Introduction
Cardiomyopathy (CM) is a common chronic disease that
leads to heart failure (HF). Parathyroid hormone (PTH), a 4-amino
acid peptide secreted by parathyroid cells, is the major regulator
of blood calcium and phosphorus metabolism and regulates the
cardiovascular system via the G-protein-coupled parathyroid hormone
receptor (1,2). Extensive studies have been performed to
investigate the association between PTH and HF, particularly the
therapeutic potential of PTH in HF and myocardial injury (3–6).
Previous studies have indicated that PTH enhances myocardial
contractility and improves cardiac function by influencing the
shortening fraction and autorhythmicity (7,8).
Additionally, PTH directly dilates the coronary artery and promotes
myocardial microcirculation, thereby improving the myocardial
oxygen supply and cardiac pump function (9–12).
Furthermore, PTH regulates the secretion and expression of stromal
derived factor 1, matrix metalloproteinase 9 and granulocyte colony
stimulating factor in the bone marrow and induces the mobilization
and homing of cluster of differentiation (CD)34/CD45-positive bone
marrow stem cells, thereby promoting the release of vascular
endothelial growth factor and angiogenesis (13–16).
Some studies using animal-model systems have verified that
injection of PTH promotes the production of endothelium-derived
colony stimulating factor, angiogenesis and cell viability, thereby
reducing myocardial necrosis in rats with myocardial infarction
(17–20). Therefore, PTH may be applicable as a
therapeutic agent for acute myocardial infarction and ischemic CM.
However, the potential therapeutic value of PTH for non-ischemic CM
has not been determined.
In the present study, a rat model of Adriamycin
(ADR)-induced CM was established. Recombinant PTH (rPTH) was
administered and its effects on cardiac function and the underlying
mechanisms were evaluated. Results from the study provide a
theoretical basis for the potential application of PTH as a
treatment for non-ischemic CM.
Materials and methods
Animals
A total of 30 Sprague-Dawley rats (age, 12–16 weeks;
male; weight, 250 g) were purchased from Nanjing Qingzilan
Technology (Nanjing, China) and randomly divided into a normal
control (NC) group (n=6) and an experimental group (n=24). The
ADR-induced CM in a rat model was established in the experimental
group of animals according to the method previously described by
Teraoka et al (21). Briefly,
at total of 5 injections of 2 mg/kg ADR (Shanghai Dibo Chemical
Technology Co., Ltd., Shanghai, China) was administered
intraperitoneally every third day for a total of 15 days followed
by a further injection every week for 5 weeks, for a total
cumulative dose of 20 mg/kg. Age-matched rats in the NC group
received intraperitoneal injections of normal saline. The rats were
housed at a temperature and humidity of 20–25°C and 40–70%,
respectively, with a 12 h light/dark cycle and ad libitum
access to food and water. Under these conditions, rat physical
activity, food intake, urine-output volume and mental status were
monitored. Cardiac ultrasonography and plasma B-type natriuretic
peptide (BNP) levels were assessed to confirm the successful
establishment of CM. Following a total of 10 weeks, rats in the
experimental group were randomly subdivided into the PTH-untreated
CM group and three CM treatment groups. In the NC and PTH-untreated
CM group, rats received daily mock-treatments for 7 days consisting
of subcutaneous injections of normal saline. For the CM treatment
groups, CM-induced rats were subdivided into three equal subgroups
and received daily subcutaneous injections of rPTH (Rattus
norvegicus, Residues Ala32-Gln115; Cloud-Clone Corp., Wuhan,
China) at doses of 5, 10 or 20 µg/kg for 7 days. The subgroups were
termed PTH-5, PTH-10, and PTH-20, respectively.
The present study was approved by the Animal Care
and Use Committee of Anhui Medical University (Wuxi, China) and all
animals received care compliant with standards of the Guide for the
Care and Use of Laboratory Animals published in 1988 by The
National Academies.
Measurement of biochemical
indices
A total of 10 weeks following the start of the
experiment, blood was collected from the femoral arteries of 5
randomly selected rats from each of the NC and experimental groups
and the sera were processed. Concentrations of PTH, BNP, C-reactive
protein (CRP), troponin T and electrolytes in the sera were
determined prior to the establishment of the five subgroups. At 11
weeks following the start of the experiment, blood samples of rats
in the five subgroups were collected again and the sera was
analyzed for PTH, BNP, CRP, troponin T and electrolyte
concentrations. The levels of PTH (cat. no. E-EL-R0714c), BNP (cat.
no. E-EL-R0126c), and troponin T (cat. no. E-EL-R0054c) were
determined using the appropriate rat ELISA kits (Wuhan Elabscience
Biotechnology Co., Ltd., Wuhan, China). The biochemical indices
were determined using an automated biochemical analyzer (AU480;
Beckman Coulter, Inc., Brea, CA, USA).
Cardiac ultrasonography
Following 10 weeks of starting of the experiments,
prior to the establishment of the five subgroups, 5 randomly
selected rats from each of the NC and experimental groups were
evaluated using a high-resolution ultrasound system for small
animal imaging (Vevo 2100; VisualSonics Inc., Toronto, ON, Canada).
Ultrasonic determination of the left atrial diameter,
interventricular septal thickness, left ventricular end-diastolic
volume (LVEDV), left ventricular end-systolic volume (LVESV), left
ventricular fractional shortening (LVFS) and left ventricular
ejection fraction (LVEF) was performed for each rat analyzed.
Cardiac ultrasonography was performed again at week 11.
