larson CL,
Oakes TR, Lindgren KA, Holden JE, Perlman SB, Turski PA, Krahn DD,
Benca RM and Davidson RJ: Metabolic rate in the right amygdala
predicts negative affect in depressed patients. Neuroreport.
9:3301–3307. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weyts K, Vernooij M, Steketee R, Valkema R
and Smits M: Qualitative agreement and diagnostic performance of
arterial spin labelling MRI and FDG PET-CT in suspected early-stage
dementia: Comparison of arterial spin labelling MRI and FDG PET-CT
in suspected dementia. Clin Imaging. 45:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kamm J, Ponto Boles LL, Manzel K,
Gaasedelen OJ, Nagahama Y, Abel T and Tranel D: Temporal lobe
asymmetry in FDG-PET uptake predicts neuropsychological and seizure
outcomes after temporal lobectomy. Epilepsy Behav. 78:62–67. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fu C: Regional homogeneity and FDG uptake
in patients with major depressive disorder. J Nucl Med.
58:12942017.
|