Introduction
Bronchial asthma is a kind of chronic airway disease
that affects patients worldwide. According to the report of the
World Health Organization (WHO), the number of patients with
bronchial asthma is up to 30 million in China, showing an
increasing trend year by year. Bronchial asthma is mainly
manifested as acute bronchial asthma that often occurs in children
(1–3). The main symptoms of bronchial asthma
are airway smooth muscle spasm and airway inflammatory
infiltration, and its incidence is jointly affected by genetic
factors and environmental factors, but its specific pathogenesis
remains unclear. Studies have reported that it is closely related
to the airway inflammation, allergic reaction, neurohumor and other
factors (4,5). Glucocorticoid therapy is the most
common therapeutic regimen for acute bronchial asthma, which has
strong anti-inflammatory and immunosuppressive effects. However,
the long-term application of glucocorticoid leads to obvious
adverse reactions and withdrawal effect (6). In recent years, a large number of
clinical reports in China and foreign countries have suggested that
montelukast can be used to replace glucocorticoid in the treatment
of acute bronchial asthma (7,8).
Montelukast is a kind of antagonist of cysteinyl leukotriene
(Cys-LT) receptor that can regulate the leukotriene pathway, which
is clinically used in the acute and long-term treatment of asthma
and widely applied in the treatment of asthma children currently
(9). Moreover, montelukast can block
the pro-inflammatory effect mediated by Cys-LT1 by binding to the
Cys-LT1 receptor on target cells (10). CD4+CD25+
regulatory T cells are cells derived from thymus used to regulate
the immune balance and mediate immune tolerance. It is reported
that CD4+CD25+ T cells can inhibit the
production of T helper 2 (Th2) cytokines and aggregation of airway
eosinophils (EOS) (11). Therefore,
investigations on the role of CD4+CD25+
regulatory T cells in bronchial asthma and its molecular mechanism
can be a feasible research direction for the targeted therapy of
bronchial asthma.
In this study, the therapeutical effect of
montelukast on children with acute bronchial asthma and the
regulating effect on CD4+CD25+ regulatory T
cells were investigated, and its mechanism was further studied, so
as to provide a theoretical basis for the application of
montelukast in the treatment of acute bronchial asthma.
Materials and methods
Subjects of study
In this study, a total of 56 child patients with
acute asthma aged 7–13 years treated in Pneumology Department of
Shangluo Central Hospital from March 2015 to March 2016 were
selected, and all patients met the diagnostic criteria in the
Guidelines for Prevention and Treatment of Bronchial Asthma: i)
recurrent wheezing, shortness of breath, chest tightness and cough;
ii) expiratory-phase wheezing rale can be heard in asthmatic
attack; iii) the above symptoms can be relieved after treatment or
spontaneously. The above patients were randomly divided into the
control group (n=28) and treatment group (n=28). The control group,
including 17 males and 11 females with an average age of 10.25±3.82
years, was treated with conventional therapy (anti-infection
combined with aminophylline therapy), while the treatment group,
including 18 males and 10 females with an average age of 9.85±3.68
years, took montelukast sodium tablets (Sichuan Otsuka
Pharmaceutical Co., Ltd., Sichuan, China; NMPN H20064370) orally
once per day (5 mg/time) before sleep. Patients in the two groups
were treated for one week. None of the patients suffered from other
autoimmune diseases, lung infection and other wasting diseases, and
they did not take glucocorticoid and leukotriene receptor
antagonist within one month. There were no statistically
significant differences in age, sex, condition and course of
disease between the two groups.
Parents or their guardians signed the written
informed consent. The experimental scheme was approved by the
Ethics Committee of Shangluo Central Hospital (Shangluo, China) and
the above patients had the complete clinical and pathological data
and the complete therapeutic regimen.
Determination of lung function
The lung function of all the patients was detected
by the physician in the Pneumology Department of our hospital using
the lung function apparatus (Jaeger Master Diffusion; Jaeger,
Hoechberg, Germany). The lung function parameters were recorded in
detail: the ratio of forced expiratory volume in 1 sec to forced
vital capacity (FEV1/FVC), peak expiratory flow (PEF) and 25% PEF
(PEF25). The above lung function parameters were statistically
analyzed by experienced physicians who were not involved in the
previous experiment.
