Introduction
Degenerative musculoskeletal and spine conditions
are significant causes of pain and disability in patients worldwide
(1). In recent decades, advances in
regenerative medicine and cell-based therapies, particularly the
transplantation of mesenchymal stem cells (MSCs), have led to
numerous studies and clinical trials utilizing these biological
therapies to treat degenerative conditions, frequently reporting
favorable outcomes (2). The increase
in the number of therapeutic MSC procedures in the peripheral joint
and in spine interventions is causing a growing concern regarding
the potential toxicity of co-injectable drugs to MSCs.
Intra-articular administration of amide-type local
anesthetics (LAs) is routinely performed during arthroscopic joint
surgery (3). In orthopedic cartilage
repair operations, the delivery of human MSCs is often required via
intra-articular injection and it is common to introduce LAs prior
to, during and following this procedure (4). However, a number of in vitro
experimental studies using the same concentrations of LAs as in the
clinical setting suggested its cytotoxic effect on articular
chondrocytes (5) and human MSCs
(6–8). While multiple in vitro trials
have been performed, they were frequently limited by small sample
size, heterogeneity in study design and conflicting outcomes, and
therefore, the impact of LAs on adipose-derived mesenchymal stem
cells (ADMSCs) remains to be elucidated.
To the best of our knowledge, the response of ADMSCs
to LAs during chondrogenic differentiation has remained largely
elusive. Breu et al (9)
demonstrated that LAs have cytotoxic effects on bone marrow-derived
MSCs (BMSCs) undergoing chondrogenesis, particularly in the
superficial layers. Bupivacaine, ropivacaine and mepivacaine did
not differ in cytotoxicity on MSC in aggregates. To the best of our
knowledge, the present study was the first to report the
cytotoxicity of LAs to human MSCs during chondrogenesis. The study
by Breu et al had several limitations. First, only three
types of LA were used and lidocaine was not included. In addition,
the BMSCs were exposed to LAs at 7 days after chondrogenesis, which
is not an ideal time-point to replicate clinical conditions, as
MSCs are always injected following LA-induced anesthesia. Finally,
the evaluation index for cell viability and response to LA exposure
during chondrogenesis was limited as cell viability, apoptosis and
necrosis were only evaluated after LA exposure.
Therefore, the present in vitro study was
designed to determine the cytotoxic effect of different types of LA
on rabbit ADMSCs (rADMSCs) during early chondrogenesis. The present
study aimed to identify a safe type and concentration of LA for
ADMSCs. The results of the present study may assist clinicians in
selecting the optimal LA concentration during regenerative
procedures and serve as a foundation for future research examining
the cytotoxicity of LAs to ADMSCs during early chondrogenic
differentiation.
Materials and methods
General design
rADMSCs were exposed to LA: 1% lidocaine, 0.5%
bupivacaine (both Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China), 0.5% ropivacaine (AuroMedics Pharma LLC
Pharma LLC., East Windsor, NJ, USA) or 2% mepivacaine (Hospira
Inc., Lake Forest, IL, USA) for 60 min. The control groups were
treated with complete PBS for 60 min. Following the incubations,
the cells were removed from the solutions and incubated in culture
medium [Dulbecco's modified Eagle's medium supplemented with 10%
fetal bovine serum (Hyclone; GE Healthcare, Little Chalfont, UK)]
Analyses of cell viability, apoptosis, morphological alterations
and chondrogenesis-associated gene expression were performed early
after exposure (day 3) and later (day 7).
Cell isolation, culture conditions and
in vitro chondrogenic differentiation
A total of 5 healthy New Zealand rabbits weighing
2.5–3.0 kg and aged 2–3 months were provided by the Experimental
Animal Centre of Zhejiang University Affiliated with Sir Run Run
Shaw Hospital (SRRSH; Hangzhou, China). The experimental protocol
was approved by the Animal Ethics Committee of Zhejiang University
Affiliated with SRRSH Hospital (Hangzhou, China).
Primary rADMSCs were harvested from the adipose
tissue in the groin of the New Zealand rabbits as previously
described (10). Adipose tissue was
digested with Type I collagenase (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 1.5 h at 37°C, centrifuged at 400 × g for 5
min, rinsed with PBS and passed through 100-µm cell strainers
(Shanghai Bolting Cloth Manufacturing Co., Ltd., Shanghai, China).