Preparation of samples
At the end of the experiments, the rats were
sacrificed by cervical dislocation and the hearts were harvested
for paraffin-embedded sectioning and histological analyses. In
brief, 10% formalin-fixed (4°C, ~48 h) and paraffin-embedded heart
tissues were transversely cut into 4-µm thick sections.
Immunohistochemical staining of PTH on the tissue sections was
performed at room temperature for 200–240 min with assistance from
personnel from the Department of Pathology at the Third People's
Hospital of Zhenjiang (Zhenjiang, China). PTH expression was
observed and photographed under an optical microscope (cat. no.
CX41-32C02; Olympus Corporation, Tokyo, Japan; magnification,
×40-400). Hematoxylin and eosin (HE) staining (cat. no. G1005) and
Masson's trichome staining (cat. no. G1006) of paraffin sections
were performed using kits (Wuhan Goodbio Technology Co., Ltd.
Wuhan, China). HE staining was performed at room temperature for
~120 min and Masson's trichome staining was performed at room
temperature for ~130 min. Pictures were obtained using a digital
slice scanning analysis system (Pannoramic P250; 3DHISTECH Ltd.,
Budapest, Hungary).
Furthermore, western blot analysis was also
performed to detect the protein expression of PTH in the myocardial
tissues. Total cell lysates for western blot analysis were prepared
using radioimmunoprecipitation assay lysis buffer (pH=8.0; 150 mM
NaCl, 0.5% sodium deoxycholate, 1.0% Triton x-100, 50 mM Tris, and
0.1% SDS). Protein concentrations were measured using a BCA Protein
Assay Kit (Thermo Scientific, Inc.). Cell lysates containing 20 µg
of protein were boiled for 10 min in sample loading buffer mixed
with reducing reagent (Thermo Fisher Scientific, Inc.) prior to
separation by SDS-PAGE. The protein samples were
electrophoretically separated on NuPAGE Novex 10% Bis-Tris gels
commercially available from Thermo Fisher Scientific, Inc. and then
transferred to polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membrane blots were blocked in 5% non-fat
dry milk in 0.05% PBST at room temperature for 1 h, and incubated
with the anti-PTH polyclonal antibody (Cloud-Clone Corp.; cat. no.
Ala32-Gln115; 1:1,000) at 4°C for 2 h. After washing with 0.05%
PBST, membranes were then incubated with the horseradish peroxidase
conjugated-anti-Rabbit secondary antibody (Jackson ImmunoResearch
Europe Ltd.,. Cambridgeshire, UK; cat. no. 111-035-003; 1:5,000) at
room temperature for 1 h. Western blots were then developed using
an ECL reagent (Merck KGaA, Darmstadt, Germany) and detected using
a Tanon 5200 Multi System (Tanon Science and Technology Co., Ltd.,
Shanghai, China). The housekeeping β-actin gene was used as a
control reference gene.
The mRNA expression levels of PTH and BNP were
measured using RT-qPCR. Briefly, total RNA was extracted using
TRIzol (Thermo Fisher Scientific., Inc.) and the cDNA was
synthesized using a First-Strand cDNA Synthesis Kit (Thermo Fisher
Scientific, Inc.). PCR mixtures contained 10 µl SYBR Green I
(Takara Bio Inc., Otsu, Japan), 0.2 µM sense primer, 0.2 µM
antisense primer, 2 µl cDNA and 7.6 µl H2O in a total
volume of 20 µl. Primers are indicated in Table I. The primers were synthesized by
Shanghai Rui Mian Biological Technology Co., Ltd. (Shanghai,
China). GAPDH was used as an internal control reference gene.
 | Table I.Details of primers used in the present
study. |
Table I.
Details of primers used in the present
study.
| Gene | Primer (5′-3′) | Product size (base
pairs) |
|---|
| PTH |
F:TGGCAGTTTGTCTCCT |
|
|
|
R:TTCCTCCTTCTTGGTG | 218 |
| BNP |
F:AGGTCACTCCCATCCC |
|
|
|
R:TCTATCTTCTGCCCAAA | 246 |
| GAPDH |
F:CAAGTTCAACGGCACAG |
|
|
|
R:CCAGTAGACTCCACGACAT | 138 |
Statistical analysis
All statistical analyses were performed using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA). Quantitative
data are presented as mean ± standard deviation. To compare the
differences between the groups, one-way analysis of variance
(ANOVA) and least significant difference post hoc tests were
applied. P<0.05 was considered to indicate a statistically
significant difference.
Results
Induction of the CM model using ADR
and the preparation of samples
During establishment of ADR-induced CM, 2 rats from
the experimental group died; no rats from the control group died.
At 10 weeks following the start of the experiments, 15 of the
surviving rats from the experimental group were randomly selected
and equally divided into three treatment subgroups (n=5/treatment
group) and termed PTH-5, PTH-10 and PTH-20, respectively. The
remaining 7 rats were placed into the PTH-untreated CM group.
Following completion of the treatment regimen, myocardial tissue
samples were collected from 5 rats of the NC group, PTH-untreated
CM group and the three PTH-5, PTH-10, and PTH-20 groups (n=25
total).
Changes in rat biochemistry upon CM
induction using ADR
The serum concentrations of BNP, troponin T, CRP,
creatinine and phosphorus were all increased in the rats with
ADR-induced CM compared with those concentrations in the NC group;
however, the serum concentrations of PTH and calcium were decreased
in the rats with ADR-induced CM compared with those concentrations
in the NC group (data not shown). Notably, compared with CM group,
the administration of PTH decreased the serum levels of BNP, CRP,
troponin T, creatinine and phosphorus and increased the serum
levels of PTH and calcium in a dose-dependent manner. ANOVA results
indicated that the overall differences for these biochemistry
indexes among the five experimental groups all reached statistical
significance (P<0.01; Table
II).
 | Table II.BNP, PTH, electrolyte and cardiac
ultrasound results. |
Table II.