Evaluation of therapeutic effects
The treatment effects on acute bronchial asthma in
the above patients were evaluated: remarkably effective (3 points):
dyspnea and other symptoms were improved and wheezing rale in lung
disappeared within 3 days of treatment; effective (2 points):
dyspnea and other symptoms were improved and wheezing rale in lung
disappeared within 7 days of treatment; ineffective (1 point):
serious cough and wheezing still occurred and wheezing rale in lung
still existed after 1 week of treatment.
Separation and detection of peripheral
CD4+CD25+ regulatory T cells
Fasting peripheral blood (5 ml) was drawn from all
the patients, added and mixed with the anticoagulant, and the
mixture was labeled using CD4 and CD25 multicolor fluorescent
antibodies. IgG1-fluorescein isothiocyanate (FITC) and
IgG1-PE were used as negative controls. The mixture was
incubated in the dark at room temperature for 10 min and detected
using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA,
USA). The positive rate (%) of CD4+CD25+
regulatory T cells in peripheral blood was calculated, and the
absolute number of CD4+CD25+ regulatory T
cells in 1 µl peripheral blood was also calculated, followed by
signal acquisition and results analysis using Cellquest Pro
software.
Inflammatory factor detection
The serum was separated from the peripheral blood of
patients, and the concentrations of interleukin-4 (IL-4), IL-5,
IL-6, transferrin-γ (TFN-γ), IL-10 and immunoglobulin E (IgE) were
detected using the corresponding enzyme-linked immunosorbent assay
(ELISA) kits. The standard sample was added onto an ELISA plate to