The remaining rADMSCs (those which passed through the strainer)
were cultured in culture medium. The culture medium was replaced
every 3 days and non-adherent cells were removed. In the present
study, adherent primary rADMSCs exhibited a fibroblastoid and
typical spindle-shaped morphology. The presence of CD29 and CD44
was detected by flow cytometry to confirm the identity of the
rADMSCs (data not shown). All rADMSCs used in the subsequent
experiments were of passage 7.
The cells were cultured in 175 ml flasks at 37°C in
a humidified atmosphere containing 5% CO2 until 90%
confluence. Subsequently, they were trypsinized (TrypLE Express;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and seeded onto
96-well plates (for MTS analysis), and onto 6-well plates [for flow
cytometry, Safranine Fast Green double staining and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis] at a density of 10,000 cells/cm2. The rADMSCs
were maintained in chondrogenic differentiation media which
consisted of culture media supplemented with 6.25 µg/ml insulin
(Shanghai Orgchem Co., Ltd., Shanghai, China), 6.25 µg/ml
transferrin (Shanghai Orgchem Co., Ltd.), 50 µg/ml ascorbic acid
(Sigma-Aldrich; Merck KGaA), 10 ng/ml transforming growth factor
(TGF)-β1 (Peprotech, Inc., Rocky Hill, NJ, USA) and 0.1 µmol/l
dexamethasone (Sigma-Aldrich; Merck KGaA). To evaluate the response
of rADMSCs to exposure to LAs, all cells were allowed to
differentiate for up to 3 days in chondrogenic differentiation
media prior to LA treatment.
Treatment groups
rADMSCs were exposed to 1% lidocaine, 0.5%
bupivacaine, 0.5% ropivacaine, 2% mepivacaine or complete PBS as a
control. rADMSC cultures were subdivided into triplicate wells per
condition and exposed to LAs for 60 min. Following exposure, the
cells were washed with 1X PBS and returned to the incubator in
fresh chondrogenesis culture media. rADMSC viability was measured
on days 3 and 7 post-exposure.
Assessment of ADMSC viability
To assess the metabolic activity of ADMSCs post-LA
exposure, MTS colorimetric assays (Promega Corp., Madison, WI, USA)
were performed at days 3 and 7 post-exposure. MTS assays (11) were evaluated using a Spectra Max plus
Plate Reader (Molecular Devices, Sunnyvale, CA, USA) at a
wavelength of 490 nm. The assay is based on the reduction of the
levels of the MTS tetrazolium compound by viable cells to generate
a colored formazan product that is soluble in cell culture media.
This conversion is performed by NADPH-dependent dehydrogenase
enzymes in metabolically active cells. The formazan dye produced by
viable cells may be quantified by measuring the absorbance at 490
nm.
Analysis of rADMSC apoptosis
Cell apoptosis was measured by flow cytometry. An
Annexin V-FITC Apoptosis Detection kit (Accuri C6; BD Biosciences,
Franklin Lakes, NJ, USA) was used to evaluate the percentage of
apoptotic cells. rADMSCs were treated with trypsin (without EDTA),
washed two times with PBS and centrifuged (750 × g, 5 min, 37°C) to
collect the pellets. Subsequently, 5×104 treated cells
were re-suspended in 300 µl cold 1X binding buffer, after which 5
µl Annexin V-FITC and 10 µl propidium iodide were added and the
suspension was analyzed by flow cytometry (FACScan; BD
Biosciences).
Morphological alterations during
chondrogenic differentiation
Cell morphological alterations during chondrogenic
differentiation were measured by Safranine Fast Green double
staining. A total of 8×104 cells were seeded into each
well of a 6-well plate containing microscopic slides and cultured
to 80% confluency at 37°C with 5% CO2. Following
culture, the cells were rinsed with PBS three times for 1 min each
and fixed for 2 h in cold paraformaldehyde solution. The slides
were placed in histology cassettes and stored in 70% ethanol.
Subsequently, 1% safranin (Solarbio, Beijing, China) and 0.02% Fast
Green (CAS no. 2353-45-9; Jianglai Bio, Shanghai, China) double
staining was performed (12).
Finally, after dewaxing with xylene and sealing with neutral
glycerol, the slides were observed under a light microscope (Nikon,
Tokyo, Japan).