BNP, PTH, electrolyte and cardiac
ultrasound results.
| Variables | NC group (n=5) | CM group (n=5) | PTH-5 group
(n=5) | PTH-10 group
(n=5) | PTH-20 group
(n=5) | F | P-value |
|---|
| PTH (ng/l) | 29.90±3.42 |
25.78±3.09b | 30.46±1.44 | 31.84±2.87 |
36.00±2.87b | 8.531 | <0.01 |
| BNP (ng/l) |
29.60±5.94a |
108.20±9.81b | 96.00±11.34 | 87.60±6.80 | 78.40±9.10 | 58.656 | <0.01 |
| Troponin
(ng/l) | 4.37±0.66 | 6.14±0.72 | 6.21±0.48 | 5.78±0.98 | 5.31±0.97 | 4.655 | <0.01 |
| CRP (mg/ml) |
16.04±2.63b | 25.86±3.10 | 23.60±1.37 | 21.92±1.72 | 20.38±2.01 | 13.441 | <0.01 |
| Creatinine
(µmol/l) |
53.00±6.20a | 135.40±10.45 | 123.40±10.78 | 119.00±10.25 |
103.82±9.71b | 55.925 | <0.01 |
| Calcium
(mmol/l) |
5.41±0.96b | 2.11±0.16 | 2.81±0.60 | 3.23±0.26 |
4.27±0.74b | 21.666 | <0.01 |
| Phosphate
(mmol/l) | 2.67±0.38 |
5.95±0.88b | 4.84±0.78 | 4.11±0.12 | 3.20±0.44 | 24.398 | <0.01 |
| LVEF |
74.86±1.95a |
56.06±3.46a | 63.03±2.06 | 65.83±2.58 | 67.38±2.76 | 33.998 | <0.01 |
| LVFS |
45.31±1.71a |
30.50±2.55a | 35.52±1.54 | 37.73±2.06 | 38.97±2.11 | 35.423 | <0.01 |
| LV mass (mg) | 898.62±152.40 | 845.96±86.70 | 824.06±142.45 | 941.07±80.89 | 951.35±218.79 | 0.753 | >0.05 |
| LVEDV (µl) | 349.74±54.23 | 361.22±29.85 | 360.04±37.63 | 375.10±49.44 | 376.79±49.68 | 0.315 | >0.05 |
| LVESV (µl) |
88.36±19.12b |
158.20±20.60b | 133.22±16.60 | 128.17±19.01 | 131.30±16.78 | 9.223 | <0.01 |
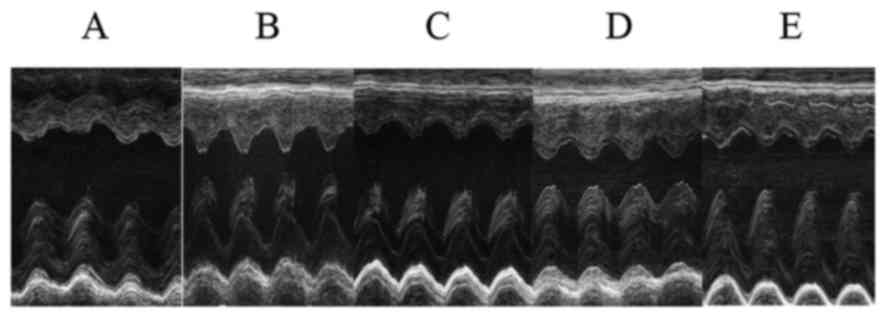
Cardiac ultrasonography
The LVEF and LVFS were markedly decreased and the
LVESV was significantly increased in the CM group compared with
that in the NC group (P<0.01). The LVEF and LVFS were gradually
resolved with increasing doses of PTH, whereas the LVESV exhibited
no consistent change with PTH treatment. The differences of the
LVEF, LVFS and LVESV among the five groups reached statistical
significance (P<0.01). There were trends for dose-dependent
increases in LV mass and LVEDV with PTH-treatment, but these
differences failed to reach statistical significance (P>0.05).
The details of the findings are indicated in Table II and Fig. 1.
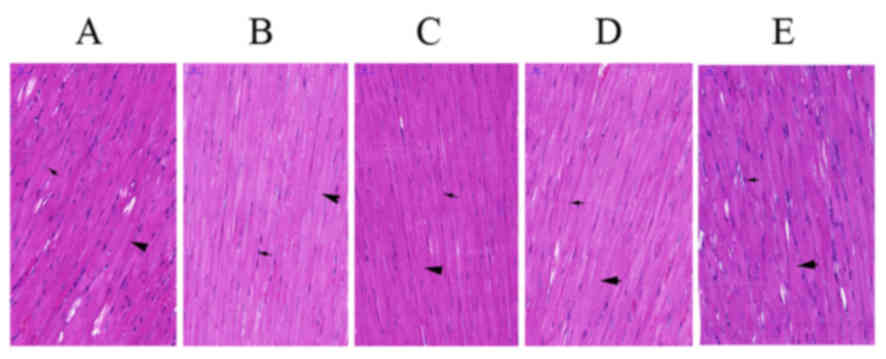
Changes in myocardial tissue
morphology of rats
Results from HE staining of heart tissue specimens
from the experimental rats revealed that myocardial fibers
(indicated by a bold black arrow; Fig.