prepare the standard curve. The serum sample in each group was
diluted 10-fold using the sample diluent was added into each well,
and the plate was sealed with sealing membrane for reaction at room
temperature for 60 min. The solution was discarded, and the
corresponding biotin-labeled antibody was added for reaction at
37°C for 60 min. The plate was then washed with cleaning solution 3
times (1 min/time). Avidin-peroxidase complex (100 µl) was added
for reaction at 37°C for 30 min. The liquid waste was discarded,
and the plate was washed again with cleaning solution and added
with 100 µl stop buffer to terminate the reaction. The optical
density (OD) value of each well was measured using a microplate
reader and the standard curve was drawn using the software.
Finally, the serum IL-4, IL-5, IL-6, TFN-γ, IL-10 and IgE levels in
each group were calculated through the standard curve.
Statistical analysis
Graphpad Prism 6.0 was used for the statistical
analysis of data, and data were presented as mean ± SD. The t-test
was used for analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Changes in lung function
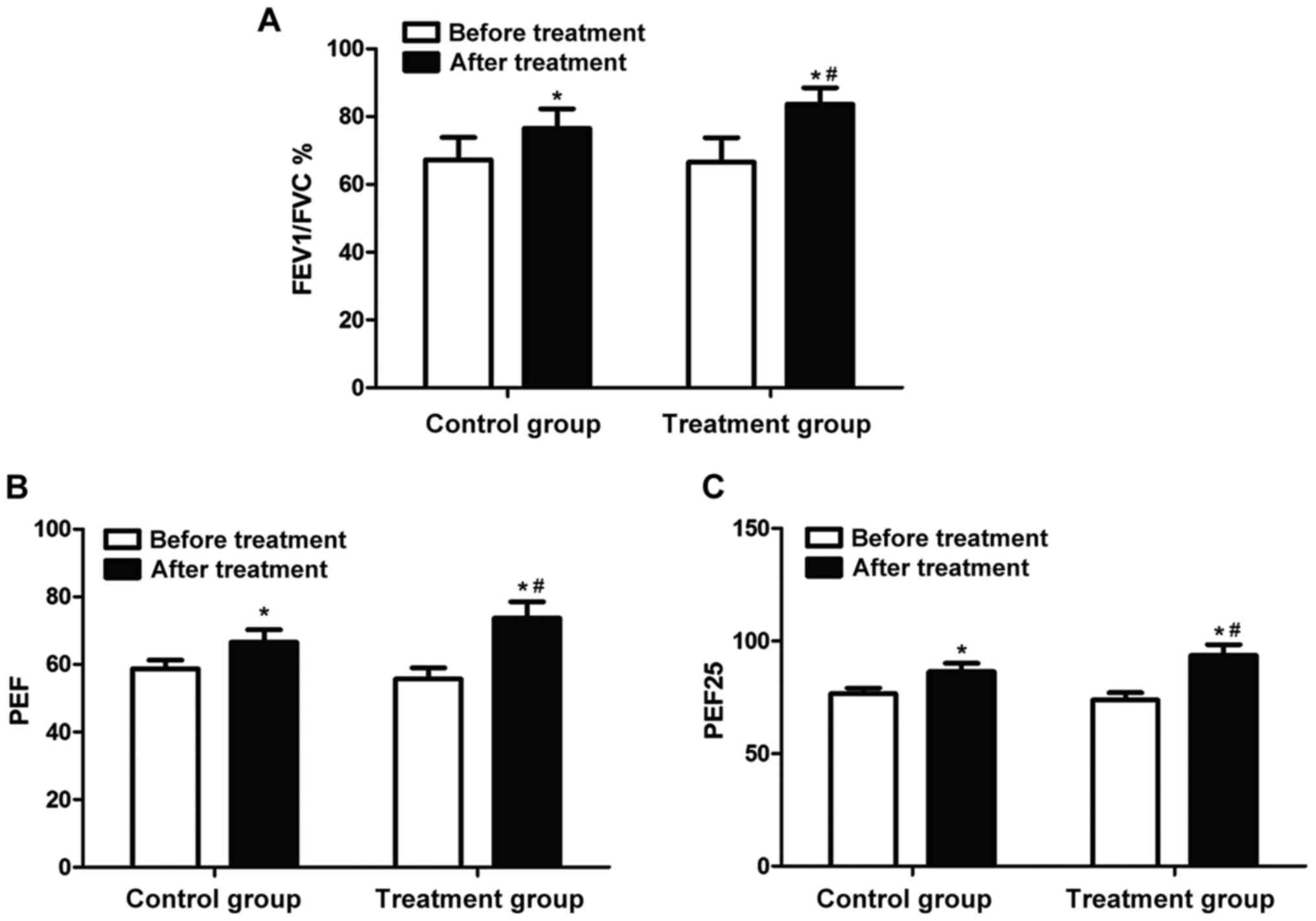
The lung function of patients in each group was
detected using the lung function monitor. The results (Fig. 1) showed that there were no
statistically significant differences in FEV1/FVC, PEF and PEF25
between the two groups before treatment (P>0.05). FEV1/FVC, PEF
and PEF25 in both groups were significantly increased after
treatment for 7 days (P<0.05); FEV1/FVC, PEF and PEF25 in the
treatment group were obviously higher than those in the control
group (P<0.05).