Genetic analysis of the response of
rADMSCs to exposure to LAs
To determine the effect of LA exposure on rADMSCs
during chondrogenic differentiation for clinical applications in
cartilage regeneration, rADMSCs were subjected to RT-qPCR analysis
of chondrogenic-associated gene expression. Prior to RT-qPCR, RNA
was isolated and reverse transcribed using the RT-qPCR kit
(TransGen Biotech, Beijing, China). Subsequently, the complementary
DNA obtained was amplified by RT-qPCR (CFX384 Real-Time System;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) with SYBR Green
detection using primers specific to chondrogenesis-associated
genes, including collagen I, collagen III and sex-determining
region Y box (SOX)9, whose sequences are listed in Table I. Gene expression levels were
quantified using the 2−ΔΔCq method (13). The mRNA expression of the target
genes was normalized to the housekeeping gene GAPDH. All of the
samples were assayed in triplicate.
 | Table I.Primer sequences used for polymerase
chain reaction. |
Table I.
Primer sequences used for polymerase
chain reaction.
|
|
| Primer sequence
(5′-3′) |
|---|
|
|
|
|
|---|
| Gene | Implication | Forward | Reverse |
|---|
| Collagen I | Chondrogenesis |
CTGGCCCTAATGGATTCGCT |
TCACCCTTAGGTCCCTTGGT |
| Collagen III | Chondrogenesis |
TGGAGACTGGGGAAACATGC |
CAGAGTCCGTCCACCACTTC |
| Sex-determining
region | Chondrogenesis |
TCTGGAGACTGCTGAACGAG |
CTGCCCATTCTTCACCGACTT |
| Y box 9 |
| GAPDH | Housekeeping
gene |
ACTGCTGAACGAGAGCGAGAA |
CTGCCCATTCTTCACCGACTTC |
Statistical analysis
Descriptive statistics were utilized to demonstrate
the effects of LA exposure on rADMSC viability (mitochondrial
activity, MTS results), the apoptotic rate and the mRNA expression
on days 3 and 7 post-exposure. The results were analyzed by one-way
analysis of variance followed by a least-significant differences
post-hoc test using GraphPad Prism software (version 6.0; GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference. Values are
expressed as the mean ± standard deviation.
Results
Effect of LAs on rADMSC viability
The viability of rADMSC based on their mitochondrial
activity was assessed by an MTS assay. It was determined that the
mitochondrial activity was decreased at 3 days following treatment
with LAs compared with that in the PBS control (P<0.05; Fig. 1). Treatment with 1% lidocaine
resulted in reduced toxic effects on mitochondrial activity
compared with the other LAs (P<0.05). Furthermore, mitochondrial
activity of rADMSCs recovered to a certain extent at day 7 in all
LA exposure groups, but remained decreased in all LA treatment
groups compared with the PBS control (P<0.05). On day 7, the
mitochondrial activity in the 1% lidocaine- and 2%
mepivacaine-treated groups was higher than that in the groups
treated with 0.5% ropivacaine or 0.5% bupivacaine. Cells treated
with 0.5% bupivacaine had markedly lower mitochondrial activity
compared with the other 3 LAs. Therefore, the recovery abilities of
the cells treated with 1% lidocaine and 2% mepivacaine were
improved when compared with those in the other two LA groups
(P<0.05).
Effect of LAs on rADMSC apoptosis
The apoptotic rate of rADMSCs was measured by flow
cytometry. It was determined that the apoptotic rate increased on
days 3 and 7 after treatment with LAs compared with that in the
PBS-treated control (P<0.05; Fig.
2). The apoptotic rate in the 1% lidocaine and 0.5% ropivacaine
groups was lower compared with that in the groups treated with 2%
mepivacaine- and 0.5% bupivacaine groups (on day 7). Therefore,
treatment with 1% lidocaine or 0.5% ropivacaine resulted in a lower
apoptotic rate compared with that in the groups treated with the
other LAs (P<0.05; Fig. 2).
