Introduction
Acute heart failure (AHF) is a major public health
issue due to its high incidence and poor prognosis, while the
number of hospitalizations for AHF continues to increase due to the
aging population (1–4). Efficient and timely diagnosis is
critical for improving the prognosis, as well as reducing the
mortality rate, length of hospital stay and treatment costs
(5,6). Compared with other detection methods
for diagnosing AHF, plasma markers have certain advantages,
including the simple and easy detection. Amino-terminal pro-brain
natriuretic peptide (NT-proBNP) is one of the most commonly used
markers in AHF diagnosis (7–9). However, renal dysfunction is a common
comorbidity in AHF patients (10).
In addition, patients with chronic kidney disease (CKD) present
increased risks of accelerated atherosclerosis, nonfatal myocardial
infarction, congestive heart failure (CHF), atrial and ventricular
arrhythmias, and cardiac death (11). Although NT-proBNP is considered as a
marker in AHF diagnosis, the diagnostic value of NT-proBNP in
patients with renal insufficiency is still debated (12–15).
Therefore, a more effective plasma marker is required for the
diagnosis of AHF patients with renal insufficiency.
A multimarker strategy may help diagnose and
determine the prognosis of patients with AHF (16), particularly in patients with AHF and
mild to moderate renal impairment, while multimarker approach based
on a panel of serially evaluated biomarkers may provide the
greatest prognostic improvement. A previous study demonstrated that
seven circulating biomarkers, including NT-proBNP, high sensitivity
cardiac troponin T, soluble ST2, growth differentiation factor 15,
cystatin-C, galectin-3 and high-sensitivity C-reactive protein
(CRP), measured at baseline and on days 2, 5, 14 and 60 in 1,161
patients provided an improved quick diagnosis (17).
The adipocyte-secreted protein adiponectin (ADPN) is
a 247 amino acid peptide, predominantly secreted by adipocytes
(18,19). Plasma ADPN is typically high in cats,
which are overweight and functions by regulating the level of
leptin (20). ADPN is considered as
a useful biomarker in metabolic diseases, such as diabetes,
high-fat-associated disease and dementia (21–23). In
addition, it has been observed that ADPN exerts several protective
functions in the peripheral tissues, including insulin sensitizing,
anti-inflammatory and anti-oxidative effects, which may benefit
various neurodegenerative diseases, such as Alzheimer's disease
(24). The role of ADPN in heart
diseases has been investigated in numerous studies. For instance, a
cross-sectional study revealed that a low ADPN level was associated
with diastolic dysfunction in women (25). Another study demonstrated that
natriuretic peptides, which are promising candidates for the
treatment of CHF, may have a beneficial effect on cardiomyocytes in
patients through enhancing the ADPN production by human adipocytes
in vitro and in patients with CHF (26). Furthermore, the circulating ADPN
concentration increased in patients with chronic heart failure
(HF), and thus it is regarded as a useful novel biomarker in CHF
and AHF (27,28). A previous study suggested that when
the ADPN level is used in conjunction with NT-proBNP in chronic HF,
the prognostic value may be improved when compared with the use of
each biomarker alone (29). Another
study also identified ADPN as a robust biomarker and appropriate
therapeutic targets in HF (30).
These aforementioned studies prompt the hypothesis
that ADPN may be a useful biomarker in diagnosing AHF patients.
However, there are a limited number of studies on whether the
circulating level of ADPN was affected by glomerular filtration
rate (GFR) and, thus, whether it has superiority in diagnosing AHF
patients with renal insufficiency. The aim of the present study was
to examine the value of ADPN in the diagnosis of these patients. In
total, 407 participants were enrolled into the current study,
including 218 participants diagnosed with AHF and 189 participants
serving as the control group. ADPN was measured in the participants
using an in-house sandwich enzyme-linked immunosorbent assay
(ELISA) and NT-proBNP was measured by electrochemiluminescence
immunoassay. The levels of circulating ADPN and NT-proBNP in the
patients of AHF were also compared between different New York
Health Association (NYHA) classes, as well as ischemic and
non-ischemic AHF. The correlation between the renal function and
the two biomarkers of all participants were compared by Spearman's
correlation. Finally, the diagnostic efficiency of ADPN and
NT-proBNP was evaluated in patients with and without renal
insufficiency.
Patients and methods
Clinical cohort
The present study enrolled 407 patients with
suspected cardiac-associated dyspnea who were admitted to the
Emergency Department of the Xiamen Cardiovascular Hospital (Xiamen,
China) between April 2015 and June 2015. Among them, 218 patients
(53%) were diagnosed with AHF based on the ‘European Society of
Cardiology guidelines’ (31) and
classified according to the NYHA system, as follows: Class I, the
patient suffers from heart disease, however, ordinary activities
are not restricted by cardiac function; Class II, mild restrictions
on the physical activity of patients with heart disease and no
symptoms at rest, although patients may experience fatigue, dyspnea
or angina following physical activity; Class III, severe
restrictions on the physical activity of patients with heart
disease with cardiac failure symptoms; and Class IV, patient is
unable to participate in any physical activities. In light of the
guidelines from the European Society of Cardiology guidelines, the
following levels of NT-proBNP were used for excluding AHF patients:
450 pg/ml in patients aged <50 years; 900 pg/ml between the ages
of 50 and 75 years; and 1,800 pg/ml in patients aged >75 years
(31). Furthermore, cardiovascular
magnetic resonance was conducted to determine whether ischemic AHF
was present. The remaining 189 participants presented a normal
cardiac function and served as the controls, including patients
with bronchial asthma and other respiratory system diseases.
Patients receiving dialysis within the three months prior to
admission were excluded from the current study since ADPN and
NT-proBNP levels are affected by dialysis. The study was approved
by the Ethics Committee of Zhongshan Hospital, Xiamen University
(Xiamen, China), and was conducted according to the Declaration of
Helsinki (2008). Written informed consent was obtained from all
participants.
