Introduction
Acute pancreatitis (AP) is a common inflammatory
disorder of the pancreas that may involve surrounding tissues or
remote organs and lead to considerable morbidity; it has a
mortality rate of ~5% (1). In the
US, AP is the leading cause of gastrointestinal disease associated
with hospital admission and accounts for ~0.27 million hospital
admissions per year and >2.5 billion dollars in medical expenses
(2,3). Upon the establishment of the diagnosis
of AP, the severity is determined according to the 2012 revision of
the Atlanta classification of acute pancreatitis as follows:
Severe, moderately severe or mild (4). An estimated 20–30% of AP cases progress
to severe acute pancreatitis (SAP), which rapidly progresses to
include local complications and multiple organ failure (5). The mortality rate associated with SAP
is ≤20–30% in spite of diagnostic and therapeutic advances, and
there is a great requirement for emergency resuscitation and
supportive treatment (6). The
antiprotease drug ulinastatin has been demonstrated to be a
critical therapeutic medicine for the clinical management of AP in
China and Japan (7,8). Previous studies have reported the
predictive role of C-reactive protein (CRP) in pancreatitis as a
biochemical marker, and the sensitivity and specificity for
predicting SAP may be 80 and 76%, respectively (9,10).
CRP belongs to the pentraxin family of proteins with
a hepatic origin, and it serves as a major component of any
inflammatory reaction (11). In
addition, CRP is an acute-phase protein that demonstrates a rapid
increase in plasma concentration when responding to infection,
acute inflammation and tissue damage (12). Specifically, CRP is secreted in
response to the pro-inflammatory cytokines, including interleukin
(IL)-6 and tumor necrosis factor α (TNF-α), and it is implicated in
innate immunity by facilitating phagocytosis of damaged and foreign
cells, and activation of the complement pathway (13). CRP has been highlighted as a factor
with prognostic significance for pancreatitis and a factor involved
in the severity score for the management of AP (14). Of note, CRP levels have been
indicated to be associated with the progression of SAP and have
predictive value regarding mortality associated with multiple organ
failure (15). Although multiple
studies have focused on searching for novel biomarkers with better
predictive value for SAP, none of them identified a superior marker
to CRP (16). Therefore, the present
study was performed with the major objective of exploring the serum
CRP levels in SAP patients and its value in determining the
severity of gastrointestinal failure, as well as predicting the
therapeutic effects of ulinastatin combined with somatostatin.
Materials and methods
Study subjects
From June 2011 to June 2015, a total of 260 patients
diagnosed with SAP were recruited at The First People's Hospital of
Kunming (Kunming, China). A total of 172 males and 88 females (age,
22–77 years; mean age, 44.54±12.96 years; median age, 44.5 years)
were enrolled. The cohort comprised 108 patients with acute
alcoholic pancreatitis, 116 patients with acute biliary
pancreatitis and 36 patients with unknown causes. All patients had
clinical symptoms, including paroxysmal abdominal pain, nausea,
fever and vomiting, which are the diagnostic criteria for SAP
according to the guidelines for diagnosis and treatment of SAP
published by the Chinese Society of Gastroenterology and the
Chinese Medical Association (17).
Patients were included if they met at least two of the following
three criteria: i) Patients with upper abdominal pain and serum
amylase levels that increased to more than 3 times the normal
limit; ii) computed tomography (CT) and magnetic resonance imaging
(MRI) results indicating changes in the patient's condition
consistent with extensive pancreatic necrosis and a Ranson's score
of >3; and iii) patients with organ failure and with an acute
physiology and chronic health evaluation II (APACHE II) score of
>8. Patients were excluded if they met the following criteria:
i) Age, <18 or >80 years; ii) patients who had acute
circulatory failure, were treated with high doses of vasoactive
drugs and had arterial blood lactate of >4 mmol/l; iii)
triglyceride (TG) levels >8 mmol/l; iv) patients who were
pregnant, had diseases of the immune system or were treated with
immune enhancers or immunosuppressive agents; and v) patients who
were affected by other inflammatory reactions or were treated with
immunomodulatory drugs, and patients who underwent associated
treatment, including bedside blood purification and thymic
peptide.