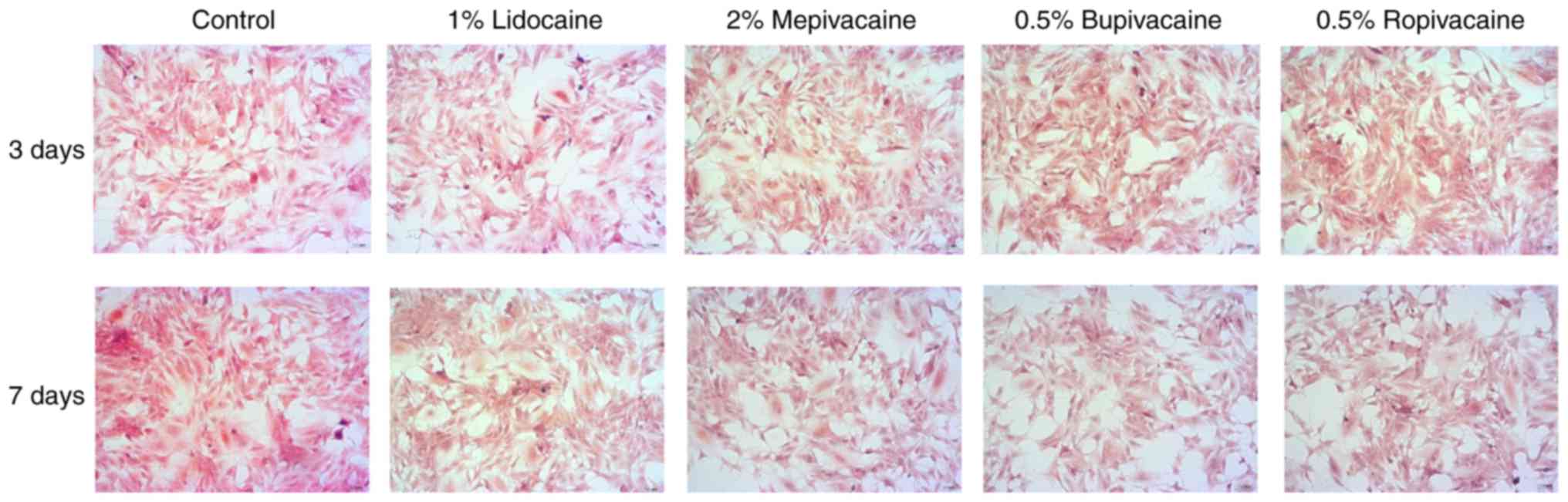
Effect of LAs on morphological
alterations of chondrogenic differentiation
rADMSCs cultured in chondrogenic medium formed
aggregates and exhibited typical pink staining for
glycosaminoglycans and morphological features, including enlarged
lacunae around cells, typical of cartilage (safranine Fast Green
double staining). In all LA treatment groups, less mature cartilage
compared with that in the PBS group was identified using the
staining on days 3 and 7 after the 60-min exposure (Fig. 3). By contrast, the aggregates in the
1% lidocaine treatment group resembled more mature cartilage
compared with that in the group treated with the other LAs, as
demonstrated by the staining (Fig.
3).
Effect of LAs on
chondrogenesis-associated mRNA expression
The effect of a 60-min LA exposure on
chondrogenesis-associated mRNA expression was evaluated on days 3
and 7. As expected, chondrogenesis-associated markers, including
collagen I, collagen III and SOX9, were all decreased at day 3
after LA exposure compared with those in the control group
(P<0.05; Fig. 4). In comparison
with day 3, these chondrogenesis-associated genes began to increase
in all groups on day 7 after exposure but remained lower compared
with those in the control group (P<0.05; Fig. 4). Exposure to LAs decreased the
chondrogenic ability of rADMSCs. The mRNA expression levels of
collagen III and SOX9 were higher in the lidocaine group and,
collagen I, collagen III and SOX9 were higher in the mepivacaine
group compared with that in the other LA-treated groups. These
results demonstrates that lidocaine and mepivacaine exhibited a
less pronounced influence on chondrogenesis-associated mRNA
expression and the course of recovery from the 60-min exposure was
better compared with that in the other LA-treated groups.
Discussion
The results of the present study demonstrated that
the viability and apoptotic rate of rADMSCs were decreased and
increased, respectively, following a brief exposure to LAs in a
concentration- and substance-specific manner in monolayer cell
culture experiments during early chondrogenesis. Furthermore, the
ADMSC viability and genetic analysis results demonstrated that
lidocaine (1%) and mepivacaine (2%) appeared to be less toxic to
rADMSCs during early chondrogenesis.