Evaluation of therapeutic effects
after treatment
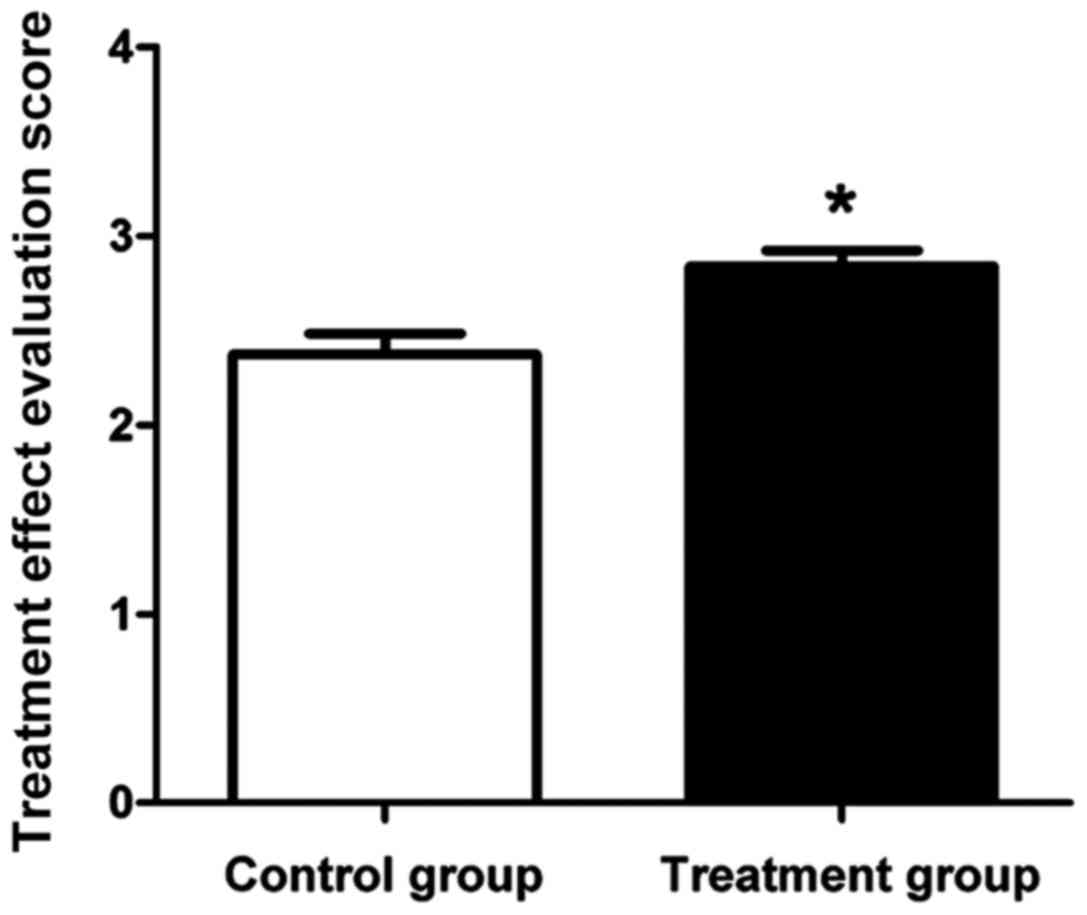
The therapeutic effects on acute bronchial asthma in
patients in both groups after treatment for one week were
evaluated. The effective treatment rate (Table I) and therapeutic effect score
(Fig. 2) showed that the effective
treatment rate in treatment group was significantly higher than
that in the control group (P<0.05). The therapeutic effect score
in the treatment group was also significantly higher to that in the
control group (P<0.05).
 | Table I.Effective treatment rate of acute
bronchial asthma. |
Table I.
Effective treatment rate of acute
bronchial asthma.
| Groups | Remarkably effective
(n) | Effective (n) | Ineffective (n) | Total effective rate
(%) |
|---|
| Control group | 22 | 4 | 2 | 92.85 |
| Treatment group | 15 | 4 | 9 | 67.85 |
| P-value | <0.05 | >0.05 | <0.05 | <0.05 |
| t-test | 0.832 | 0.126 | 0.765 | 0.693 |
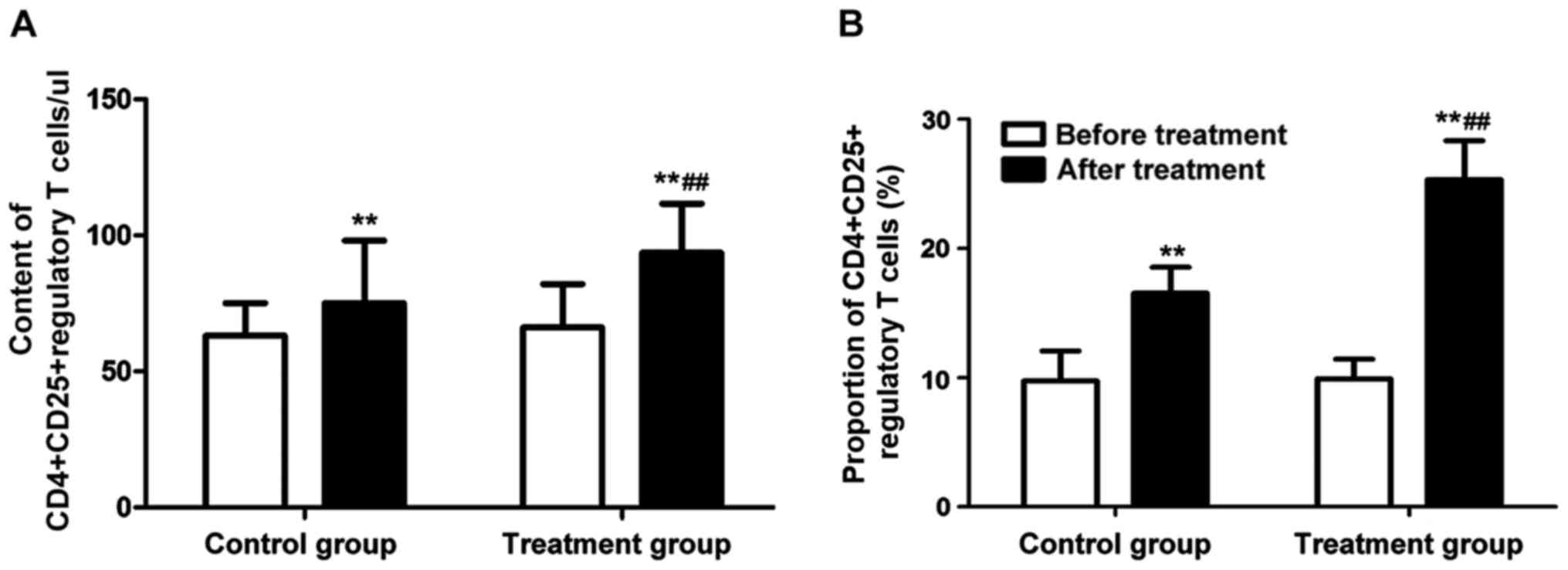
Changes in
CD4+CD25+ regulatory T cells
CD4+CD25+ regulatory T cells
in peripheral blood of patients in each group were detected before
and after treatment. The results revealed that the content of
CD4+CD25+ regulatory T cells in peripheral
blood and its proportion in T lymphocytes in patients with acute
bronchial asthma after treatment were obviously increased
(P<0.01) (Fig. 3). In addition,
the content of CD4+CD25+ regulatory T cells
in peripheral blood and its proportion in T lymphocytes in patients
in the treatment group were significantly higher than those in the
control group, and the differences were statistically significant
(P<0.01).
