Introduction
With the development of modern society and changes
in people's life style and diet structure, incidence of diabetes
has been increased significantly during the last several decades
(1). Besides diabetes itself,
complications of this disease also seriously affects human health
(2). As one of the common
complications of diabetes, diabetic retinopathy, which is caused by
the long-term hyperglycemia, is the most serious eye disease that
may eventually lead to blindness (3). It has been reported that more than 80%
of diabetic patients with a course of disease longer than 20 years
will develop diabetic retinopathy (4). Although disease conditions in almost
90% of patients with diabetic retinopathy can been improved with
proper treatment, blindness will happen in the remaining 10% due to
unexplained reasons (5). Therefore,
the identification of novel treatment targets for diabetic
retinopathy is always needed to improve treatment outcomes of this
disease.
Long non-coding RNA (lncRNA) refers to a group of
RNA transcripts composed of more than 200 nucleotides that will not
be translated into protein products (6). Since its discovery, lncRNAs have been
proved to play pivotal roles in almost all important physiological
processes in animal, plant and the human body (7). Besides that, lncRNAs also participate
in the progression of various pathological conditions, including
the development of human diseases, such as cancer (8), and heart diseases (9). Studies in last serval years also showed
that the development of diabetic retinopathy also requires the
participation of lncRNAs (10). As a
tumor suppressor gene, functionality of lncRNA maternally expressed
gene 3 (MEG3) has been widely studied in different types of human
cancer (11,12). It has been reported that lncRNA MEG3
can suppress neovascularization, which is a key step of the
development of diabetic retinopathy, indicating the potential
inhibitory effects of MEG3 on diabetic retinopathy. However,
functionality of MEG3 in diabetic retinopathy remains unclear.
In this study, serum levels of MEG3 in patients with
diabetic retinopathy, diabetic patients without retinopathy as well
as healthy people were measured. Interactions between MEG3, and
vascular endothelial growth factor (VEGF) and transforming growth
factor-β1 (TGF-β1), which are two key players in diabetic
retinopathy were explored. There report is as follow:
Materials and methods
Patients
A total of 33 patients with diabetic retinopathy
were selected in Affiliated Hospital of Beihua University (Jilin,
China) from January 2014 to January 2017. All those patients were
diagnosed according to the diagnostic criteria established by
Chinese Medical Association in 2014. Patients with other types of
retinopathy were excluded. Those patients included 15 males and 17
females, and age ranged from 44 to 71 years, with an average age of
56±11.3 years. During the same time period, 28 diabetic patients
without retinopathy were also selected. Those diabetic patients
included 12 males and 16 females, and age ranged from 39 to 77
years, with an average age of 53±14.1 years. At the same time, 30
healthy people were selected as control group. Control group
included 11 males and 19 females, and the age ranged from 40 to 72
years, with an average age of 54±13.7 years. No significant
differences in age and gender were found among three groups. This
study was approved by the Ethics Committee of Affiliated Hospital
of Beihua University. All patients signed informed consent.
Preparation of serum samples
Fasting blood (20 ml) was extracted from each
participant in the morning. Blood samples were kept at room
temperature for 1 h, followed by centrifugation at 2,500 × g for 20
min to collect serum samples. Serum samples were stored at −80°C
before use.
ELISA
Serum levels of VEGF and TGF-β1 were detected using
corresponding ELISA kit provided by (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) according to the instructions.
Cell line and cell culture
Human retinal pigment epithelial cell line ARPE-19
was provided by American Type Culture Collection (Manassas, VA,
USA). ARPE-19 cells were cultured under conditions recommended by
ATCC. Cells were harvested at logarithmic growth phase.