Intra-articular administration of amide-type LAs is
routinely performed during arthroscopic joint surgery (3). During the last two decades, the use of
allogeneic and autologous human MSCs for tissue repair has become
increasingly popular. Stem cell-based methods to treat cartilage
defects include marrow-stimulating techniques, including
microfracture (14), intra-articular
MSC injection (15) and implantation
of scaffolding material containing MSCs (16,17). In
these instances, the stem cells come into direct contact with
intra-articular administered LAs. Different cytotoxic effects of
different local anesthetics have been widely reported in patients
and animal models. Previous studies have indicated that ropivacaine
at its commonly used concentration (0.5%) was significantly less
toxic than other anesthetics (0.5% bupivacaine or 1% lidocaine)
(6,8), and that bupivacaine was more toxic to
cartilage cells than ropivacaine and lidocaine (18). However, Keck et al (19) demonstrated that treatment with 2%
lidocaine resulted in a significant reduction in the live cell
count compared with that in all other LA-treated groups. The
present study demonstrated that lidocaine, bupivacaine, ropivacaine
and mepivacaine were cytotoxic to rADMSCs during early
chondrogenesis. Lidocaine (1%) in the present study induced
significantly less cytotoxicity compared with ropivacaine,
bupivacaine and mepivacaine. Lidocaine and mepivacaine exerted a
less pronounced influence on chondrogenesis-associated mRNA
expression and the course of recovery from the 60-min exposure was
better compared with that in the groups treated with the other LAs.
These results may conflict those of previous studies (8,9) as the
cytotoxicity effects of LAs on mesenchymal stem cells were
different, this may be for the following reasons: Firstly, each of
the studies used the LAs at different concentrations; secondly,
different methods of analysis, types of MSCs and differentiation
conditions were used. Breu et al (9) investigated the cytotoxic potency of LAs
on human BMSCs during chondrogenesis. In this experiment, the cells
were embedded in varying amounts and structures of
cartilage-specific extracellular matrix. In the study by Breu et
al (9), it was revealed that
bupivacaine, ropivacaine and mepivacaine did not differ in the
extent of cytotoxicity induced on MSCs in aggregates during
chondrogenesis. It is not unusual that different concentrations and
MSC conditions (e.g. cell types and direction of differentiation)
lead to different results. In clinical practice, 1% lidocaine, 0.5%
bupivacaine, 0.5% ropivacaine and 2% mepivacaine is utilized at our
institution. To mimic the clinical situation, the cytotoxicity of
these anesthetics was compared at these concentrations. The present
study assessed the cytotoxic effects of LAs with equivalent
anesthetic potencies. To model the conditions of early
chondrogenesis, the rADMSCs were allowed to differentiate for up to
3 days in chondrogenic differentiation media and were subsequently
exposed to LAs for 60 min. Therefore, the experimental conditions
used in the present study are closest to the ones used in clinical
practice.
All local anesthetics used in the present study are
members of the amide family. The cytotoxicity of LAs on different
cell types, including neurons, has been addressed by several
studies (20,21). Three major mechanisms have been
hypothesized to explain the cytotoxic effects of LAs on stem cells.
First, the toxic actions of local anesthetics have been
demonstrated to be associated with their lipophilic properties
(22,23). Bupivacaine and ropivacaine are highly
lipophilic molecules, whereas lidocaine is only slightly lipophilic
(24). Furthermore, MSCs are
sensitive to LAs that may inhibit cell-to-cell communication, which
is critical in proliferation and migration, and inhibition of
mitochondrial respiration with subsequent depletion of cellular ATP
and formation of reactive oxygen species may occur (25,26). LAs
may also affect inflammatory processes. Bupivacaine was reported to
induce nitric oxide synthase-2 activity (27). This suggested that bupivacaine
toxicity may be in part attributable to the production of nitric
oxide during ongoing inflammation. In terms of cell viability and
apoptosis, 1% lidocaine exhibited less cytotoxicity on ADMSCs than
the other LAs used in the present study. This result is similar to
those of previous studies. Shoshani et al (28) investigated the effect of lidocaine on
the viability of injected adipose tissue in nude mice. A local
anesthetic solution, consisting of lidocaine and epinephrine, did
not alter the acceptance of fat grafts, and had no influence on the
viability of adipocytes. However, in terms of gene expression, 2%
mepivacaine appeared to have a low impact on the expression of
cartilage differentiation-associated genes. These results imply
that different local anesthetics exert toxic effects on ADMSCs
during cartilage differentiation via different mechanisms.