Laboratory measurements
The levels of NT-proBNP and the renal function were
detected immediately upon presentation to the Emergency Department.
Plasma was collected in an EDTA-K3 anticoagulation tube and
centrifuged at 1,006 × g at 4°C for 15 min. NT-proBNP was detected
using the Elecsy proBNP II electrochemiluminescence immunoassay
(Roche Diagnostics, Basel, Switzerland) and the Cobas E601 analyzer
(Roche Diagnostics). The residual plasma samples were preserved at
−80°C for ADPN level detection.
Subsequently, the plasma ADPN levels were detected
using a sandwich ELISA kit (Xiamen Innovax Biotech Co., Ltd.,
Xiamen, China). The serum samples were diluted by 100 times during
the ELISA procedure, while the total ADPN level was the sum of
several polymers, including trimers, hexamers and high
polymers.
Renal function tests, including creatinine and GFR,
were performed with an auto-biochemistry analyzer (Cobas C701;
Roche Diagnostics). The GFR was estimated using the Modification of
Diet in Renal Disease equation as previously described (32). A GFR value of <60 ml/min/1.73
m2 indicated renal insufficiency, while a GFR value of
≥60 ml/min/1.73 m2 was considered to indicate normal
renal function.
Statistical analysis
The SPSS version 19.0 statistical software package
(IBM Corp., Armonk, NY, USA) was used to analyze the data. The
Skewness-Kurtosis test was used to determine the distribution of
data, and the non-parametric Kruskal-Wallis test was performed if
the data were not distributed normally and the values were reported
as the median (25th quantile and 75th quantile). Enumeration data
were compared using a χ2 test. The Nemenyi post-hoc test
was used to compare between multiple sets of data. Spearman's
correlation co-efficient was calculated for the correlation of
renal function with the ADPN and NT-proBNP levels. Receiver
operating characteristic (ROC) curve analyses were performed and
the area under the ROC curve (AUC) was calculated to evaluate the
diagnostic accuracy of ADPN and NT-proBNP. Data were analyzed
separately for participants with normal and impaired renal
functions, and subsequently compared. The Z-test was also performed
to determine the statistical difference of the ROC curve. A
two-sided P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics of the
participants
A total of 407 participants were enrolled into the
present study, including 262 males and 145 females, among them 218
participants were accurately diagnosed with AHF. The clinical
characteristics of all participants are listed in Table I. In the AHF group, the plasma
creatinine levels of the 218 participants were 0.87 (0.71, 1.13)
mg/dl, while the estimated GFR was 86.26 (60.20, 106.98)
ml/min/1.73 m2. In the control group, the plasma
creatinine levels of the 189 participants were 0.97 (0.80, 1.34)
mg/dl and the estimated GFR was 75.04 (54.18, 95.08) ml/min/1.73
m2. The reference values for creatinine and GFR were
0.60–1.09 mg/dl and 90–120 ml/min/1.73 m2, respectively.
Renal insufficiency was detected in 111 participants, among which
52 were diagnosed with AHF. Renal insufficiency was detected in 59
individuals within the control group. In addition, the plasma ADPN
level was 11.05 (6.51, 16.28) µg/ml and the NT-proBNP was 2,158
(1,032, 4,930) pg/ml in the AHF group, whereas the ADPN level was
5.56 (3.43, 7.39) µg/ml and the NT-proBNP was 220 (56, 690) pg/ml
in the control group.
 | Table I.Clinical characteristics of 407
participants. |
Table I.
Clinical characteristics of 407
participants.
|
Characteristics | AHF | Control | P-value |
|---|
| No. of cases
(n) | 218 | 189 | 0.80 |
| Diabetes mellitus
(n) | 123 | 109 | 0.31 |
| Smoking (n) | 58 | 42 |
|
| Age
(years)a | 63 (51, 74) | 63 (55, 71) | 0.14 |
| Weight
(kg)a | 62 (53, 72) | 63 (57, 76) | 0.21 |
| Height
(m)a | 1.65 (1.61,
1.73) | 1.67 (1.63,
1.74) | 0.18 |
| BMI
(kg/m2)a | 23.34 (21.39,
26.76) | 22.78 (20.97,
25.47) | 0.27 |
| NT-proBNP
(pg/ml)a | 2,158 (1,032,
4,930) | 220 (56, 690) | <0.001 |
| ADPN
(µg/ml)a | 11.05 (6.51,
16.28) | 5.56 (3.43,
7.39) | <0.001 |
| Renal
functiona |
| CREA
(mg/dl) | 0.87 (0.71,
1.13) | 0.97 (0.80,
1.34) |
|
| GFR
(ml/min/1.73 m2) | 86.26 (60.2,
106.98) | 75.04 (54.18,
95.08) |
|
| Renal
insufficiency | 52 | 59 |
|
| Normal renal
function | 166 | 130 |
|
| Systolic pressure
(mmHg)a | 124 (108, 143) | 129 (114, 147) |
|
| Pathogenesis
(n) |
|
Non-ischemic | 138 | – |
|
|
Ischemic | 80 | – |
|
| NYHA class (n) |
|
|
|
| I | 9 | – |
|
| II | 61 | – |
|
|
III | 91 | – |
|
| IV | 57 | – |
|
ADPN levels are significantly
associated with the NYHA class, and may assist in distinguishing
non-ischemic from ischemic AHF
The results revealed that ADPN and NT-proBNP levels
were significantly higher in participants with an advanced NYHA
class (Table II; Fig. 1A and B). Statistically significant
differences were detected in the ADPN levels between the control
group and the NYHA class I (P=0.04); however, there was no
significant difference in the NT-proBNP level between these groups
(P=0.26). In terms of the plasma ADPN level, significant
differences were detected between class I and II (P=0.12), class II
and III (P=0.00), and class III and IV (P=0.04). Regarding the
plasma NT-proBNP levels, marked differences were observed between
class I and II (P=0.02) and class III and IV (P=0.02).
 | Table II.Levels of ADPN and NT-proBNP in the
control group and in heart failure patients with different NYHA
cardiac function classifications. |
Table II.