2A) were disordered and the distribution density of myocardial
nuclei (indicated by a thin black arrow; Fig. 2A) was increased in the PTH-untreated
CM group (Fig. 2A) compared with
those in the NC group (Fig. 2B).
Administration of rPTH caused the myocardial permutation to become
regular and the distribution density of myocardial nuclei was
decreased (Fig. 2C-E). As indicated
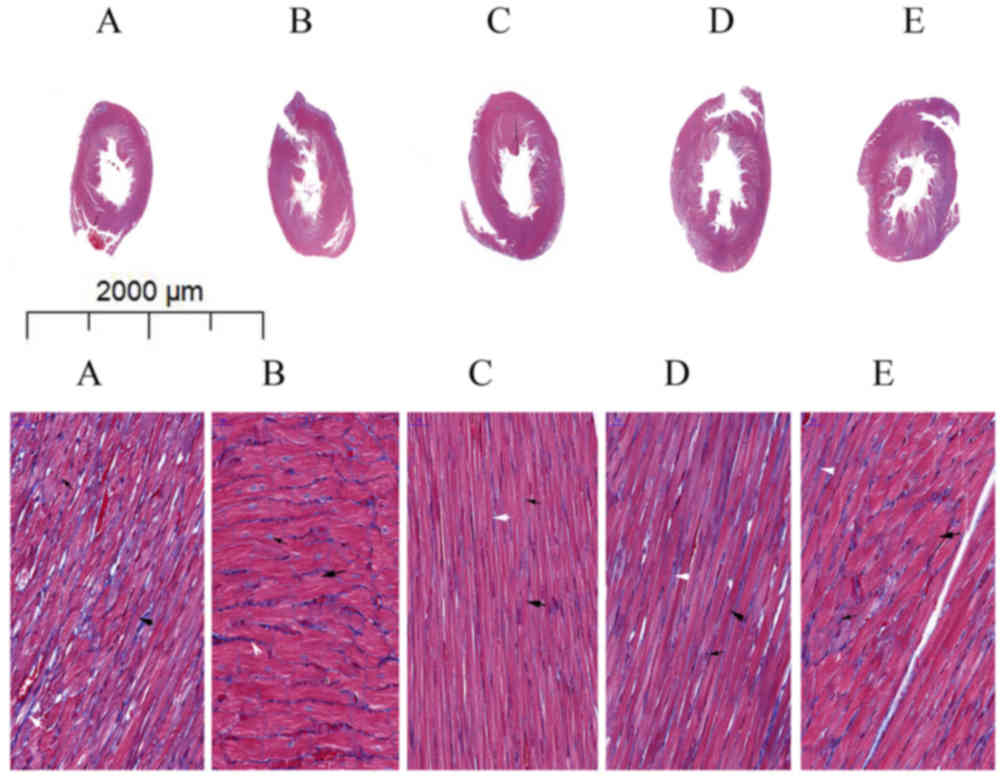
in Masson's trichome staining of heart tissue specimens from the
experimental rats, myocardial fibers (indicated by a bold black
arrow; Fig. 3A) were thinner and
disordered in the PTH-untreated CM group (Fig. 3A) compared with those in the NC group
(Fig. 3B), whereas the number of
collagen fibers (indicated by a white arrow; Fig. 3A) had increased. Administration of
rPTH in a dose-dependent manner resulted in thicker, regular
myocardial fibers (Fig. 3C-E).
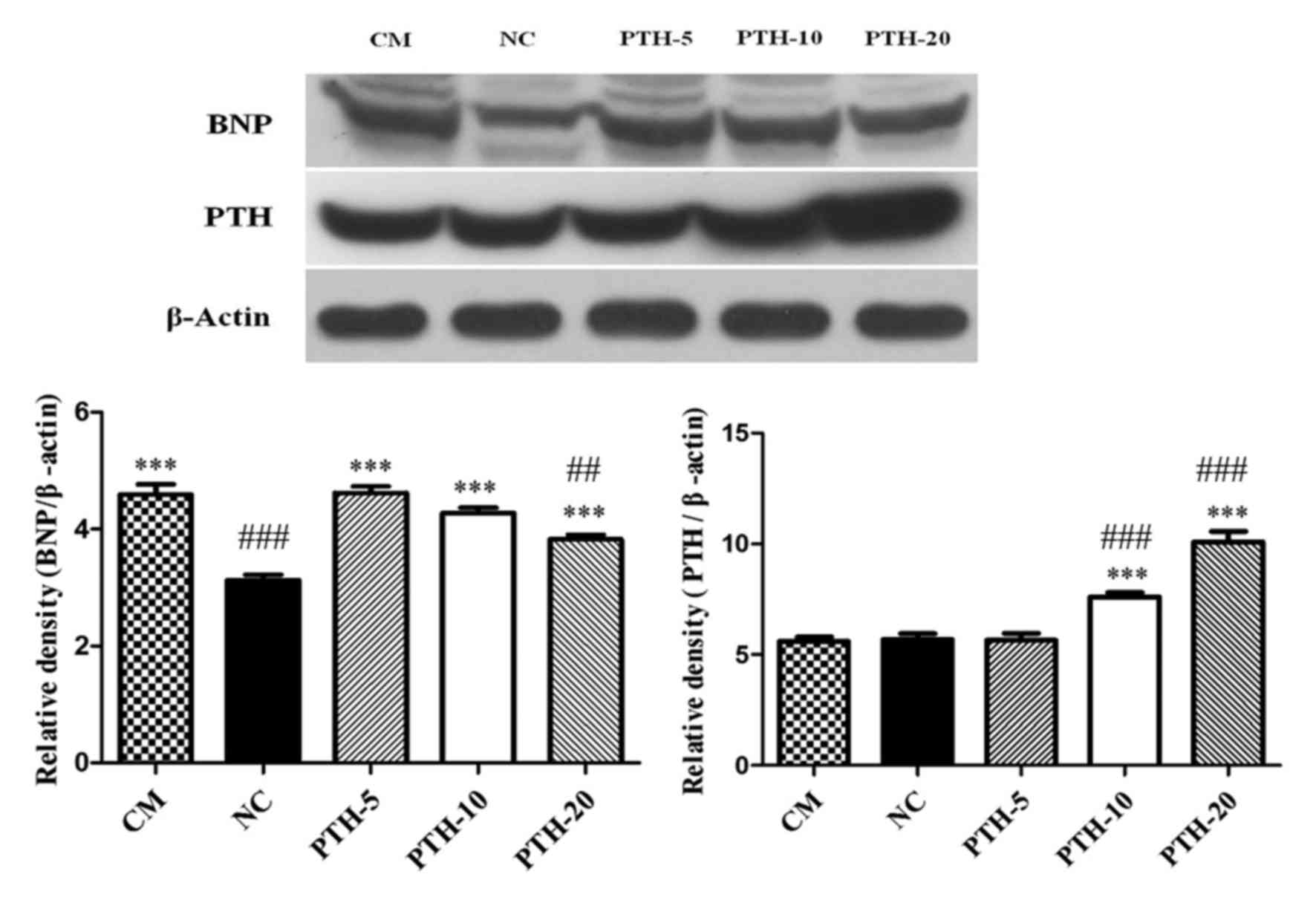
Changes in expression levels of PTH
protein and mRNA in myocardial tissue
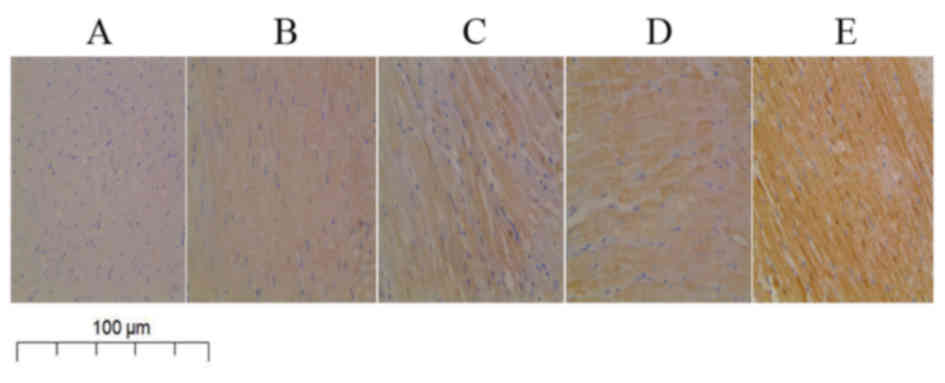
Results from immunohistochemical analysis of heart
tissue specimens from the experimental rats revealed that the
expression of PTH protein in the cytoplasm of myocardial cells was
markedly decreased in the PTH-untreated CM group compared with that
in the NC group (Fig. 4A and B).
Furthermore, administration of rPTH enhanced the expression of PTH
protein in a dose-dependent manner (Fig.
4C-E). Consistent with the immunohistochemistry results,
western blot analysis also demonstrated that the expression levels
PTH protein in the myocardial tissue of the PTH-untreated CM group
was slightly lower compared with the levels expressed in the NC
control group (P>0.05). Additionally, 10 and 20 µg/kg PTH
treatment in rats with ADR-induced CM significantly increased the
expression levels of PTH protein (all P<0.001 vs. NC group and
CM group). However, the PTH protein expression levels in the PTH-5
group compared with the PTH-untreated CM group and the NC group
failed to reach a statistically significant difference (P>0.05;
Fig. 5).
Consistent with the detected protein expression
levels, the PTH mRNA expression levels in the myocardial tissue
were decreased in the PTH-untreated CM group compared with the NC
group (P>0.05). However, compared with the NC group and the CM
group, treatment with rPTH in the PTH-10 and PTH-20 groups
significantly elevated the expression levels of PTH mRNA (all
P<0.001). The levels of PTH mRNA in the PTH-5 group were also
significantly elevated compared with the CM group (P<0.01),
while only slightly elevated compared with the NC group. The
results are presented in Fig.
6A.