Establishment of MEG3 overexpression
cell line
MEG3 cDNA (V0728, GeneCopoeia) was inserted into
pIRSE2-EGFP vector (Clontech Laboratories, Inc., Mountainview, CA,
USA) to make MEG3 expression vector. Cells were cultured overnight
to reach 80–90% confluent, and transfection was performed using
Lipofectamine 2000 reagent (11668-019; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from serum and in
vitro cultured cells using Trizol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). RNA quality was checked using NanoDrop™
2000 Spectrophotometers (Thermo Fisher Scientific, USA), and the
ones with a A260/A280 ratio between 1.8 and 2.0 were used in
reverse transcription to synthesize cDNA. Following primers were
used in PCR reactions: 5′-GCATTAAGCCCTGACCTTTG-3′ (forward) and
5′-TCCAGTTTGCTAGCAGGTGA-3′ (reverse) for MEG3;
5′-AAATGCTTTCTCCGCTCTGA-3′ (forward) and 5′-CCCACTGAGGAGTCCAACAT-3′
(reverse) for VEGF; 5′-CCCAGCATCTGCAAAGCTC-3′ (forward) and
5′-GTCAATGTACAGCTGCCGCA-3′ (reverse) for TGF-β1;
GACCTCTATGCCAACACAGT (forward) and AGTACTTGCGCTCAGGAGGA (reverse)
for β-actin. PCR reaction conditions were as follow: 95°C for 40
sec, followed by 40 cycles of 95°C for 15 sec and 60°C for 45 sec.
Obtained data were processed using 2−ΔΔCq method, and
expression of each gene was normalized to endougenous control
β-actin.
Western blot analysis
Total protein extraction from in vitro
cultured cells was performed using cell lysis buffer (clontech,
USA). Protein samples were quantified using BCA. Then 10% SDS-PAGE
gel electrophoresis was carried out using 20 µg of protein from
each sample. After gel transfer, PVDF membranes were blocked with
5% skimmed milk at room temperature for 1 h. After washing,
membranes were incubated with primary antibodies including rabbit
anti-VEGF antibody (1:2,000, ab46154; Abcam, Cambridge, UK),
anti-TGF-β1 antibody (1:2,000, ab9758; Abcam), and anti-GAPDH
antibody (1:1,000, ab9485; Abcam) overnight at 4°C. After washing,
membranes were further incubated with anti-rabbit IgG-HRP secondary
antibody (1:1,000, MBS435036; MyBioSource, Inc., San Diego, CA,
USA) at room temperature for 1 h. After washing, signal detection
was performed by ECL (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) method. Image J software was used to normalize relative
expression level of each protein to GAPDH.
Statistical analysis
SPSS19.0 (SPSS, Inc., Chicago, IL, USA) was used.
Normal distribution data were expressed as mean ± standard
deviation), and comparisons among multiple groups were performed by
one-way analysis of variance followed by the LSD test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Serum levels of MEG3 in three
groups
As shown in Fig. 1,
serum levels of MEG3 were significantly lower in patients with
diabetic retinopathy and diabetic patients than in healthy control
(P<0.05). Serum levels of MEG3 were slightly lower in patients
with diabetic retinopathy than in diabetic patients, but the
difference was not statistically significant.
Serum levels of VEGF and TGF-β1 in
three groups
VEGF and TGF-β1 play pivotal roles in the
development of diabetic retinopathy. Therefore, serum levels of
VEGF and TGF-β1 were detected by ELISA and compared among groups.
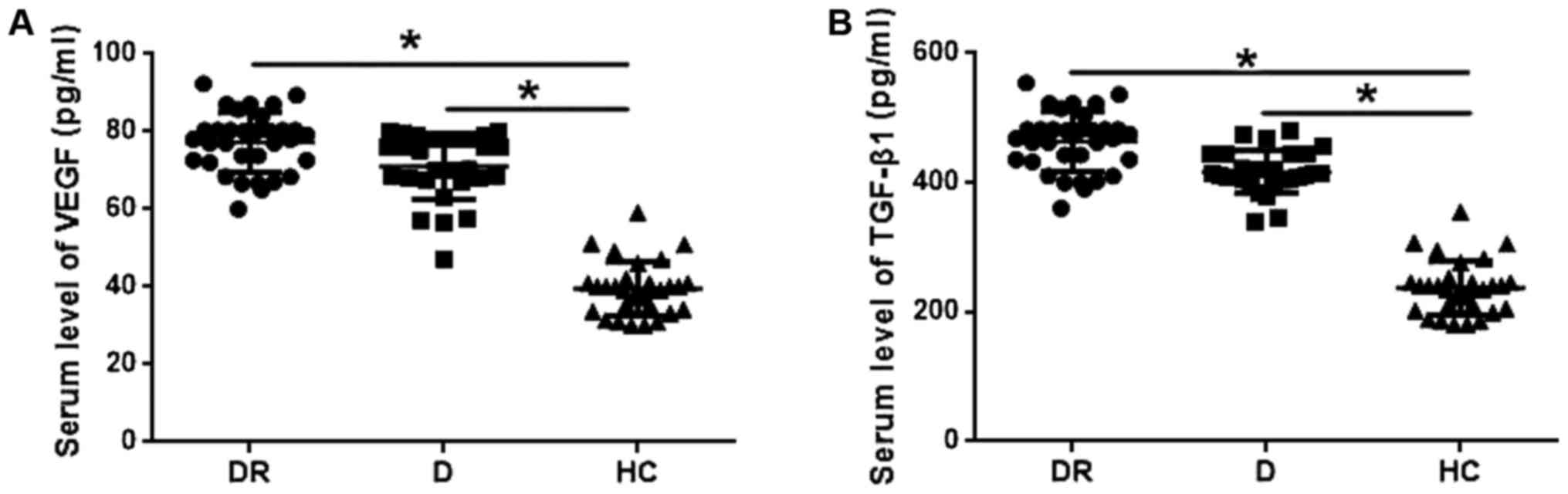
As shown in Fig. 2A, serum levels of
VEGF were significantly higher in patients with diabetic
retinopathy and diabetic patients without retinopathy than in
healthy controls (P<0.05). In addition, serum levels of VEGF
were slightly higher in patients with diabetic retinopathy than in
diabetic patients, but the difference was not statistically