Levels of ADPN and NT-proBNP in the
control group and in heart failure patients with different NYHA
cardiac function classifications.
| Group | NT-proBNP
(pg/ml) | ADPN (µg/ml) |
|---|
| Control
(n=189) | 219.5 (55.5,
690.0) | 5.6 (3.4, 7.4) |
| NYHA class |
| I
(n=9) | 152.0 (119.0,
664.0) | 8.2 (5.6, 8.6) |
| II
(n=61) | 1,367.0 (759.0,
3706.0) | 10.0 (5.7,
11.2) |
| III
(n=91) | 2,158.0 (1,105.5,
4,152.0) | 11.5 (6.0,
16.6) |
| IV
(n=57) | 3,986.0 (1,798.0,
9,233.0) | 14.8 (10.1,
22.8) |
Participants with AHF were further divided into the
ischemic and non-ischemic HF groups based on the disease
pathogenesis of AHF. The non-ischemic group included cases of
valvular heart disease, hypertension, dilated cardiomyopathy,
hypertrophic cardiomyopathy and hypertensive heart disease. The
ischemic group included coronary artery disease and atherosclerosis
heart disease cases. As shown in Table
III and Fig. 1C and D, the ADPN
levels in the ischemic group were significantly reduced when
compared with those in the non-ischemic group (P=0.02). However,
there was no statistically significant difference detected in the
NT-proBNP levels between the two groups (P=0.81). The combination
of these two indicators may help clinicians to establish a timely
and accurate diagnosis of AHF, and to subsequently select the
appropriate treatment.
 | Table III.Levels of ADPN and NT-proBNP in the
serum of normal, ischemic and non-ischemic groups. |
Table III.
Levels of ADPN and NT-proBNP in the
serum of normal, ischemic and non-ischemic groups.
| Group | ADPN µg/ml | NT-proBNP
pg/ml |
|---|
| Normal (n=189) | 5.6 (3.4, 7.4) | 219.5 (55.5,
690.0) |
| Ischemic
(n=80) | 9.8 (6.0,
13.2) | 2,376.0 (935.5,
5,896.5) |
| No-ischemic
(n=138) | 11.2 (6.9,
18.7) | 1,898.0 (1,116.5,
3,697.0) |
Circulating ADPN levels are affected
by renal function to a lesser extent as compared with NT-proBNP
levels
The GFR value of the participants was determined to
examine the renal function (renal insufficiency: GFR≥60). The
levels of NT-proBNP (pg/ml) in both the AHF and control (normal
cardiac function) patients were significantly higher in individuals
with renal insufficiency as compared with those with normal renal
function [AHF renal insufficiency 4,697.0 (1,878.5, 10,744.5) vs.
normal renal function 1,892.0 (892.5, 3,870.0) and Control renal
insufficiency 656.0 (316.8, 2,172.5) vs. normal renal function
104.0 (37.0, 331.0), respectively; both P<0.01; Fig. 2A and B]. Similarly, the levels of
ADPN (µg/ml) in AHF patients were also significantly affected by
the renal function, since the ADPN level in AHF patients with renal
insufficiency was significantly higher compared with those with
normal renal function [AHF renal insufficiency 12.9 (8.6, 16.5) vs.
normal renal function 10.6 (6.2, 15.7); P=0.04; Fig. 2C]. By contrast, in control patients
(normal cardiac function), there was no evident difference in the
ADPN level between the normal and abnormal renal function groups
[renal insufficiency 5.8 (3.7, 7.5) vs. normal renal function 5.3
(3.3, 7.2); P=0.62; Fig. 2D).
The correlation between AHF serum biomarkers
(NT-proBNP and ADPN) and renal function were then further
investigated. In the present study, the levels of creatinine and
GFR were considered to reflect the renal function. As shown in
Fig. 3A and B, the NT-proBNP levels
were significantly correlated with the renal function. The
NT-proBNP levels were positively correlated with creatinine and
negatively correlated with GFR in both the AHF and control groups.
The correlation coefficients in the control and AHF groups were
0.386 (P<0.01) and 0.343 (P<0.01) for creatinine,
respectively, and −0.488 (P<0.01) and −0.412 (P<0.01) for
GFR, respectively. By contrast, as shown in Fig. 3C and D, ADPN was not significantly
correlated with creatinine and GFR. In the control and AHF groups,
the correlation coefficients for creatinine were observed to be
0.124 (P=0.12) and 0.073 (P=0.29), respectively, while for GFR, the
coefficients were 0.008 (P=0.21) and −0.177 (P=0.16), respectively.
These findings indicated that the ADPN level was affected to a
lesser extent by impaired renal function as compared with the
NT-proBNP level, which may be helpful in diagnosing AHF patients
with impaired renal function.
 | Figure 3.Comparative correlation of ADPN or
NT-proBNP with renal function in the control and AHF groups. Renal
function was examined according to the creatinine level and GFR.
Correlation between NT-proBNP and (A) creatinine or (B) GFR in the
control and AHF groups. The correlation coefficients were 0.386
(P<0.01) and 0.343 (P<0.01) for creatinine, respectively, and
−0.488 (P<0.01) and −0.412 (P<0.01) for GFR, respectively.