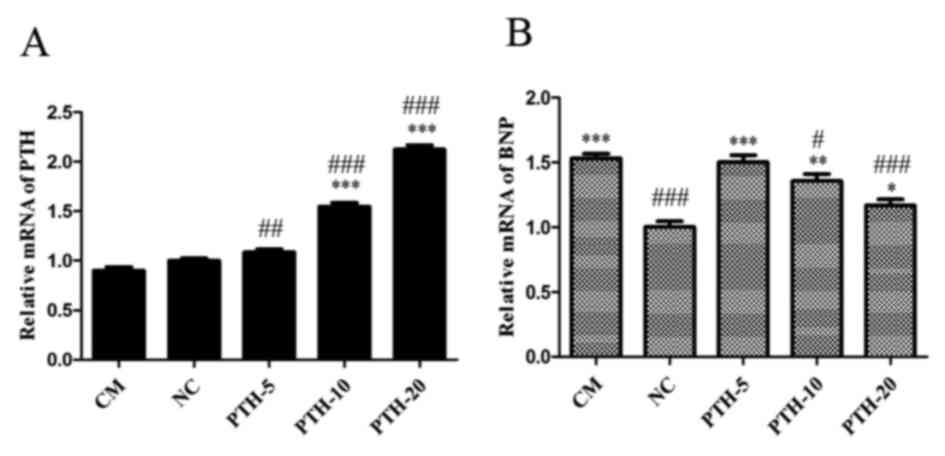 | Figure 6.mRNA expression levels of (A) PTH and
(B) BNP in myocardial tissue (n=5 for each group). *P<0.05,
**P<0.01, ***P<0.001 vs. NC group. #P<0.05,
##P<0.01 and ###P<0.001 vs. CM group.
BNP, B-type natriuretic peptide; PTH, parathyroid hormone; CM,
PTH-untreated cardiomyopathy group; NC, normal control group;
PTH-5, 5 µg/kg recombinant parathyroid hormone treatment group;
PTH-10, 10 µg/kg recombinant parathyroid hormone treatment group;
PTH-20, 20 µg/kg recombinant parathyroid hormone treatment
group. |
Changes in the expression levels of
BNP protein and mRNA in myocardial tissue
Western blot analysis of cell lysates generated from
heart tissue from the experimental rats demonstrated that BNP
protein expression in myocardial tissue was significantly increased
in the PTH-untreated CM group compared with that in the NC group
(P<0.001), and rPTH treatment in ADR-induced CM rats gradually
decreased the expression levels of BNP protein in a dose-dependent
manner (all P<0.001 vs. NC group). However, compared with the CM
group, treatment with rPTH only in the PTH-20 group significantly
decreased the expression levels of BNP protein (P<0.01; Fig. 5).
Notably, the expression levels of BNP mRNA in
myocardial tissue were also significantly increased in the
PTH-untreated CM group compared with the NC group (P<0.001).
Furthermore, consistent with the indicated protein expression
levels, rPTH treatment decreased the BNP mRNA expression levels in
a dose-dependent manner (P<0.001, P<0.01 and P<0.05 vs. NC
group, respectively). However, compared with the CM group, the
expression of BNP mRNA in the PTH-10 and PTH-20 groups were
significantly decreased (P<0.05 and P<0.001, respectively),
while those in the PTH-5 group were only slightly decreased. The
results were presented in Fig.
6B.
Discussion
In the present study, an ADR-induced CM rat model
was established to observe the effects of PTH on myocardial
pathology and cardiac function. Results indicated that ADR
treatment increased serum levels of BNP and decreased LVEF, which
suggested the successful establishment of CM in the animal model.
Conversely, treatment with rPTH significantly decreased serum BNP,
and cardiac ultrasonography indicated that the rPTH decreased the
LVESV and enhanced the LVEF, suggesting improved overall cardiac
function in rats with ADR-induced CM. PTH exerts direct
hypertrophic effects on myocardium (22). In the present study, HE staining of
paraffin sections revealed that the distribution density of the
myocardial nuclei of rats in PTH-treated CM groups was decreased
compared with the PTH-untreated CM rats, which may be explained by
the thickening of the myocardial fibers. Masson's trichome staining
of paraffin sections further confirmed that myocardial fibers of
rats in the PTH-treated CM groups were thicker and more regular
compared with those of the PTH-untreated CM rats. These results
were consistent with those of a previous study (22). Notably, there were consistent trends
for dose-dependent increases in LV mass following PTH-treatment,
although these differences did not reach statistical significance.
The short observation time and small sample size may have
contributed to the lack of detectable statistically significant
differences. In addition, immunohistochemistry and western blot
analysis revealed that the expression of PTH protein in myocardial
tissue was significantly elevated following PTH treatment,
suggesting that PTH acted on myocardial tissue to improve the
myocardial remodeling and cardiac function in non-ischemic CM.
Furthermore, the expression of BNP protein in myocardial tissue of
the PTH-untreated CM group was significantly elevated, and
treatment with rPTH decreased the expression of BNP in a
dose-dependent manner, further suggesting that PTH could improve
cardiac function in non-ischemic CM. Therefore, results from the
present study effectively supported the protective effect of PTH on
ischemic CM in rats. Interestingly, the present data suggested that
20 µg/kg/day as a treatment dose produced a positive therapeutic
effect. Notably, this dose was lower than the typical dose, which
was used in a previous myocardial infarction study in rats
(20).
PTH influences myocardium and cardiac function via
expansion of blood vessels to decrease peripheral resistance,
positive inotropic action and reduction of left ventricle thickness
and volume to improve ventricular remodeling (19). In addition, PTH activates PTH 1
receptor on smooth muscle cells to increase cyclic AMP synthesis,
which reduces calcium influx and leads to the expansion of blood
vessels (12,23). This expansion subsequently decreases
cardiac load and improves cardiac pump function (4). Additionally, PTH enhances myocardial
contractility through the activation of voltage-dependent calcium
channel-dependent calcium influx (24,25),
elevating the autorhythmicity of the sinoatrial node and the heart
rate. In the present study, cardiac ultrasonography indicated that
PTH significantly reduced the LVESV of rats, suggesting an
inhibitory role for PTH in ventricular remodeling. Such effects may
be associated with the persistent expansion of peripheral vessels,
decreased arterial elasticity and subsequent reduced peripheral
resistance (26,27). Conversely, PTH interacts with
adrenergic signals mediated by G-protein coupled receptor kinases,
including β-adrenoreceptor kinase, which can influence ventricular
remodeling (5,28). As PTH has multiple targets of action,
namely smooth muscle and the myocardium, it may improve cardiac
function by decreasing the cardiac load, enhancing myocardial
contractility and inhibiting the nervous system (29). Because of this diversity, PTH has
pronounced therapeutic potential for treating HF resulting from
various causes.