significant. Similarly, serum levels of TGF-β1 were significantly
higher in patients with diabetic retinopathy and diabetic patients
without retinopathy than in healthy control (P<0.05), but no
significant differences in serum levels of TGF-β1 were found
between patients with diabetic retinopathy and diabetic
patients.
Glucose regulates the expression of
VEGF, TGF-β1 and MEG3 in the ARPE-19 cell line
In this study, two different concentrations (15 and
30 mM) of d-glucose were used to treat ARPE-19 cells, and 5 mM
d-glucose was used as control. Expression of VEGF, TGF-β1 and MEG3
in ARPE-19 cells was detected by qRT-PCR. As shown in Fig. 3, d-glucose treatment significantly
increased the serum level of VEGF (Fig.
3A) and TGF-β1 (Fig. 3B) in a
dose and time-dependent manner (P<0.05). In contrast, d-glucose
treatment significantly decreased the serum level of MEG3 in a dose
and time-dependent manner (P<0.05; Fig. 3C).
MEG3 overexpression reduced the
increased VEGF expression in the ARPE-19 cell line induced by
glucose
In this study, expression level of VEGF was
increased for more than 5-fold after treatment with 30 mM d-glucose
for 48 h. Previous studies have shown that MEG3 expression level is
negatively correlated with the expression of VEGF, indicating that
MEG3 may negatively regulate the expression of VEGF. In this study,
MEG3 overexpression ARPE-19 cell line was established (Fig. 4A) and incubated with 30 mM d-glucose
for 4 h to explore the effects of MEG3 overexpression on VEGF
expression. As shown in Fig. 4, MEG3
overexpression significantly reduced the 30 mM d-glucose-induced
increased expression level of VEGF at both mRNA (Fig. 4B) and protein levels (Fig. 4C).
MEG3 overexpression reduced the
increased TGF-β1 expression in the ARPE-19 cell line induced by
glucose
It is well known that high glucose can induced the
expression of TGF-β1 (13). In this
study, expression level of TGF-β1 mRNA was increased for more than
7-fold after treatment with 30 mM D-glucose for 48 h (Fig. 5A), and expression level of TGF-β1
protein was increased for more than 4-fold (Fig. 5B). MEG3 overexpression significantly
reduced the increased expression levels of TGF-β1 induced by
treatment with 30 mM d-glucose for 48 h at both mRNA and protein
levels. TGF-β1 plays pivotal roles in the pathogenesis of diabetic
retinopathy. Those data suggest that MEG3 overexpression may
improve diabetic retinopathy by inhibiting TGF-β1 expression.
Discussion
At present, diabetes affects more than 350 million
people worldwide, and the incidence of this disease is still
increasing (1). Incidence of
diabetes in China was used to be low, while with the changes in
people's life style and popularization of western dining culture,
more and more people are suffering from this disease now (14). As a type of chronic metabolic
disease, diabetes also induces the occurrence of many types of
complications during its long-term course of disease (2), severely impairing people's quality of
life. As one of the most common complications of diabetes, diabetic
retinopathy may cause reduced vision, visual symptoms or even
blindness by damaging small blood vessels in the retina (3). Oxidative stress and high blood glucose
level now are considered to be major causes of diabetic retinopathy
(15). Besides that, reduced levels
of endostatin, which is an anti-angiogenic protein, have also been
proved to contribute to the development of this disease (13). In spite of the progresses that have
been made in understanding the mechanism of diabetic retinopathy,
pathogenesis of this disease still hasn't been fully elucidated,
leading to poor treatment outcomes in some extreme cases.