The correlation of ADPN with (C) creatinine or (D) GFR in the
control and AHF groups is also displayed. The correlation
coefficients were 0.124 (P=0.12) and 0.073 (P=0.29) for creatinine,
respectively, and 0.008 (P=0.21) and −0.177 (P=0.16) for GFR,
respectively. AHF, acute heart failure; NT-proBNP, amino-terminal
pro-brain natriuretic peptide; ADPN, adiponectin; GFR, glomerular
filtration rate. |
Diagnostic value of ADPN and NT-proBNP
for AHF in normal renal function and impaired renal function
patients
To determine whether the plasma levels of ADPN and
NT-proBNP had diagnostic values in AHF, the ROC curve analysis was
applied to examine their diagnostic efficiency. As shown in
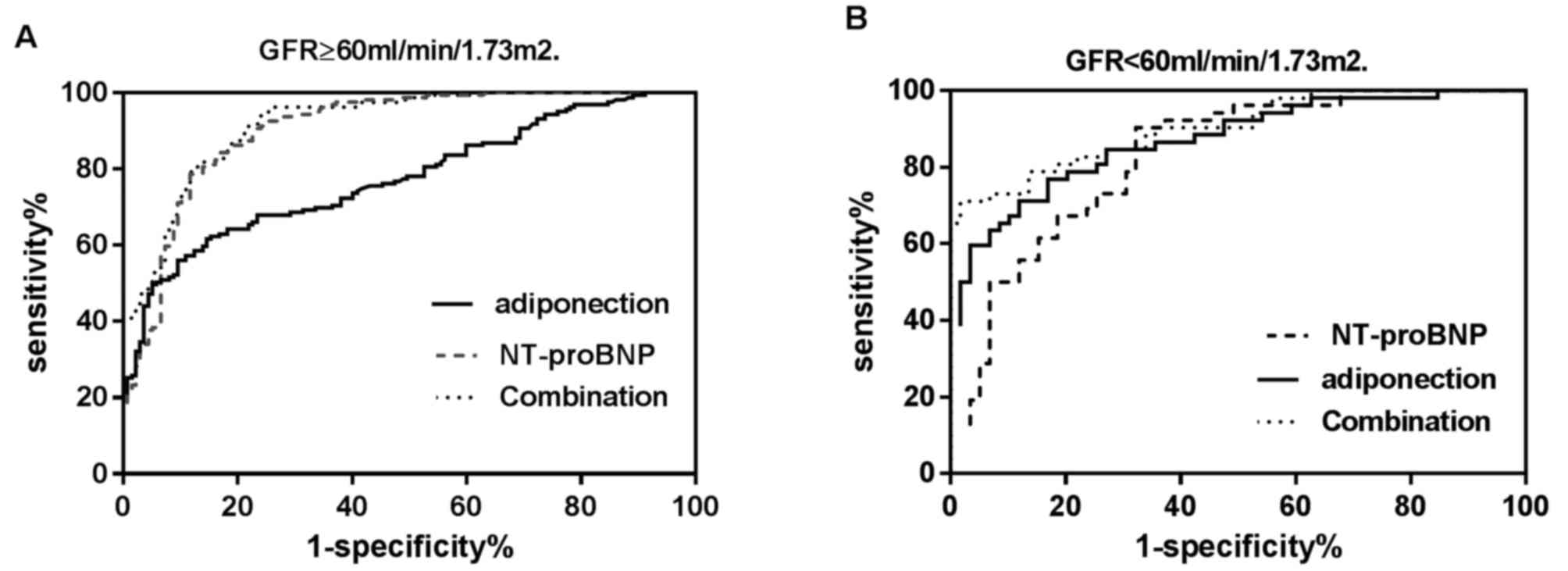
Table IV and Fig. 4A, among the patients with a normal
renal function (n=296), NT-proBNP presented a significantly better
diagnostic efficiency for AHF (AUC=0.905; 95% CI=0.866–0.936) in
comparison with that of ADPN (AUC=0.775; 95% CI=0.723–0.821;
P<0.001). The optimal cutoff value for NT-proBNP was 296.0
pg/ml, while the sensitivity and specificity were 92.1 and 72.3%,
respectively. ADPN achieved an optimal diagnostic efficiency at the
threshold of 10.6 µg/ml, while the sensitivity and specificity were
43.9 and 94.9%, respectively. Compared with NT-proBNP alone, the
combination of the two biomarkers did not exhibit a higher
diagnostic efficiency (AUC=0.920; 95% CI, 0.883–0.948; P=0.12).
 | Table IV.Diagnostic value of ADPN, NT-proBNP
and their combination in acute heart failure patients with impaired
or normal renal function. |
Table IV.
Diagnostic value of ADPN, NT-proBNP
and their combination in acute heart failure patients with impaired
or normal renal function.
|
| ADPN | NT-proBNP | Combination |
|---|
|
|
|
|
|
|---|
| Parameter | Normal renal
function | Renal
insufficiency | Normal renal
function | Renal
insufficiency | Normal renal
functiona | Renal
insufficiencyb |
|---|
| Diagnosis
efficiency | 77.5% | 87.2% | 90.5% | 82.8% | 92.0% | 90.0% |
| CI | 72.3–82.1% | 79.5–89.3% | 86.6–93.6% | 74.5–89.3% | 88.3–94.8% | 83.3–95.3% |
| Cut-off | 10.6 µg/ml | 10.6 µg/ml | 296.0 pg/ml | 1,129.0 pg/ml | 35.0% | 75.0% |
| Sensitivity | 43.9% | 65.4% | 93.1% | 90.4% | 95.0% | 71.2% |
| Specificity | 94.9% | 91.5% | 72.3% | 67.8% | 75.9% | 98.3% |
| PPV | 91.8% | 87.2% | 79.6% | 71.2% | 92.9% | 93.8% |
| NPV | 61.9% | 75.0% | 81.8% | 88.9% | 82.1% | 61.1% |
As shown in Table IV
and Fig. 4B, among patients with an
abnormal renal function (n=111), ADPN presented a better diagnostic
efficiency (AUROC=0.872; 95% CI, 0.795–0.928) in comparison with
that of NT-proBNP (AUROC=0.828, 95%CI: 0.745–0.893), although the
difference was not statistically significant (P=0.34). The optimal
cutoff value for NT-proBNP in the renal insufficiency patients was
1,129.0 pg/ml, with sensitivity and specificity of 90.4 and 67.8%,
respectively. The optimal threshold for ADPN was also 10.6 µg/ml,
and the sensitivity and specificity were 65.4 and 91.5%,
respectively. Compared with NT-proBNP alone, combination of the two
biomarkers significantly increased the diagnostic efficiency
(AUC=0.90; 95% CI, 0.833–0.951; P=0.02). Compared with ADPN alone,
combination of the two biomarkers also significantly increased the
diagnostic efficiency (P=0.04). Combined analysis of the two
biomarkers demonstrated a higher area under the curve (AUC=0.90)
and specificity (98.3%), but with lower sensitivity (70.8%), in
diagnosing AHF in patients.