Currently, PTH is primarily used in the treatment of
patients with osteoporosis (30).
Further investigation into the role of PTH in CM and HF is
required. The present study revealed that PTH was predominantly
expressed in the cytoplasm of myocardial cells; however, the
specific signaling pathways in myocardial cells that may be
involved and the potential interaction of PTH with organelles also
requires further study.
One of the limitations of the current study was the
small sample size. Secondly, only 5 rats from each group were
randomly selected for the collection of blood samples and used for
cardiac ultrasonography analysis rather than all of the rats, which
may have resulted in less exacting conclusions. Thirdly, the data
collected were simplified; more objective indicators of cardiac
function, such as left ventricular filling pressure, were not
analyzed. Therefore, the primary endpoints of the present study
were relatively simplistic. As mentioned above, further studies
should be conducted to identify the specific signaling pathways on
which PTH interacts with in myocardial cells.
In conclusion, the present findings demonstrated
that PTH improved the cardiac function in rats with ADR-induced CM
by affecting myocardial contractility and remodeling. These
findings provide promise for the development of a PTH-based
clinical treatment of non-ischemic CM.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific
Research Project of the Wuxi Municipal Health and Family Planning
Commission (grant nos. MS201638 and Z201608).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW and GZ conceived and designed the current study.
GW, TW, BX, YS, XZ and XW performed the experiments. GW, TW, BX and
YS performed data analysis. GW drafted the manuscript and ZC
created the figures and was involved in data analysis. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Anhui Medical University (Wuxi, China) and all
animals received care compliant with standards of the Guide for the
Care and Use of Laboratory Animals published in 1988 by The
National Academies.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu G, Wang X, Wang X, Jiang H, Wang L,
Wang T, Liu J, An D, Cao L, Xia Y and Zong G: Serum parathyroid
hormone levels predict discharge and readmission for heart failure.
Genet Test Mol Biomarkers. 20:328–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altay H and Colkesen Y: Parathyroid
hormone and heart failure: Novel biomarker strategy. Endocr Metab
Immune Disord Drug Targets. 13:100–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kubiak GM, Kolaszko A and
Nowalany-Kozielska E: Parathyroid hormone serum concentration in
Central European patients with non-ischemic heart failure as a
potential marker of disease severity and poor prognosis. Endokrynol
Pol. 68:299–305. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ballane GT, Sfeir JG, Dakik HA, Brown EM
and El-Hajj Fuleihan G: Use of recombinant human parathyroid
hormone in hypocalcemic cardiomyopathy. Eur J Endocrinol.
166:1113–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian J, Colbert MC, Witte D, Kuan CY,
Gruenstein E, Osinka H, Lanske B, Kronenberg HM and Clemens TL:
Midgestational lethality in mice lacking the Parathyroid hormone
(PTH)/PTH-related peptide receptor is associated with abrupt
cardiomyocyte death. Endocrinology. 144:1053–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monego G, Arena V, Pasquini S, Stigliano
E, Fiaccavento R, Leone O, Arpesella G, Potena L, Ranelletti FO, Di
Nardo P and Capelli A: Ischemic injury activates PTHrP and PTH1R
expression in human ventricular cardiomyocytes. Basic Res Cardiol.
104:427–434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zittermann A, Ernst JB, Pilz S, Dreier J,
Kuhn J, Knabbe C, Gummert JF, Morshuis M and Milting H:
Calciotropic and phosphaturic hormones in end-stage heart failure
patients supported by a left-ventricular assist device. PLoS One.
11:e01644592016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi YH, Cowan DB, Wahlers TC, Hetzer R,
Del Nido PJ and Stamm C: Calcium sensitisation impairs diastolic
relaxation in post-ischaemic myocardium: Implications for the use
of Ca(2+) sensitising inotropes after cardiac surgery. Eur J
Cardiothorac Surg. 37:376–383. 2010.PubMed/NCBI
|
|
9
|
Osto E, Fallo F, Pelizzo MR, Maddalozzo A,
Sorgato N, Corbetti F, Montisci R, Famoso G, Bellu R, Lüscher TF,
et al: Coronary microvascular dysfunction induced by primary
hyperparathyroidism is restored after parathyroidectomy.
Circulation. 126:1031–1039. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Capitanio S, Sambuceti G, Giusti M,
Morbelli S, Murialdo G, Garibotto G, Vera L, Ameri P, Repetto B,
Naseri M, et al: 1,25-Dihydroxy vitamin D and coronary
microvascular function. Eur J Nucl Med Mol Imaging. 40:280–289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verdoia M, Pergolini P, Rolla R, Nardin M,
Barbieri L, Schaffer A, Bellomo G, Marino P, Suryapranata H and De
Luca G: Novara Atherosclerosis Study Group (NAS): Parathyroid
hormone levels and high-residual platelet reactivity in patients
receiving dual antiplatelet therapy with acetylsalicylic acid and
clopidogrel or ticagrelor. Cardiovasc Ther. 34:209–215. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schreckenberg R, Wenzel S, da Costa Rebelo
RM, Röthig A, Meyer R and Schlüter KD: Cell-specific effects of
nitric oxide deficiency on parathyroid hormone-related peptide
(PTHrP) responsiveness and PTH1 receptor expression in
cardiovascular cells. Endocrinology. 150:3735–3741. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang LL, Chen D, Lee J, Gu X, Alaaeddine
G, Li J, Wei L and Yu SP: Mobilization of endogenous bone marrow
derived endothelial progenitor cells and therapeutic potential of
parathyroid hormone after ischemic stroke in mice. PLoS One.
