Introduction
Stroke is associated with high morbidity and
mortality, with ischemic stroke being the most common type
(1,2). Cerebellar infarction is a less common
subtype of ischemic stroke, which may arise from thrombophilia
causing both deep venous thrombosis and pulmonary embolism. Abraham
et al (3) indicated that 25%
of strokes occur in young patients aged <40 years. Stroke in
young individuals combined with disability may cause a heavy burden
on their family, as they are frequently the major economic and
labor source of their family. Protein C, a glycoprotein encoded by
the PROC gene, is synthesized in the liver and activated by
thrombin on the surface of endothelial cells. Protein S has an
anti-coagulation role in the presence of protein C by inhibiting
factors Va and VIIIa (4,5). Studies have indicated that inherited or
acquired protein C and protein S deficiency may be a cause of
thrombophilia. Patients with protein C/S deficiency usually develop
deep venous thrombosis (DVT), including lower limb DVT, while
arterial thrombosis is relatively rare. The present study reported
on an unusual case of hereditary protein C deficiency due to a
c.565C>T heterozygous mutation in the PROC gene in a young woman
who experienced recurrent ischemic stroke. Further pedigree
validation analysis was performed, which confirmed that the same
gene locus mutation was present in the patient's father.
Case report
Methods
A 35-year-old Asian woman was admitted to the First
Affiliated Hospital of Guangxi Medical University (Nanning, China)
in January 2017, with symptoms of weakness in the right side of her
limb and fatigue for 19 days. Initially, she was able to hold
objects with her right arm but walked with a dragging step. The
weakness of her right limb progressed and she was unable to walk
independently and hold chopsticks. At 9 days prior to presentation,
she started vomiting and experienced dizziness. It became so severe
that she was admitted to the local hospital. She was treated with
anti-nausea drugs and stomach protection drugs, but experienced no
obvious improvements in the weakness of the right side of her limb.
Her symptoms persisted to the point where ambulation was difficult,
thus prompting her to visit the neurological outpatient
department.
A review of the patient's medical history revealed
the diagnosis of portal vein and superior mesenteric artery
occlusion 4 years ago, and a successful surgery had been performed.
Furthermore, the patient suffered from systemic lupus erythematosus
(SLE) and received regular treatment with aspirin and
hydroxychloroquine sulfate pills. She also suffered from
hyperthyroidism and was treated with propylthiouracil tablets. No
obesity, diabetes or hypertension were present. The patient was
allergic to animal hair, but no drug allergies were detectable.
Tobacco and alcohol use was denied. Furthermore, no hereditary
disease was recorded for the patient's family.
The patient's vital signs were normal when she
arrived at the department of neurology with an axillary temperature
of 36.3°C, heart rate of 78 beats per minute, blood pressure of
127/72 mmHg and respiratory rate of 19 breaths per minute. No
positive signs were observed on general examination. Nervous system
examination indicated vague speech and her tongue lagged to the
right side. A muscle strength test indicated that her right-side
strength was decreased (level IV, according to the UK Medical
Research Council RT01 trial) (6).
Results
Laboratory test results indicated a low glucose 6
phosphate dehydrogenase activity of 5.02 (normal range,
6.80–20.50). Auto-antibody tests revealed positive results for
anti-nuclear antibody. Blood coagulation function and D-dimer level
was normal, but clotting factor activity was abnormal. The level of
clotting factor XII was decreased (57.5%; normal range, 78–112).
However, clotting factor VIII (152%; normal range, 78–128) and
factor XI (118.9%; normal range, 82–118) exhibited increased
activity. The results of her routine blood test, blood glucose,
electrolytes and thyroid function were normal. Electrocardiogram
results were normal. The long-range electrocardiogram results
revealed sinus arrhythmia. Cerebrovascular Doppler examination was
normal.
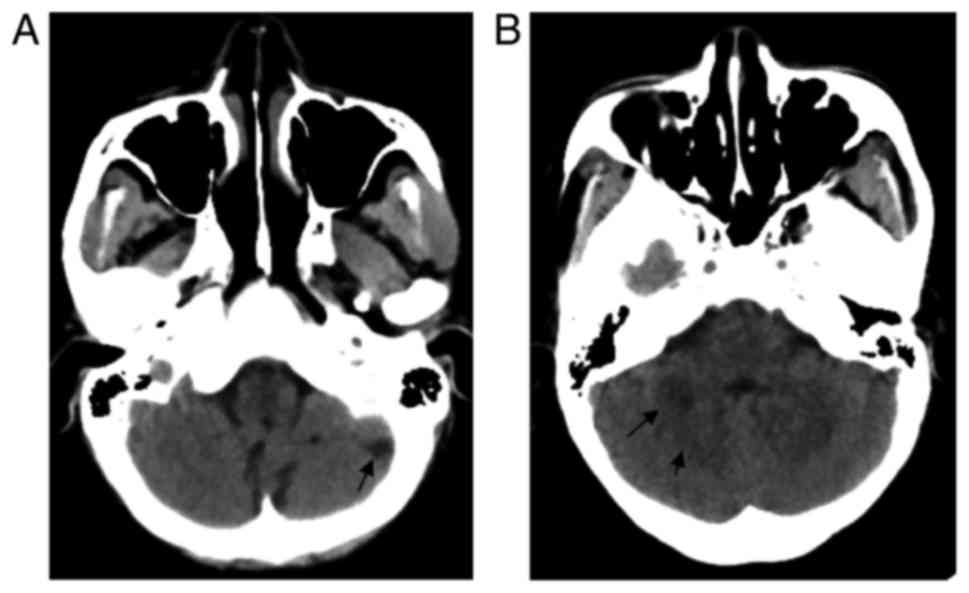
A computed tomography (CT) scan of the brain was
performed immediately and indicated multiple low-density lesions on
the bilateral sides of the cerebellar hemisphere and cerebellar
vermis (Fig. 1). Magnetic resonance
imaging (MRI) was performed to confirm ischemic lesions (Fig. 2), which was indicative of acute
cerebral infarction of the right cerebellar hemisphere and
cerebellar vermis, and a previous cerebral infarction of the left
cerebellar hemisphere. CT angiography (CTA) was also performed to
identify whether any of the neck and intracranial arteries were
narrow; however, the results indicated that no obvious narrow
artery was detectable (Fig. 3).