Diagnostic efficiency of ADPN in AHF
is less affected by age than NT-proBNP
As shown in Table V,
the optimal diagnostic cutoff values of NT-proBNP and ADPN in
different age groups were analyzed. Patients with a normal renal
function (n=218) were divided into three groups according to their
age, namely the groups of ≥75, 50–75 and ≤50 years. The plasma
NT-proBNP levels were affected by the age of patients. The present
study revealed that the optimal cut-off values of NT-proBNP in
diagnosing AHF were 221.5, 335.0 and 1,497.0 pg/ml in these groups,
respectively.
 | Table V.Comparative diagnostic efficiencies
of ADPN and NT-proBNP in participants in different age groups. |
Table V.
Comparative diagnostic efficiencies
of ADPN and NT-proBNP in participants in different age groups.
|
| ADPN | NT-proBNP | Combination |
|---|
|
|
|
|
|
|---|
| Parameter | >75 years | 50–75 years | <50 years | >75 years | 50–75 years | <50 years |
|---|
| AUC (%) | 78.9 | 75.9 | 79.7 | 90.6 | 90.8 | 91.3 |
| Cut-off | 10.6 | 10.6 | 10.6 | 1,497.0 | 335.0 | 221.5 |
| Sensitivity
(%) | 67.9 | 44.1 | 52.6 | 78.6 | 94.6 | 92.1 |
| Specificity
(%) | 87.5 | 95.1 | 100 | 93.7 | 78.8 | 72.7 |
| PPV (%) | 71.4 | 78.4 | 95.8 | 73.9 | 80.7 | 85.3 |
| NPV (%) | 56.5 | 70.3 | 58.3 | 71.4 | 94.0 | 84.2 |
The AUC in individuals aged >75 years was 78.9%
when ADPN was 10.6 µg/ml, and the sensitivity and specificity were
67.9 and 87.5%, respectively (Table
V). The positive and negative predictive rates were 71.4 and
56.5%, respectively. Within the group aged 50–75 years, the AUC was
75.9%, the sensitivity and specificity were 44.1 and 95.1%,
respectively and the positive and negative predictive rates were
78.4 and 70.3%, respectively. In the group aged <50 years, the
sensitivity and specificity were 52.6 and 100.0%, respectively and
the positive and negative predictive rates were 95.8 and 58.3%
respectively. These results suggest that as a diagnostic marker
ADPN was insensitive to age, however it had a lower diagnosis
efficiency compared with NT-proBNP.
Discussion
AHF is the most common cause of unplanned hospital
admissions, and is associated with high mortality rates (2,33), with
the cost of managing AHF being a global burden (34). Efficient and timely diagnosis is
critical for reducing the mortality rates (35). NT-proBNP has long been used as a
routine and rapid diagnostic method for patients with AHF (7–9);
however, the use of NT-proBNP in diagnosing AHF in patients with
renal insufficiency is controversial, since its level is markedly
affected by the renal function (13–15,35). A
significant proportion of patients exhibit functional impairment of
both the heart and kidneys (36,37).
Thus, diagnosing AHF patients with renal insufficiency rapidly and
correctly is challenging in clinical practice.
ADPN is a 247 amino acid peptide that is
predominantly secreted by adipocytes (18) and is recognized as a useful biomarker
in numerous diseases, including fat-associated and heart diseases
(19,29,30,38). As
an adipokine, ADPN has anti-inflammatory and cardioprotective
effects (39). The present study
results demonstrated that plasma ADPN levels increased with the
increase of the NYHA class of patients (Fig. 1A), which was consistent with the
findings of previous studies (28–30). In
addition, previous results indicated that ADPN may have an
anti-inflammatory and anti-atherosclerotic role (40,41).
Another study revealed that regular aerobic exercise decreased the
potential risk of coronary heart disease by improving the plasma
levels of interleukin-6, ADPN, leptin and CRP (42). Furthermore, a cohort study performing
long-term follow-up of the glucose tolerance in patients with acute
myocardial infarction indicated that elevated levels of ADPN
predicted the outcome following acute myocardial infarction
(43), while low ADPN levels may,
indicate coronary artery disease (25,44). In
the present study, the levels of ADPN were found to be lower in
ischemic heart disease as compared with those in non-ischemic AHF
(Fig. 1C and Table III), which was consistent with the
aforementioned results. It was also observed that the level of ADPN
in NYHA cardiac function I participants was higher compared with
that of the control group, the difference was statistically
significant between the two groups (P=0.04); by contrast, NT-proBNP
did not exhibit a marked difference between these two groups.
In the present study, the NT-proBNP levels were
observed to be significantly correlated with the renal function and
age of participants (Tables IV and
V), and the cut-off value for
diagnosing AHF evidently varied in the presence of renal
insufficiency, which made it difficult for physicians to determine
whether the nephropathy was associated with HF. In addition, the
cut-off value of ADPN was not altered in the presence of renal
insufficiency, which facilitated the diagnosis. The use of ADPN as
a marker of AHF exhibited superiority since it was not affected by
the age and renal function of the patients (Tables IV and V). The ADPN receptor 2 is mainly
distributed in the liver, and only a small amount will be filtered
out through the kidneys, indicating that ADPN is less susceptible
to the GFR in comparison with NT-proBNP (45,46). In
the current study, compared with NT-proBNP, the same cut-off value
of ADPN could be utilized to diagnose patients with AHF.