Therefore, further in vitro and in vivo studies using
equipotent LAs are required to confirm the mechanisms of
cytotoxicity on ADMSCs during cartilage differentiation.
One limitation of the present study is the fact that
the experiments were performed in vitro. The drug
concentration was constant over the 60-min exposure time. However,
drug concentrations slowly decrease over time in clinical practice.
Therefore, further experiments are required to assess the in
vivo cytotoxic effects of LAs on ADMSCs.
In conclusion, the present study demonstrated that
several common amide-type LAs are cytotoxic to rADMSCs during early
chondrogenesis in a drug type-dependent manner. Furthermore, 1%
lidocaine exerted relatively lower cytotoxic effects on ADMSCs, and
2% mepivacaine and 1% lidocaine appeared to exhibit a less
pronounced effect on cartilage differentiation-associated gene
expression. In the light of the results of the present study, it is
of critical importance that clinicians are cautious when selecting
an amide-type LA to be used during rADMSC therapy in future, in
order to avoid compromising the integrity and potency of cell-based
therapies.
Acknowledgements
The authors of the present study give thanks to Dr.
Jinjin Ben (Pathology Department, Nanjing Medical University,
Nanjing, China) for their technical assistance.
Funding
This study was supported by the Natural Science
Foundation of Zhejiang Province, China (2016–2018; grant no.
LY16H170001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TW and JL designed and directed the experiment. ZS,
HS and YL performed the experiments. ZS performed the statistical
analysis. TW and JL wrote the manuscript. ZS and HS reviewed and
edited the manuscript. The final version of the manuscript has been
read and approved by all authors, and each author believes that the
manuscript represents honest work.
Ethical approval and consent to
participate
The experimental protocol was approved by the Animal
Ethics Committee of Zhejiang University Affiliated with SRRSH
(Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Vos T, Flaxman AD, Naghavi M, Lozano R,
Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V,
et al: Years lived with disability (YLDs) for 1160 sequelae of 289
diseases and injuries 1990–2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2163–2196. 2010.
View Article : Google Scholar
|
|
2
|
Wu T, Song HX, Dong Y and Li JH:
Cell-based therapies for lumbar discogenic low back Pain:
Systematic review and single-arm meta-analysis. Spine (Phila Pa
1976). 43:49–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Møiniche S, Mikkelsen S, Wetterslev J and
Dahl JB: A systematic review of intra-articular local anesthesia
for postoperative pain relief after arthroscopic knee surgery. Reg
Anesth Pain Med. 24:430–437. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ballieul RJ, Jacobs TF, Herregods S, Van
Sint Jan P, Wyler B, Vereecke H, Almqvist F and Herregods L: The
peri-operative use of intra-articular local anesthetics: A review.
Acta Anaesthesiol Belg. 60:101–108. 2009.PubMed/NCBI
|
|
5
|
Chu CR, Izzo NJ, Coyle CH, Papas NE and
Logar A: The in vitro effects of bupivacaine on articular
chondrocytes. J Bone Joint Surg Br. 90:814–820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dregalla RC, Lyons NF, Reischling PD and
Centeno CJ: Amide-type local anesthetics and human mesenchymal stem
cells: Clinical implications for stem cell therapy. Stem Cells
Transl Med. 3:365–374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lucchinetti E, Awad AE, Rahman M, Feng J,
Lou PH, Zhang L, Ionescu L, Lemieux H, Thébaud B and Zaugg M:
Antiproliferative effects of local anesthetics on mesenchymal stem
cells: Potential implications for tumor spreading and wound
healing. Anesthesiology. 116:841–856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Breu A, Eckl S, Zink W, Kujat R and Angele
P: Cytotoxicity of local anesthetics on human mesenchymal stem
cells in vitro. Arthroscopy. 29:1676–1684¸. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Breu A, Scheidhammer I, Kujat R, Graf B
and Angele P: Local anesthetic cytotoxicity on human mesenchymal
stem cells during chondrogenic differentiation. Knee Surg Sports
Traumatol Arthrosc. 23:937–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Y, Liu T, Song K, Fan X, Ma X and Cui
Z: Adipose-derived stem cell: A better stem cell than BMSC. Cell
Biochem Funct. 26:664–675. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romeo R, Carnovale C, Giofrè SV, Monciino
G, Chiacchio MA, Sanfilippo C and Macchi B: Enantiomerically pure
phosphonated carbocyclic 2′-oxa-3′-azanucleosides: Synthesis and
biological evaluation. Molecules. 19:14406–14016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garrido Pascual C, Hakimiyan AA, Rappoport
L, Oegema TR, Wimmer MA and Chubinskaya S: Anti-apoptotic
treatments prevent cartilage degradation after acute trauma to
human ankle cartilage. Osteoarthritis Cartilage. 17:1244–1251.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asik M, Ciftci F, Sen C, Erdil M and
Atalar A: The microfracture technique for the treatment of
full-thickness articular cartilage lesions of the knee: Midterm
results. Arthroscopy. 24:1214–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia P, Wang X, Lin Q and Li X: Efficacy of
mesenchymal stem cells injection for the management of knee
osteoarthritis: A systematic review and meta-analysis. Int Orthop.