9:e872842014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang ST, Gao YJ, Duan CC, Li DD, Tian XC,
Zhang QL, Guo B and Yue ZP: Effects of PTHrP on expression of MMP9
and MMP13 in sika deer antler chondrocytes. Cell Biol Int.
37:1300–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Zou D, Li C, Meng H, Sui W, Feng S,
Cheng T, Zhai Q and Qiu L: Targeting stem cell niche can protect
hematopoietic stem cells from chemotherapy and G-CSF treatment.
Stem Cell Res Ther. 6:1752015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cusano NE, Rubin MR, Zhang C, Anderson L,
Levy E, Costa AG, Irani D and Bilezikian JP: Parathyroid hormone
1–84 alters circulating vascular endothelial growth factor levels
in hypoparathyroidism. J Clin Endocrinol Metab. 99:E2025–E2028.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Engelmann MG, Theiss HD, Hennig-Theiss C,
Huber A, Wintersperger BJ, Werle-Ruedinger AE, Schoenberg SO,
Steinbeck G and Franz WM: Autologous bone marrow stem cell
mobilization induced by granulocyte colony-stimulating factor after
subacute ST-segment elevation myocardial infarction undergoing late
revascularization: Final results from the G-CSF-STEMI (Granulocyte
Colony-Stimulating Factor ST-Segment Elevation Myocardial
Infarction) trial. J Am Coll Cardiol. 48:1712–1721. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zohlnhöfer D, Ott I, Mehilli J, Schömig K,
Michalk F, Ibrahim T, Meisetschläger G, von Wedel J, Bollwein H,
Seyfarth M, et al: Stem cell mobilization by granulocyte
colony-stimulating factor in patients with acute myocardial
infarction: A randomized controlled trial. JAMA. 295:1003–1010.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaruba MM, Huber BC, Brunner S, Deindl E,
David R, Fischer R, Assmann G, Herbach N, Grundmann S, Wanke R, et
al: Parathyroid hormone treatment after myocardial infarction
promotes cardiac repair by enhanced neovascularization and cell
survival. Cardiovasc Res. 77:722–731. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brunner S, Weinberger T, Huber BC, Segeth
A, Zaruba MM, Theiss HD, Assmann G, Herbach N, Wanke R,
Mueller-Hoecker J and Franz WM: The cardioprotective effects of
parathyroid hormone are independent of endogenous
granulocyte-colony stimulating factor release. Cardiovasc Res.
93:330–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teraoka K, Hirano M, Yamaguchi K and
Yamashina A: Progressive cardiac dysfunction in adriamycin-induced
cardiomyopathy rats. Eur J Heart Fail. 2:373–378. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schlüter KD and Piper HM: Cardiovascular
actions of parathyroid hormone and parathyroid hormone-related
peptide. Cardiovasc Res. 37:34–41. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noda M, Katoh T, Takuwa N, Kumada M,
Kurokawa K and Takuwa Y: Synergistic stimulation of parathyroid
hormone-related peptide gene expression by mechanical stretch and
angiotensin II in rat aortic smooth muscle cells. J Biol Chem.
269:17911–17917. 1994.PubMed/NCBI
|
|
24
|
Wu P, Xie F, Xue M, Xu X, He S, Lin M and
Bai L: Advanced oxidation protein products decrease the expression
of calcium transport channels in small intestinal epithelium via
the p44/42 MAPK signaling pathway. Eur J Cell Biol. 94:190–203.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Selim AA, Mahon M, Juppner H, Bringhurst
FR and Divieti P: Role of calcium channels in carboxyl-terminal
parathyroid hormone receptor signaling. Am J Physiol Cell Physiol.
291:C114–C121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hagström E, Ahlström T, Ärnlöv J, Larsson
A, Melhus H, Hellman P and Lind L: Parathyroid hormone and calcium
are independently associated with subclinical vascular disease in a
community-based cohort. Atherosclerosis. 238:420–426. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rocha-Singh KJ, Zeller T and Jaff MR:
Peripheral arterial calcification: Prevalence, mechanism,
detection, and clinical implications. Catheter Cardiovasc Interv.
83:E212–E220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seeland U, Selejan S, Engelhardt S, Müller
P, Lohse MJ and Böhm M: Interstitial remodeling in beta1-adrenergic
receptor transgenic mice. Basic Res Cardiol. 102:183–193. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hong ZR, Gil HW, Yang JO, Lee EY, Ahn JO
and Hong SY: Associations between sympathetic activity, plasma
concentrations of renin, aldosterone, and parathyroid hormone, and
the degree of intractability of blood pressure control in
modialysis patients. J Korean Med Sci. 22:604–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng ZY, Ye T, Ling QY, Wu T, Wu GY and
Zong GJ: Parathyroid hormone promotes osteoblastic differentiation
of endothelial cells via the extracellular signal-regulated protein
kinase 1/2 and nuclear factor-κB signaling pathways. Exp Ther Med.
15:1754–1760. 2018.PubMed/NCBI
|