Furthermore, the combination of ADPN and NT-proBNP achieved a
significantly higher diagnostic value compared with NT-proBNP alone
in patients with renal sufficiency. Thus, ADPN may serve as a novel
biomarker in the diagnosis of AHF in patients with abnormal renal
function.
Through the ROC curve analysis of the diagnostic
value of ADPN and NT-proBNP, the results of the present study
indicated that ADPN was not associated with the renal function and
age. While the sensitivity of NT-proBNP was higher compared with
that of ADPN, the specificity of NT-proBNP was lower than that of
ADPN. ADPN and NT-proBNP were used to obtain a logistic regression
equation, and the predictor of the equation was used to diagnose
AHF. The efficiency of the combination of ADPN and NT-proBNP was
better in diagnosing AHF patients with renal insufficiency.
Although the influence of dialysis on the diagnostic efficiency of
these markers was not discussed in the present study, there was no
doubt in assessing the diagnostic efficiency of ADPN for AHF
patients with renal insufficiency based on the aforementioned
results.
Several studies have revealed the association of
serum ADPN levels with the degree of renal failure. For instance,
the association of ADPN with CKD was demonstrated recently in a
case-control study conducted by Lim et al (47) in 450 CKD cases and 920 controls
involving Chinese and Indian adults aged 40–80 years. The authors
observed that a higher level of serum ADPN was positively
associated with CKD independently of traditional risk factors in
the examined Asian population (48).
In other studies, higher ADPN levels were reported in end-stage
renal disease (49,50), whereas the role of ADPN in mildly
impaired renal function was inconsistent, with a previous study
demonstrating lower levels of ADPN were associated with CKD
(51), while others reporting that
higher levels were associated with CKD or no significant
association was observed as discussed by Lim et al (47).
The present study has certain limitations. Firstly,
only two markers were measured for diagnosing AHF. In addition, the
sample size of the study and the number of participants with renal
insufficiency were small. Furthermore, patients with severe renal
impairment were not represented adequately in the current study.
Thus, a large-scale trial comparing several different markers of
AHF is required in order to further evaluate the diagnostic
accuracy of ADPN and NT-proBNP in patients with renal
insufficiency. Finally, patients with hemodialysis need to be
enrolled in order to further evaluate the diagnostic accuracy of
each biomarker.
In conclusion, NT-proBNP demonstrated a higher
diagnostic efficiency compared with ADPN in AHF patients without
renal insufficiency, while ADPN presented a better diagnostic
value. Therefore, a combination of these two biomarkers may provide
improved efficacy in the diagnosis of AHF with renal insufficiency.
In addition, the present study observed that ADPN was less affected
by the renal function and age of patients, and can be used for
diagnosing AHF, particularly in patients with impaired renal
function.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Major Project
of Department of Science and Technology of Fujian Province (grant
no. 2011D018), the Scientific and Technological Project of Xiamen
City (grant no. 3502Z20130006), and the Youth Foundation of Fujian
Province Health Department (grant no. 2012-2-83).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZD, YZ, HY and ZZ conceived and designed the study.
ZD, YZ, HY, GZ, HJ, ZC, YY, ZC, XT, JZ, XL, HX, PL and SG performed
the experiments. ZD and ZZ wrote the paper. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee of Zhongshan Hospital, Xiamen University (Xiamen,
China) and written informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Heidenreich PA, Albert NM, Allen LA,
Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam
MA, Maddox TM, et al: Forecasting the impact of heart failure in
the United States: A policy statement from the American Heart
Association. Circ Heart Fail. 6:606–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marti CN, Georgiopoulou VV and
Kalogeropoulos AP: Acute heart failure: Patient characteristics and
pathophysiology. Curr Heart Fail Rep. 10:427–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ,
Howard VJ, et al: Heart disease and stroke statistics-2015 update:
A report from the American Heart Association. Circulation.
131:e29–e322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fabbri A, Marchesini G, Carbone G,
Cosentini R, Ferrari A, Chiesa M, Bertini A and Rea F: Acute heart
failure in the emergency department: A follow-up study. Intern
Emerg Med. 11:115–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Collins S, Storrow AB, Albert NM, Butler
J, Ezekowitz J, Felker GM, Fermann GJ, Fonarow GC, Givertz MM,
Hiestand B, et al: Early management of patients with acute heart
failure: state of the art and future directions. A consensus
document from the society for academic emergency medicine/heart
failure society of America acute heart failure working group. J
Card Fail. 21:27–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Madika AL, Fertin M, Hebbar E and Lamblin
N: 0202: Predictive factors of left ventricular recovery in acute
heart failure revealing reduced left ventricular ejection fraction.
Arch Cardiovas Dis Suppl. 8:262016.
|
|
7
|
Januzzi JL Jr, Camargo CA, Anwaruddin S,
Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT,
Chae CU, et al: The N-terminal Pro-BNP investigation of dyspnea in
the emergency department (PRIDE) study. Am J Cardiol. 95:948–954.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roberts E, Ludman AJ, Dworzynski K,
Al-Mohammad A, Cowie MR, McMurray JJ and Mant J: NICE Guideline
Development Group for Acute Heart Failure: The diagnostic accuracy
of the natriuretic peptides in heart failure: Systematic review and
diagnostic meta-analysis in the acute care setting. BMJ.
350:h9102015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steinhart B, Thorpe KE, Bayoumi AM, Moe G,
Januzzi JL Jr and Mazer CD: Improving the diagnosis of acute heart
failure using a validated prediction model. J Am Coll Cardiol.