LnRNAs now are considered to be major plays in the
development of various human diseases. A recent study has shown
that the development of diabetic retinopathy is accompanied with
the changes in genome-wide expression profile of lncRNA (10), and the expression level of lncRNA
MALAT1, which play critical roles in vascularization and angiogenic
response of endothelial cells, can reflect the severity of this
disease (10). In another study,
lncRNA ANRIL has been proved to participate in the pathogenesis of
diabetic retinopathy by affecting expression of
vascularization-related factors (16). LncRNA MEG3 plays a role as tumor
suppressor gene in development of different types of cancer
(11). In the study of ischemic
brain injury, Liu et al reported that reduced expression
level of MEG3 is responsible for accelerated angiogenesis (17), which is also a key step for the
development of diabetic retinopathy, indicating the possible
involvement of MEG3 in this disease. In this study, serum levels of
MEG3 were significantly lower in patients with diabetic retinopathy
and diabetic patients without retinopathy than in healthy control.
In addition, although no significant differences were found, serum
levels of MEG3 were slightly lower in patients with diabetic
retinopathy than in diabetic patients without retinopathy. Those
data suggest that, downregulation of MEG3 may participate in the
development of diabetic retinopathy as well as other complications
of diabetes.
Vascular endothelial growth factor (VEGF), which is
a key player in neovascularization, is closely correlated with the
development of diabetic retinopathy (18), and anti-VEGF therapy has been widely
used in treatment of diabetic retinopathy (18). TGF-β1 is also a critical component of
the pathogenesis of diabetic nephropathy (19). Consistent with previous studies, in
this study, expression levels of VEGF and TGF-β1 were significantly
increased in ARPE-19 cells after treatment with high glucose. In
contrast, expression level of MEG3 was significantly decreased
after high glucose treatment. In the study of osteoarthritis, Su
et al found that expression level of MEG3 was negatively
correlated with expression level of VEGF (20), indicating the potential interactions
between them. In another study, Mondal found that MEG3 could also
interact with TGF-β1 to achieve its biological functions (21). In this study, MEG3 overexpression
significantly reduced the increased expression levels of VEGF and
TGF-β1 induced by high glucose treatment. Those results suggest
that lncRNA MEG3 overexpression may improve the conditions of
diabetic retinopathy by inhibiting the expression of VEGF and
TGF-β1. It's worth to note that we also performed electrophoretic
mobility shift assay (EMSA) to investigate the interactions of MEG3
with VEGF and TGF-β1. However, no obvious RNA-protein binding was
observed, indicating that MEG3 may indirectly regulates expression
of VEGF and TGF-β1.
In conclusion, serum levels of MEG3 were
significantly reduced, while serum of VEGF and TGF-β1 were
significantly increased in patients with diabetic retinopathy and
diabetic patients without retinopathy compared with healthy
controls. High glucose treatment upregulated the expression of VEGF
mRNA and downregulated the expression of MEG3. MEG3 overexpression
reduced the increased expression levels VEGF and TGF-β1 induced by
high glucose. Our study first reported that lncRNA MEG3 is involved
in the pathogenesis of diabetic retinopathy, and it may serve as a
promising target for the treatment of this disease. However, this
study is still limited by small sample size. In addition, all
participants in this study are Asian, and the effects caused by
ethnicity cannot be excluded. Therefore, further studies are still
needed to confirm the conclusions in this study. Besides that, due
to the limited resources, deeper investigations were not performed.
We will solve those problems in our future study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ, YL and JW designed the experiments. DZ, HQ and
LY performed the experiments. XL, LZ, DB and YM analyzed the data.
YL wrote the manuscript. All authors have read and approved the
final submitted manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Affiliated Hospital of Beihua University. All patients signed
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Imamura F, O'Connor L, Ye Z, Mursu J,
Hayashino Y, Bhupathiraju SN and Forouhi NG: Consumption of sugar
sweetened beverages, artificially sweetened beverages, and fruit
juice and incidence of type 2 diabetes: Systematic review,
meta-analysis, and estimation of population attributable fraction.