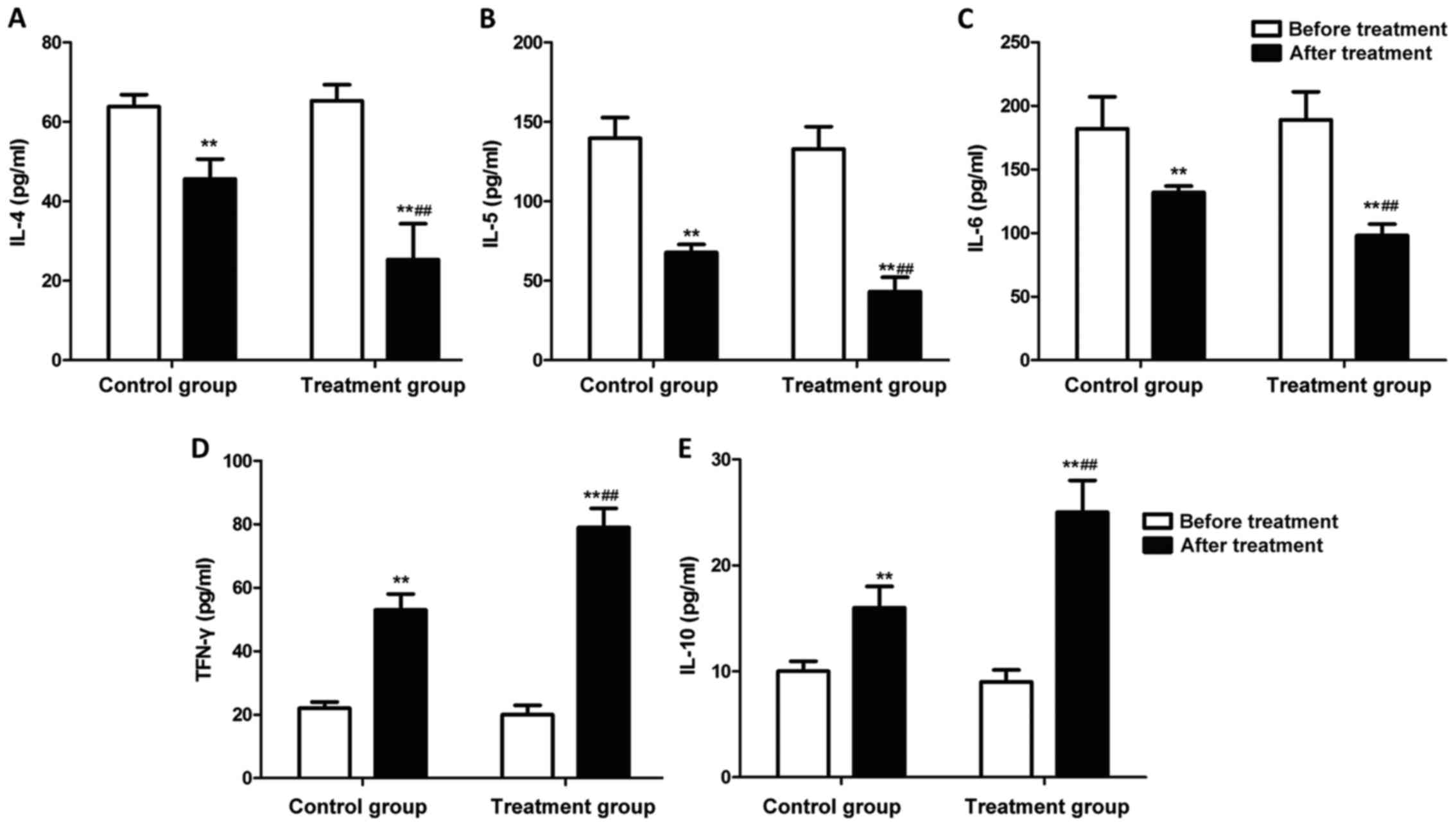
Changes in inflammatory factors
The changes in inflammatory factors in peripheral
blood of patients in each group were detected using the ELISA kit.