54:1515–1521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsue Y, Suzuki M, Torii S, Yamaguchi S,
Fukamizu S, Ono Y, Fujii H, Kitai T, Nishioka T, Sugi K, et al:
Prognostic impact of early treatment with tolvaptan in patients
with acute heart failure and renal dysfunction. Int J Cardiol.
221:188–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mcculloμgh PA: Scope of cardiovascular
complications in patients with kidney disease. Ethn Dis.
12:S3-44-82002.PubMed/NCBI
|
|
12
|
Homsak E and Ekart R: Hemodiafiltration
affects NT-proBNP but not ST2 serum concentration in end-stage
renal disease patients. Clin Biochem. 49:1159–1163. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang K, Li HL, Chen LL, Bei WJ, Lin KY,
Smyth B, Chen SQ, Guo XS, Guo W, Liu YH, et al: Association of
N-terminal pro-brain natriuretic peptide with contrast-induced
acute kidney injury and long-term mortality in patients with heart
failure and mid-range ejection fraction: An observation study.
Medicine (Baltimore). 96:e62592017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chenevier-Gobeaux C, Claessens YE, Voyer
S, Ekindjian OG, Ginsburg C and Desmoulins D: Concentrations
plasmatiques du peptide natriurétique type B (BNP) et du fragment
N-terminal du propeptide (NT-proBNP) aux urgences: Influence de la
fonction rénale. Immuno-Anal Biol Spé. 20:295–300. 2005.
|
|
15
|
Rothenburger M, Stypmann J, Wichter T, et
al: The role of NT-proBNP in chronic heart failure and renal
insufficiency. Thorac Cardiovasc Surg. 53:618–624. 2005. View Article : Google Scholar
|
|
16
|
Herrero-Puente P, Prieto-García B,
García-García M, Jacob J, Martín-Sánchez FJ, Pascual-Figal D, Bueno
H, Gil V, Llorens P, Vázquez-Alvarez J, et al: Predictive capacity
of a multimarker strategy to determine short-term mortality in
patients attending a hospital emergency Department for acute heart
failure. BIO-EAHFE study. Clin Chim Acta. 466:22–30. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Demissei BG, Cotter G, Prescott MF, Felker
GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Severin TM,
Wang Y, et al: A multimarker multi-time point-based risk
stratification strategy in acute heart failure: Results from the
RELAX-AHF trial. Eur J Heart Fail. 19:1001–1010. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scherer PE, Williams S, Fogliano M,
Baldini G and Lodish HF: A novel serum protein similar to C1q,
produced exclusively in adipocytes. J Biol Chem. 270:26746–26749.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pischon T, Girman CJ, Hotamisligil GS,
Rifai N, Hu FB and Rimm EB: Plasma adiponectin levels and risk of
myocardial infarction in men. JAMA. 291:1730–1737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zapata RC, Meachem MD, Cardoso NC, Mehain
SO, McMillan CJ, Snead ER and Chelikani PK: Differential
circulating concentrations of adipokines, glucagon and adropin in a
clinical population of lean, overweight and diabetic cats. BMC Vet
Res. 13:852017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bednarska-Makaruk M, Graban A, Wiśniewska
A, Łojkowska W, Bochyńska A, Gugała-Iwaniuk M, Sławińska K,
Ługowska A, Ryglewicz D and Wehr H: Association of adiponectin,
leptin and resistin with inflammatory markers and obesity in
dementia. Biogerontology. 18:561–580. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mazur-Bialy AI, Bilski J, Wojcik D,
Brzozowski B, Surmiak M, Hubalewska-Mazgaj M, Chmura A, Magierowski
M, Magierowska K, Mach T and Brzozowski T: Beneficial effect of
voluntary exercise on experimental colitis in mice fed a high-fat
diet: The role of irisin, adiponectin and proinflammatory
biomarkers. Nutrients. 9:pii: E410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bergmark BA, Cannon CP, White WB, Jarolim
P, Liu Y, Bonaca MP, Zannad F and Morrow DA: Baseline Baseline
adiponectin concentration and clinical outcomes among patients with
diabetes and recent acute coronary syndrome in the EXAMINE trial.
Diabetes Obes Metab. 19:962–969. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ng RC and Chan KH: Potential
neuroprotective effects of adiponectin in Alzheimer's disease. Int
J Mol Sci. 18:pii: E592. 2017. View Article : Google Scholar
|
|
25
|
Norvik JV, Schirmer H, Ytrehus K, Jenssen
TG, Zykova SN, Eggen AE, Eriksen BO and Solbu MD: Low adiponectin
is associated with diastolic dysfunction in women: a
cross-sectional study from the Tromsø Study. BMC Cardiovasc Disord.
17:792017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsukamoto O, Fujita M, Kato M, Yamazaki S,
Asano Y, Ogai A, Okazaki H, Asai M, Nagamachi Y, Maeda N, et al:
Natriuretic peptides enhance the production of adiponectin in human
adipocytes and in patients with chronic heart failure. J Am Coll
Cardiol. 53:2070–2077. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yanavitski M and Givertz MM: Novel
biomarkers in acute heart failure. Curr Heart Fail Rep. 8:206–211.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin WH, Wei J, Huang WP, Chen JW, Young MS
and Lin SJ: Prognostic value of circulating adipokine levels and
expressions of adipokines in the myocardium of patients with
chronic heart failure. Circ J. 76:2139–2147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu HP, Jen HL, Yin WH and Wei J:
Circulating adiponectin levels following treatment can predict late
clinical outcomes in chronic heart failure. Acta Cardiol Sin.
33:139–149. 2017.PubMed/NCBI
|
|
30
|
Park M and Sweeney G: Direct effects of
adipokines on the heart: Focus on adiponectin. Heart Fail Rev.