Treatment regimens
All patients were subjected to conventional therapy,
including oxygen inhalation, gastrointestinal decompression,
fasting, supplemental plasma and nutritional support. In addition,
patients were treated with 3 mg somatostatin (batch no., 10112307;
Chengdu Tiantaishan Pharmaceutical Co., Ltd., Chengdu, China) and
ulinastatin (10×105 U; batch no., 08120805; Techpool
Bio-pharma Co., Ltd., Guangzhou, China) in 250 ml 0.9% sodium
chloride for 10 days via intravenous drip (ter in die).
Observation indicators and therapeutic
effect evaluation
Venous blood samples (5 ml each time) were
separately obtained from patients on admission, days 1, 3 and 7,
and after treatment. Samples were collected in an anticoagulant
tubes and centrifuged at 1,006 × g for 10 min at 4°C. After the
serum was isolated, the samples were stored at −65°C. Serum CRP
levels were measured using the immunoturbidimetry method with kits
from Roche Diagnostics (Basel, Switzerland) in accordance with the
manufacturer's protocol. A therapeutic effects evaluation was
performed according to the guidelines for the diagnosis and
treatment of SAP published by the Chinese Society of
Gastroenterology and the Chinese Medical Association (17). The outcomes were defined as follows:
i) Cured, the patient's clinical symptoms completely disappeared
and CT scan results were normal; ii) markedly effective, the
patient's clinical symptoms improved significantly and CT scan
results were normal; iii) effective, the patient's clinical
symptoms improved to a certain extent and the amylase in the
patient's hematuria was markedly reduced, but the CT scan still
exhibited certain some abnormalities; iv) ineffective, the
patient's clinical symptoms were not alleviated and CT scan results
revealed no obvious improvement. The total efficiency was
calculated from the number of respective cases (n) as follows:
Total efficiency=(ncured + nmarkedly
effective + neffective)/ntotal ×100%.
APACHE II and computer tomography severity index (CTSI) scores were
determined to assess the patients. Each patient underwent an
enhanced CT scan on admission, and Balthazar grading and CTSI
scores were determined (18).
Indexes were determined as follows: Balthazar grades D & E were
denoted as 1 and 2 points; furthermore, necrosis was scored as
follows: No necrosis, 0; >33% necrosis, 2; 33–50% necrosis, 4;
and >50% necrosis, 6. Addition of the two scores resulted in the
CTSI score (range, 7–9 points). The criteria for the
gastrointestinal failure score were based on a previous study
(19). The gastrointestinal failure
score was graded as Table I.
 | Table I.Gastrointestinal Failure score in
patients with severe acute pancreatitis. |
Table I.
Gastrointestinal Failure score in
patients with severe acute pancreatitis.
| Clinical
symptomatology | Score |
|---|
| Normal
gastrointestinal function | 0 |
| Enteral feeding
<50% of calculated needs or no feeding 3 days after abdominal
surgery | 1 |
| Food intolerance
(enteral feeding not applicable due to high gastric aspirate
volume, vomiting, bowel distension, or severe diarrhoea) or
IAH | 2 |
| Food intolerance and
IAH | 3 |
| Abdominal compartment
syndrome | 4 |
Statistical analysis
Statistical analyses were performed using SPSS 21.0
(IBM Corp., Armonk, NY, USA) and GraphPad Prism (GraphPad Software
Inc., La Jolla, CA, USA). Values are expressed as the mean ±
standard deviation. Differences between two groups were compared
using Student's t-test. Enumeration data are expressed as n or a
ratio, and the differences between groups were analyzed using the
chi-square test. Receiver operating characteristic curve (ROC)
analysis was performed to determine the sensitivity and specificity
of CRP levels in predicting the severity of SAP and patient
prognosis. Spearman's rank correlation coefficient was determined
to assess the correlation of associated factors. Logistic
regression analysis was used to investigate the influencing factors
of the therapeutic effects. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
The SAP patients (n=260) were treated with
ulinastatin combined with somatostatin. At the end of the
treatment, the outcome was as follows: 4 cases of peripancreatic
infection, 8 cases of multiple organ failure, 8 cases of
pseudocyst, 63 cured cases, and 155 cases with markedly effective,
26 cases with effective and 16 with ineffective treatment. The
total effective rate was 93.8%. The patients were assigned to an
effective group (n=244) and an ineffective group (n=16) according
to the clinical effect. The effective group comprised 126 males and
118 females and the ineffective group comprised 10 males and 6
females. Significant differences were identified in age, CTSI
score, APACHE II score, gastrointestinal failure score, serum
albumin levels, blood urea nitrogen (BUN) and serum creatinine
levels between the two groups (P<0.05). There were no
significant differences in gender, heart rate on admission, mean
arterial pressure, 24-h urine volume, white blood cell count, the
rate of neutrophil count, blood glucose, triglycerides, blood
calcium, serum amylase, activated partial thromboplastin time,
arterial partial pressure of oxygen, blood pH and urinary albumin
(P>0.05; Table II).