Fig. 4 shows that after drug therapy
for 7 days, the levels of IL-4, IL-5 and IL-6 in patients in both
groups were significantly decreased, but the levels of TFN-γ and
IL-10 were significantly increased (P<0.01). Furthermore, the
levels of IL-4, IL-5 and IL-6 in peripheral blood in the treatment
group were lower than those in the control group, but the levels of
TFN-γ and IL-10 were higher than those in the control group
(P<0.01).
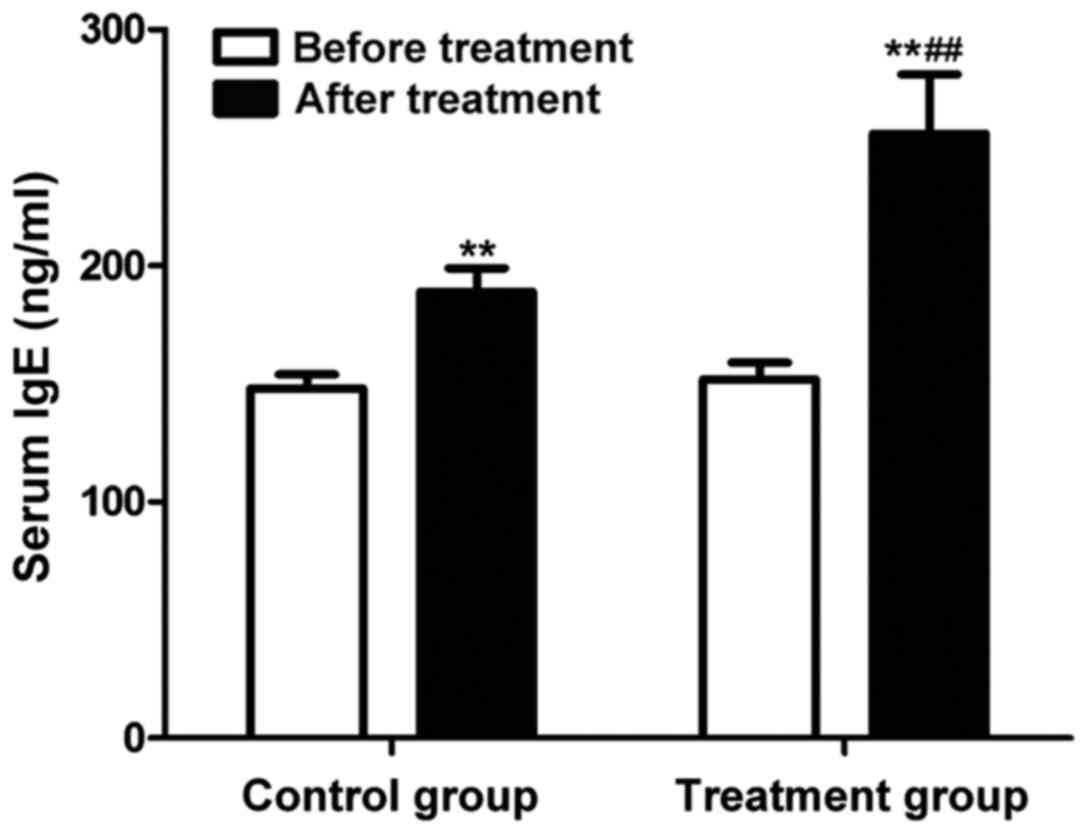
Change in serum IgE level
The change in serum IgE in peripheral blood of
patients in each group was detected using the ELISA kit. The
results (Fig. 5) showed that the
serum IgE levels were significantly increased in both groups after
drug therapy for 7 days (P<0.01). The serum IgE level in
peripheral blood of patients in the treatment group was higher than
that in the control group (P<0.01).