Considering that the patient had a previous history
of portal vein and superior mesenteric artery occlusion, as well as
asymptomatic strokes, thrombophilia (acquired or inherited) was
considered. Further evidence supported this diagnosis. Protein C
and protein S levels were detected, revealing a decreased level of
protein C (57.6%; normal range, 70–140%), but normal levels of
protein S (71.6%; normal range, 60–130%) and antithrombin III
(92.4%; normal range, 75–125%). Gene sequencing analysis associated
with thrombosis was performed by Guangzhou Kingmed Diagnostics
Group Co., Ltd. (GuangZhou, China), which analyzed the pedigree as
well as her mother and father, and revealed a heterozygous mutation
c.565C>T on the PROC gene (Fig.
4).
Once a diagnosis is available, a suitable treatment
plan is essential. In the present case, the woman was taking the
long-term anti-platelet agent aspirin. However, a recurrence of
arterial thrombosis still occurred. Heparinization therapy
replacing the oral anti-coagulant therapy was considered beneficial
for preventing further thrombosis.
Discussion
Ischemic stroke is divided into five types according
to the Trial of ORG 10172 in Acute Stroke Treatment classification:
Large artery atherosclerosis, cardioembolism, small artery
occlusion, and stroke of other determined and undetermined etiology
(7). The present case study reported
on a young woman who suffered from recurrent cerebellar infarction,
which was confirmed by CT and MRI scans. Imaging data suggested
that the patient had experienced multiple stroke lesions, including
a previous cerebral infarction, which indicated that she had a
previous asymptomatic stroke. CTA was performed, and no large
artery stenosis was noted. Since the patient had a history of
portal vein and superior mesenteric artery occlusion and recurrent
stroke, the possibility of thrombophilia was considered. Protein C
levels were decreased, and gene sequencing analysis of
thrombosis-associated genes in the patient and her family members
indicated one c.565C>T heterozygous mutation in the PROC gene in
the patient and the patient's father. However, the mother had no
mutation on the same locus, which indicated that the father was the
source of the gene mutation. Nucleotide mutation on Chr2-128183690
of the PROC gene has been reported to be associated with protein C
deficiency (8). In the Human Gene
Mutation Database, there are >270 types of mutation on different
loci of the PROC gene associated with hereditary protein C
deficiency were recorded.
A clinical feature of protein C
deficiency-associated thrombophilia is recurrent venous thrombosis.
However, associated arterial thrombosis, particularly recurrent
artery thrombosis, remains relatively rare. Numerous high-risk
situations may cause a thrombotic event, including major surgery,
oral contraceptives, pregnancy and co-existing rheumatoid immune
diseases. The case of the present study had hyperthyroidism and
SLE, which may have increased her risk of developing thrombosis.
Accordingly, the clinical manifestation of protein C deficiency is
variable among individuals and may be complicated by underlying
diseases or risk factors. Members of the patient's family,
including the patient's father who had the same PROC gene mutation
may be asymptomatic. Further studies on pedigrees with PROC gene
mutation-associated thrombophilia are required to explore the
diversity of clinical episodes of thrombophilia among affected
individuals.
A literature search of the PubMed database for
studies on cases of stroke in young individuals (<45 years old)
associated with protein C/S deficiency was then performed without
any publishing time limits but restriction to English language. The
Chinese National Knowledge Internet and Wangfang databases were
also searched for relevant studies in Chinese. A total of 9 English
case reports were acquired (9–17), but
no relevant Chinese case report was retrieved. A total of 4 studies
reported on patients without any history of disease or risk factors
who suffered a stroke (9,12,15,17).