18:631–644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McMurray JJ, Adamopoulos S, Anker SD,
Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C,
Gomez-Sanchez MA, et al: ESC guidelines for the diagnosis and
treatment of acute and chronic heart failure 2012: The task force
for the diagnosis and treatment of acute and chronic heart failure
2012 of the European Society of Cardiology. Developed in
collaboration with the heart failure association (HFA) of the ESC.
Eur J Heart Fail. 14:803–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lamb EJ, Webb MC and O'Riordan SE: Using
the modification of diet in renal disease (MDRD) and Cockcroft and
Gault equations to estimate glomerular filtration rate (GFR) in
older people. Age Ageing. 36:689–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Teixeira A, Arrigo M, Tolppanen H, Gayat
E, Laribi S, Metra M, Seronde MF, Cohen-Solal A and Mebazaa A:
Management of acute heart failure in elderly patients. Arch
Cardiovasc Dis. 109:422–430. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fonseca C, Araújo I, Marques F, Brás D and
Bettencourt P: A closer look at acute heart failure: Putting
Portμguese and European data into perspective. Rev Port Cardiol.
35:291–304. 2016.(In English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Collins SP, Storrow AB, Levy PD, Albert N,
Butler J, Ezekowitz JA, Felker GM, Fermann GJ, Fonarow GC, Givertz
MM, et al: Early management of patients with acute heart failure:
State of the art and future directions-a consensus document from
the SAEM/HFSA acute heart failure working group. Acad Emerg Med.
22:94–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Harbaum L, Hennigs JK, Baumann HJ,
Lüneburg N, Griesch E, Bokemeyer C, Grünig E and Klose H:
N-terminal pro-brain natriuretic peptide is a useful prognostic
marker in patients with pre-capillary pulmonary hypertension and
renal insufficiency. PLoS One. 9:e942632014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nishikimi T, Futoo Y, Tamano K, Takahashi
M, Suzuki T, Minami J, Honda T, Uetake S, Asakawa H, Kobayashi N,
et al: Plasma brain natriuretic peptide levels in chronic
hemodialysis patients: influence of coronary artery disease. Am J
Kidney Dis. 37:1201–1208. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McCulloμgh PA: Cardiorenal risk: An
important clinical intersection. Rev Cardiovasc Med. 3:71–76.
2002.PubMed/NCBI
|
|
39
|
Bersch-Ferreira ÂC, Sampaio GR, Gehringer
MO, Ross-Fernandes MB, Kovacs C, Alves R, Pereira JL, Magnoni CD,
Weber B and Rogero MM: Association between polyunsaturated fatty
acids and inflammatory markers in patients in secondary prevention
of cardiovascular disease. Nutrition. 37:30–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smekal A and Vaclavik J: Adipokines and
cardiovascular disease: A comprehensive review. Biomed Pap Med Fac
Univ Palacky Olomouc Czech Repub. 161:31–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baldasseroni S, Mannucci E, Orso F, Di
Serio C, Pratesi A, Bartoli N, Marella GA, Colombi C, Foschini A,
Valoti P, et al: Adiponectin in outpatients with coronary artery
disease: Independent predictors and relationship with heart
failure. Nutr Metab Cardiovasc Dis. 22:292–299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang H, Mo X, Hao Y, Huang J, Lu X, Cao J
and Gu D: Adiponectin levels and risk of coronary heart disease: A
meta-analysis of prospective studies. Am J Med Sci. 345:455–461.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akbarpour M: The effect of aerobic
training on serum adiponectin and leptin levels and inflammatory
markers of coronary heart disease in obese men. Biol Sport.
30:21–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ritsinger V, Brismar K, Malmberg K,
Mellbin L, Näsman P, Rydén L, Söderberg S, Tenerz Å and Norhammar
A: Elevated levels of adipokines predict outcome after acute
myocardial infarction: A long-term follow-up of the glucose
tolerance in patients with acute myocardial Infarction cohort. Diab
Vasc Dis Res. 14:77–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Arsenault BJ, Kohli P, Lambert G, DeMicco
DA, Laskey R, Messig MM, Kastelein JJ and Waters DD: Emerging
cardiovascular disease biomarkers and incident diabetes mellitus
risk in statin-treated patients with coronary artery disease (from
the treating to new targets [TNT] study). Am J Cardiol.
118:494–498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dries DL, Exner DV, Domanski MJ, Greenberg
B and Stevenson LW: The prognostic implications of renal
insufficiency in asymptomatic and symptomatic patients with left
ventricular systolic dysfunction. J Am Coll Cardiol. 35:681–689.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lim CC, Teo BW, Tai ES, Lim SC, Chan CM,
Sethi S, Wong TY and Sabanayagam C: Elevated serum leptin,
adiponectin and leptin to adiponectin ratio is associated with
chronic kidney disease in Asian adults. PLoS One. 10:e01220092015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Markaki A, Psylinakis E and Spyridaki A:
Adiponectin and end-stage renal disease. Hormones (Athens).
15:345–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Taherimahmoudi M, Ahmadi H, Mehrsai A and
Pourmand G: Plasma adiponectin concentration and insulin
resistance: role of successful kidney transplantation. Transplant
Proc. 42:797–800. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bakkaloglu SA, Buyan N, Funahashi T,
Pasaoglu H, Elhan AH, Hasanoglu E and Soylemezoglu O: Adiponectin
levels and atherosclerotic risk factors in pediatric chronic
peritoneal dialysis patients. Perit Dial Int. 25:357–361.
2005.PubMed/NCBI
|
|
51
|
Kamimura MA, Canziani ME, Sanches FR,
Velludo CM, Carrero JJ, Bazanelli AP, Draibe SA and Cuppari L:
Variations in adiponectin levels in patients with chronic kidney
disease: A prospective study of 12 months. J Bras Nefrol.
34:259–265. 2012. View Article : Google Scholar : PubMed/NCBI
|