39:2363–2372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Løken S1, Jakobsen RB, Arøen A, Heir S,
Shahdadfar A, Brinchmann JE, Engebretsen L and Reinholt FP: Bone
marrow mesenchymal stem cells in a hyaluronan scaffold for
treatment of an osteochondral defect in a rabbit model. Knee Surg
Sports Traumatol Arthrosc. 16:896–903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haleem AM, Singergy AA, Sabry D, Atta HM,
Rashed LA, Chu CR, El Shewy MT, Azzam A and Abdel Aziz MT: The
clinical use of human culture-expanded autologous bone marrow
mesenchymal stem cells transplanted on platelet-rich fibrin glue in
the treatment of articular cartilage defects: A pilot study and
preliminary results. Cartilage. 1:253–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piper SL, Kramer JD, Kim HT and Feeley BT:
Effects of local anesthetics on articular cartilage. Am J Sports
Med. 39:2245–2253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keck M, Zeyda M, Gollinger K, Burjak S,
Kamolz LP, Frey M and Stulnig TM: Local anesthetics have a major
impact on viability of pre-adipocytes and their differentiation
into adipocytes. Plast Reconstr Surg. 126:1500–1505. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang YS, Tseng SY, Tseng SH and Wu CL:
Cytotoxicity of lidocaine or bupivacaine on corneal endothelial
cells in a rabbit model. Cornea. 25:590–596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pollock JE: Neurotoxicity of intrathecal
local anaesthetics and transient neurological symptoms. Best Pract
Res Clin Anaesthesiol. 17:471–484. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Punke MA and Friederich P: Lipophilic and
stereospecific interactions of amino-amide local anesthetics with
human Kv1.1 channels. Anesthesiology. 109:895–904. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Werdehausen R, Fazeli S, Braun S, Hermanns
H, Essmann F, Hollmann MW, Bauer I and Stevens MF: Apoptosis
induction by different local anaesthetics in a neuroblastoma cell
line. Br J Anaesth. 103:711–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grouselle M, Tueux O, Dabadie P,
Georgescaud D and Mazat JP: Effect of local anaesthetics on
mitochondrial membrane potential in living cells. Biochem J.
271:269–272. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu X, Han B, Cai S, Lei Y, Sun T and Sheng
Z: Migration of bone marrow-derived cells induced by tumor necrosis
factor-alpha and its possible role in wound healing. Wound Repair
Regen. 17:185–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanaka Y, Maruo A, Fujii K, Nomi M,
Nakamura T, Eto S and Minami Y: Intercellular adhesion molecule 1
discriminates functionally different populations of human
osteoblasts: Characteristic involvement of cell cycle regulators. J
Bone Miner Res. 15:1912–1923. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feinstein DL, Murphy P, Sharp A, Galea E,
Gavrilyuk V and Weinberg G: Local anesthetics potentiate nitric
oxide synthase type 2 expression in rat glial cells. J Neurosurg
Anesthesiol. 13:99–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shoshani O, Berger J, Fodor L, Ramon Y,
Shupak A, Kehat I, Gilhar A and Ullmann Y: The effect of lidocaine
and adrenaline on the viability of injected adipose tissue: An
experimental study in nude mice. J Drugs Dermatol. 44:311–316.
2005.
|