BMJ. 351:h35762015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nathan DM; DCCT/EDIC Research Group, : The
diabetes control and complications trial/epidemiology of diabetes
interventions and complications study at 30 years: Overview.
Diabetes Care. 37:9–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leasher JL, Bourne RRA, Flaxman SR, Jonas
JB, Keeffe J, Naidoo K, Pesudovs K, Price H, White RA, Wong TY, et
al: Global estimates on the number of people blind or visually
impaired by diabetic retinopathy: A meta-analysis from 1990 to
2010. Diabetes Care. 39:1643–1649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kertes PJ and Johnson TM: Evidence-based
eye care. Lippincott Williams & Wilkins; Philadelphia, PA:
2007
|
|
5
|
Tapp RJ, Shaw JE, Harper CA, de Courten
MP, Balkau B, McCarty DJ, Taylor HR, Welborn TA and Zimmet PZ;
AusDiab Study Group, : The prevalence of and factors associated
with diabetic retinopathy in the Australian population. Diabetes
Care. 26:1731–1737. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perkel JM: Visiting ‘noncodarnia’.
Biotechniques. 54:301, 303–304. 2013. View Article : Google Scholar
|
|
7
|
Hung T and Chang HY: Long noncoding RNA in
genome regulation: prospects and mechanisms. RNA Biol. 7:582–585.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang G, Lu X and Yuan L: LncRNA: A link
between RNA and cancer. Biochim Biophys Acta. 1839:1097–1109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumarswamy R, Bauters C, Volkmann I, Maury
F, Fetisch J, Holzmann A, Lemesle G, de Groote P, Pinet F and Thum
T: Circulating long noncoding RNA, LIPCAR, predicts survival in
patients with heart failure. Circ Res. 114:1569–1575. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jaé N and Dimmeler S: Long noncoding RNAs
in diabetic retinopathy. Circ Res. 116:1104–1106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin R, Chen Z, Ding Y, Hao J, Hu J and Guo
F: Long non-coding RNA MEG3 inhibits the proliferation of cervical
carcinoma cells through the induction of cell cycle arrest and
apoptosis. Neoplasma. 60:486–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Y, Zhang X and Klibanski A: MEG3
noncoding RNA: A tumor suppressor. J Mol Endocrinol. 48:R45–R53.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ha H, Yu MR and Lee HB: High
glucose-induced PKC activation mediates TGF-beta 1 and fibronectin
synthesis by peritoneal mesothelial cells. Kidney Int. 59:463–470.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lopez-Galvez MI, Lavado FM and Pastor JC:
Diabetic retinopathy: An overview. Handbook of Nutrition, Diet and
the Eye. Academic Press; New York, NY: pp. 41–51. 2014, View Article : Google Scholar
|
|
16
|
Thomas AA, Feng B and Chakrabarti S:
ANRIL: A Regulator of VEGF in Diabetic Retinopathy. Invest
Ophthalmol Vis Sci. 58:470–480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Li Q, Zhang KS, Hu B, Niu X, Zhou
SM, Li SG, Luo YP, Wang Y and Deng ZF: Downregulation of the long
non-coding RNA Meg3 promotes angiogenesis after ischemic brain
injury by activating notch signaling. Mol Neurobiol. 54:8179–8190.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simó R, Sundstrom JM and Antonetti DA:
Ocular anti-VEGF therapy for diabetic retinopathy: The role of VEGF
in the pathogenesis of diabetic retinopathy. Diabetes Care.
37:893–899. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goldfarb S and Ziyadeh FN: TGF-beta: A
crucial component of the pathogenesis of diabetic nephropathy.
Trans Am Clin Climatol Assoc. 112:27–33. 2001.PubMed/NCBI
|
|
20
|
Su W, Xie W, Shang Q and Su B: The long
noncoding RNA MEG3 is downregulated and inversely associated with
VEGF levels in osteoarthritis. Biomed Res Int. 2015:3568932015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mondal T, Subhash S, Vaid R, Enroth S,
Uday S, Reinius B, Mitra S, Mohammed A, James AR, Hoberg E, et al:
MEG3 long noncoding RNA regulates the TGF-β pathway genes through
formation of RNA-DNA triplex structures. Nat Commun. 6:77432015.
View Article : Google Scholar : PubMed/NCBI
|