Discussion
Acute bronchial asthma is a kind of global disease
affecting the life and health, as well as a multi-cell and
multi-media chronic airway inflammation. Bronchial asthma is
characterized by chronic airway inflammation and airway remodeling,
involving a variety of cytokines (12). Cys-LT is the metabolite of
leukotrienes, and previous findings have shown that a high
concentration of Cys-LT is present in asthma patients, which is
thought to be one of the causes and important mediators of asthma
(13). Montelukast is a
non-steroidal anti-inflammatory drug, which, as a selective
inhibitor of Cys-LT, can relieve the airway spasm by reducing the
Cys-LT concentration in patients, thus playing a role in the
treatment of acute bronchial asthma (14). Moreover, montelukast can inhibit the
accumulation and proliferation of inflammatory cells in the airway,
reducing the glandular secretion of mucus in the body, and
affecting the activation and differentiation of lymphocytes, thus
reducing the permeability of blood vessels and playing an important
role in the treatment of pulmonary fibrosis and trachea remode ling
(15). Regulatory T cells are the
cell population that can inhibit the functions of other cells and
mediate other immune-active cells, which can be divided into
CD4+CD25+ regulatory T cells, Th3 and Tr1,
according to different cell compositions. These cells play
important roles in bronchial asthma and other allergic diseases
(16).
By studying the therapeutic effect of montelukast on
children with acute bronchitis, the therapeutic effect of
montelukast is examined from the perspective of improving clinical
symptoms and lung function. Montelukast has a significant function
in the treatment of acute bronchial asthma, which can effectively
treat the bronchial asthma, increase the effective treatment rate,
and significantly enhance the lung function of patients with
bronchial asthma. Han et al (17) studied and found that montelukast can
effectively reduce the level of airway eosinophils (EOS) in mice
induced by inhaled ovalbumin, decrease the formation of mucous plug
and inhibit the proliferation of airway smooth muscle.
Additionally, the increased Th1-induced Th1/Th2 imbalance is an
important mechanism of asthmatic attack. Th2 cytokines mainly
include IL-4 and IL-5, and TFN-γ and IL-12 are typical Th1
cytokines. IL-4 and IL-5 are increased and TFN-γ is decreased in
patients with bronchial asthma (18). The results of this study were
consistent with the aboveme ones. It was found that the montelukast
treatment for one week could significantly reduce the levels of
IL-4, IL-5 and IL-6, but increase the levels of TFN-γ and IL-10,
and regulate the Th1/Th2 balance, thus treating bronchial asthma.
Moreover, montelukast increased the level of
CD4+CD25+ regulatory T cells in patients with
bronchial asthma by affecting the Th1/Th2 balance. The functions of
EOS and epithelial cells can increase the synthesis of Cys-LT. IL-5
is an EOS differentiation factor, as well as an important stimulus
for EOS release (19). IL-4 can
upregulate the Cys-LT synthetase activity; TFN-γ can inhibit the
IL-4-induced high expression of IgE and the occurrence of allergic
reaction (20). There were still
some shortcomings in this study; for example, the specific
molecular mechanism was not deeply studied.
In conclusion, montelukast can regulate the Th1/Th2
balance, increase the expression of CD4+CD25+
regulatory T cells, inhibit the lung inflammation, and improve the
lung function, which has an important therapeutic effect on acute
bronchial asthma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XQ was involved in the conception and design of the
study. YC performed the experiments. XQ and CY analysed the data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental scheme was approved by the Ethics
Committee of Shangluo Central Hospital (Shangluo, China). Parents
or their guardians signed the written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Canonica GW, Senna G, Mitchell PD, O'Byrne
PM, Passalacqua G and Varricchi G: Therapeutic interventions in
severe asthma. World Allergy Organ J. 9:402016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung LP, Baltic S, Ferreira M, Temple S,
Waterer G and Thompson PJ: Beta2 adrenergic receptor (ADRβ2)
haplotype pair (2/4) is associated with severe asthma. PLoS One.