Matsushita et al (13)
reported on a young woman taking long-term oral anti-cancer drugs
prior to experiencing a stroke. Risk factors were noted in several
patients: A history of miscarriage or DVT (14), and accompanying transient ischemic
attack (10) or myocardial
infarction (16) were present in
certain cases. Most patients had familiar protein C deficiency, but
no further gene sequencing test was performed. To investigate the
novelty of the mutation locus on the PROC gene identified in the
present study, the single nucleotide polymorphism database
(https://www.ncbi.nlm.nih.gov/snp/)
was searched. A total of 4 public variants were retrieved using the
NM_000312.3 transcript reference sequence: PROC:c.-50A>T,
PROC:NM_000312.3:c.-50A>T, PROC:c.423G>T and
PROC:NM_000312.3NM_000312.3:c.423G>T, not including the mutation
locus identified in the present case. Therefore, in the present
study, the c.565C>T heterozygous mutation on the PROC gene
(chromosomal location, chr2-128183690; transcript reference no.
NM_000312) was discovered in the patient and the patient's father
for the first time, and pathogenicity analysis indicated a
pathogenic nature, which further confirmed the diagnosis of
hereditary protein C deficiency. However, additional study is
required to discover the mechanisms of gene mutation.
In summary, the etiology and pathogenesis of stroke
are complex, and hematologic abnormalities may contribute to stroke
in young individuals. For young stroke patients, detection of
protein C and protein S levels, as well as sequencing analysis of
pathogenic genes and validation in their family, is recommended,
even in the absence of a recorded family history of thrombotic
events. Further prospective studies should be performed to explore
the link between heterozygous protein C deficiency and ischemic
stroke, as well as the underlying mechanisms.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CQ designed the case report and revised the
manuscript critically for important content. PL acquired and
analyzed the data and drafted the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Strong K, Mathers C and Bonita R:
Preventing stroke: Saving lives around the world. Lancet Neurol.
6:182–187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warlow C, Sudlow C, Dennis M, Wardlaw J
and Sandercock P: Stroke. Lancet. 362:1211–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abraham J, Rao PS, Inbaraj SG, Shetty G
and Jose CJ: An epidemiological study of hemiplegia due to stroke
in South India. Stroke. 1:477–481. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clouse LH and Comp PC: The regulation of
hemostasis: The protein C system. N Engl J Med. 314:1298–1304.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kisiel W, Canfield WM, Ericsson LH and
Davie EW: Anticoagulant properties of bovine plasma protein C
following activation by thrombin. Biochemistry. 16:5824–5831. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sydes MR, Stephens RJ, Moore AR, Aird EG,
Bidmead AM, Fallowfield LJ, Graham J, Griffiths S, Mayles WP,
McGuire A, et al: Implementing the UK Medical Research Council
(MRC) RT01 trial (ISRCTN 47772397): Methods and practicalities of a
randomised controlled trial of conformal radiotherapy in men with
localised prostate cancer. Radiother Oncol. 72:199–211. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams H Jr and Biller J: Classification of
subtypes of ischemic stroke: History of the trial of org 10172 in
acute stroke treatment classification. Stroke. 46:e114–e117. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reitsma PH, Bernardi F, Doig RG, Gandrille
S, Greengard JS, Ireland H, Krawczak M, Lind B, Long GL, Poort SR,
et al: Protein C deficiency: A database of mutations, 1995 update.
On behalf of the subcommittee on plasma coagulation inhibitors of
the scientific and standardization committee of the ISTH. Thromb
Haemost. 73:876–889. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deguchi K, Tsukada T, Iwasaki E, Wada H,
Murashima S, Miyazaki M and Shirakawa S: Late-onset homozygous
protein C deficiency manifesting cerebral infarction as the first
symptom at age 27. Intern Med. 31:922–925. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato H, Shirahama M, Ohmori K and Sunaga
T: Cerebral infarction in a young adult associated with protein C
deficiency. A case report. Angiology. 46:169–173. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kazui S, Kuriyama Y, Sakata T, Hiroki M,
Miyashita K and Sawada T: Accelerated brain infarction in
hypertension complicated by hereditary heterozygous protein C
deficiency. Stroke. 24:2097–2103. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martinez HR, Rangel-Guerra RA and Marfil
LJ: Ischemic stroke due to deficiency of coagulation inhibitors.
Report of 10 young adults. Stroke. 24:19–25. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsushita K, Kuriyama Y, Sawada T and
Uchida K: Cerebral infarction associated with protein C deficiency.
Stroke. 23:108–111. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okon MA and Spooner SF: Protein C
deficiency and stroke in pregnancy. J Obstet Gynaecol. 18:182–183.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sultan A and Malik IH: Recurrent cerebral
infarctions in a young patient: Combined protein C and S
deficiencies. J Coll Physicians Surg Pak. 23:813–814.
2013.PubMed/NCBI
|
|
16
|
Tiong IY, Alkotob ML and Ghaffari S:
Protein C deficiency manifesting as an acute myocardial infarction
and ischaemic stroke. Heart. 89:E72003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang FC, Hsu CH, Lin JC, Chen CY and Lee
JT: Inherited protein C deficiency with acute ischemic stroke in a
young adult: A case report. Blood Coagul Fibrinolysis. 19:601–604.
2008. View Article : Google Scholar : PubMed/NCBI
|