9:e936952014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grieger JA, Wood LG and Clifton VL:
Improving asthma during pregnancy with dietary antioxidants: The
current evidence. Nutrients. 5:3212–3234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jung JW, Choi JC, Shin JW, Kim JY, Park IW
and Choi BW: Clinical characteristics according to sensitized
allergens in adult korean patients with bronchial asthma. Allergy
Asthma Immunol Res. 2:102–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akpinarli A, Tuncer A, Saraçlar Y, Sekerel
BE and Kalayci O: Effect of formoterol on clinical parameters and
lung functions in patients with bronchial asthma: A randomised
controlled trial. Arch Dis Child. 81:45–48. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scott M, Raza A, Karmaus W, Mitchell F,
Grundy J, Kurukulaaratchy RJ, Arshad SH and Roberts G: Influence of
atopy and asthma on exhaled nitric oxide in an unselected birth
cohort study. Thorax. 65:258–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scaparrotta A, Di Pillo S, Attanasi M,
Rapino D, Cingolani A, Consilvio NP, Verini M and Chiarelli F:
Montelukast versus inhaled corticosteroids in the management of
pediatric mild persistent asthma. Multidiscip Respir Med. 7:132012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jindal A, Suriyan S, Sagadevan S,
Narasimhan M, Shanmuganathan A, Vallabhaneni V and Rajalingam R:
Comparison of oral montelukast and intranasal fluticasone in
patients with asthma and allergic rhinitis. J Clin Diagn Res.
10:OC06–OC10. 2016.PubMed/NCBI
|
|
9
|
Ciebiada MG, Barylski M and Ciebiada M:
Wheal and flare reactions in skin prick tests of patients treated
with montelukast alone or in combination with antihistamines.
Inflamm Res. 63:191–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao W, Liu P, Qiu Z, Yu L, Hang J, Gao X
and Zhou X: Efficacy of add-on montelukast in nonasthmatic
eosinophilic bronchitis: The additive effect on airway
inflammation, cough and life quality. Chin Med J (Engl). 128:39–45.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee J-H, Yu H-H, Wang L-C, Yang Y-H, Lin
Y-T and Chiang B-L: The levels of CD4+CD25+
regulatory T cells in paediatric patients with allergic rhinitis
and bronchial asthma. Clin Exp Immunol. 148:53–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watanabe T, Fajt ML, Trudeau JB, Voraphani
N, Hu H, Zhou X, Holguin F and Wenzel SE: Brain-derived
neurotrophic factor expression in asthma. Association with severity
and type 2 inflammatory processes. Am J Respir Cell Mol Biol.
53:844–852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
FitzGerald JM, Foucart S, Coyle S,
Sampalis J, Haine D, Psaradellis E and McIvor RA: Montelukast as
add-on therapy to inhaled corticosteroids in the management of
asthma (the SAS trial). Can Respir J. 16 Suppl A:5A–14A. 2009.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reiss TF, Hill JB, Harman E, Zhang J,
Tanaka WK, Bronsky E, Guerreiro D and Hendeles L: Increased urinary
excretion of LTE4 after exercise and attenuation of
exercise-induced bronchospasm by montelukast, a cysteinyl
leukotriene receptor antagonist. Thorax. 52:1030–1035. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keith PK, Koch C, Djandji M, Bouchard J,
Psaradellis E, Sampalis JS, Schellenberg RR and McIvor RA:
Montelukast as add-on therapy with inhaled corticosteroids or
inhaled corticosteroids and long-acting beta-2-agonists in the
management of patients diagnosed with asthma and concurrent
allergic rhinitis (the RADAR trial). Can Respir J. 16 Suppl
A:17A–31A. 2009.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernstein JM, Lehman H, Lis M, Sands A,
Wilding GE, Shultz L, Bankert R and Bobek L: Humanized mouse model
used to monitor MUC gene expression in nasal polyps and to
preclinically evaluate the efficacy of montelukast in reducing
mucus production. Ann Otol Rhinol Laryngol. 121:307–316. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han J, Jia Y, Takeda K, Shiraishi Y,
Okamoto M, Dakhama A and Gelfand EW: Montelukast during primary
infection prevents airway hyperresponsiveness and inflammation
after reinfection with respiratory syncytial virus. Am J Respir
Crit Care Med. 182:455–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kianmehr M, Haghmorad D, Nosratabadi R,
Rezaei A, Alavinezhad A and Boskabady MH: The effect of Zataria
multiflora on Th1/Th2 and Th17/T regulatory in a mouse model of
allergic asthma. Front Pharmacol. 8:4582017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rolfes MC, Juhn YJ, Wi C-I and Sheen YH:
Asthma and the risk of rheumatoid arthritis: An insight into the
heterogeneity and phenotypes of asthma. Tuberc Respir Dis (Seoul).
80:113–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui A-H, Zhao J, Liu S-X and Hao Y-S:
Associations of IL-4, IL-6, and IL-12 levels in peripheral blood
with lung function, cellular immune function, and quality of life
in children with moderate-to-severe asthma. Medicine (Baltimore).
96:e62652017. View Article : Google Scholar : PubMed/NCBI
|