 | Table II.Baseline characteristics of patients
with severe acute pancreatitis. |
Table II.
Baseline characteristics of patients
with severe acute pancreatitis.
| Baseline
characteristics | Effective group
(n=244) | Ineffective group
(n=16) | P-value |
|---|
| Age (years) | 43.84±12.50 | 55.23±15.42 | 0.001 |
| Sex |
|
| 0.589 |
| Male | 126 (51.64%) | 10 (62.5%) |
|
|
Female | 118 (48.36%) | 6 (37.5%) |
|
| Ascites |
|
| 0.782 |
| No | 168 (68.85%) | 12 (75%) |
|
| Yes | 76 (31.15%) | 4 (25%) |
|
| Body temperature
(°C) | 36.6±1.6 | 36.9±0.8 | 0.458 |
| Heart rate (bpm) | 115.4±19.7 | 119.8±21.3 | 0.390 |
| Mean arterial
pressure (mmHg) | 97.4±19.0 | 92.8±17.8 | 0.347 |
| 24-h urine volume
(ml) | 1389.12±625.45 | 1078.10±642.36 | 0.056 |
| CTSI score | 5.34±1.89 | 6.78±2.24 | 0.004 |
| Gastrointestinal
failure score | 2.13±0.45 | 3.67±0.30 | <0.001 |
| APACHE II
score | 8.56±4.36 | 12.24±6.02 | 0.002 |
| White blood cells
(×109/l) | 17.0±12.1 | 19.1±9.0 | 0.742 |
| Rate of neutrophils
(%) | 82.25±6.74 | 83.81±11.72 | 0.397 |
| Hematocrit (%) | 0.39±0.06 | 0.39±0.07 | >0.999 |
| Serum albumin
(g/l) | 28.9±4.5 | 25.3±4.2 | 0.002 |
| Urea nitrogen
(mmol/l) | 6.47±3.04 | 15.90±6.8 | <0.001 |
| Serum creatinine
(µmol/l) | 96.4±51.2 | 136.9±16.4 | 0.002 |
| Blood glucose
(mmol/l) | 10.42±4.26 | 12.33±8.45 | 0.077 |
| Triglycerides
(mmol/l) | 7.35±7.70 | 5.26±6.34 | 0.289 |
| Blood calcium
(mmol/l) | 1.78±0.30 | 1.68±0.42 | 0.210 |
| Serum amylase
(U/l) | 624.32±472.54 | 846.26±1191.82 | 0.084 |
| APTT (sec) | 35.89±13.25 | 27.99±20.16 | 0.053 |
| Arterial partial
pressure of oxygen (mmHg) | 71.36±12.02 | 66.32±20.26 | 0.124 |
| Blood pH | 7.33±0.07 | 7.29±0.18 | 0.056 |
| Urinary
albumin |
|
| 0.102 |
|
Negative | 73 | 8 |
|
|
Positive | 171 | 8 |
|
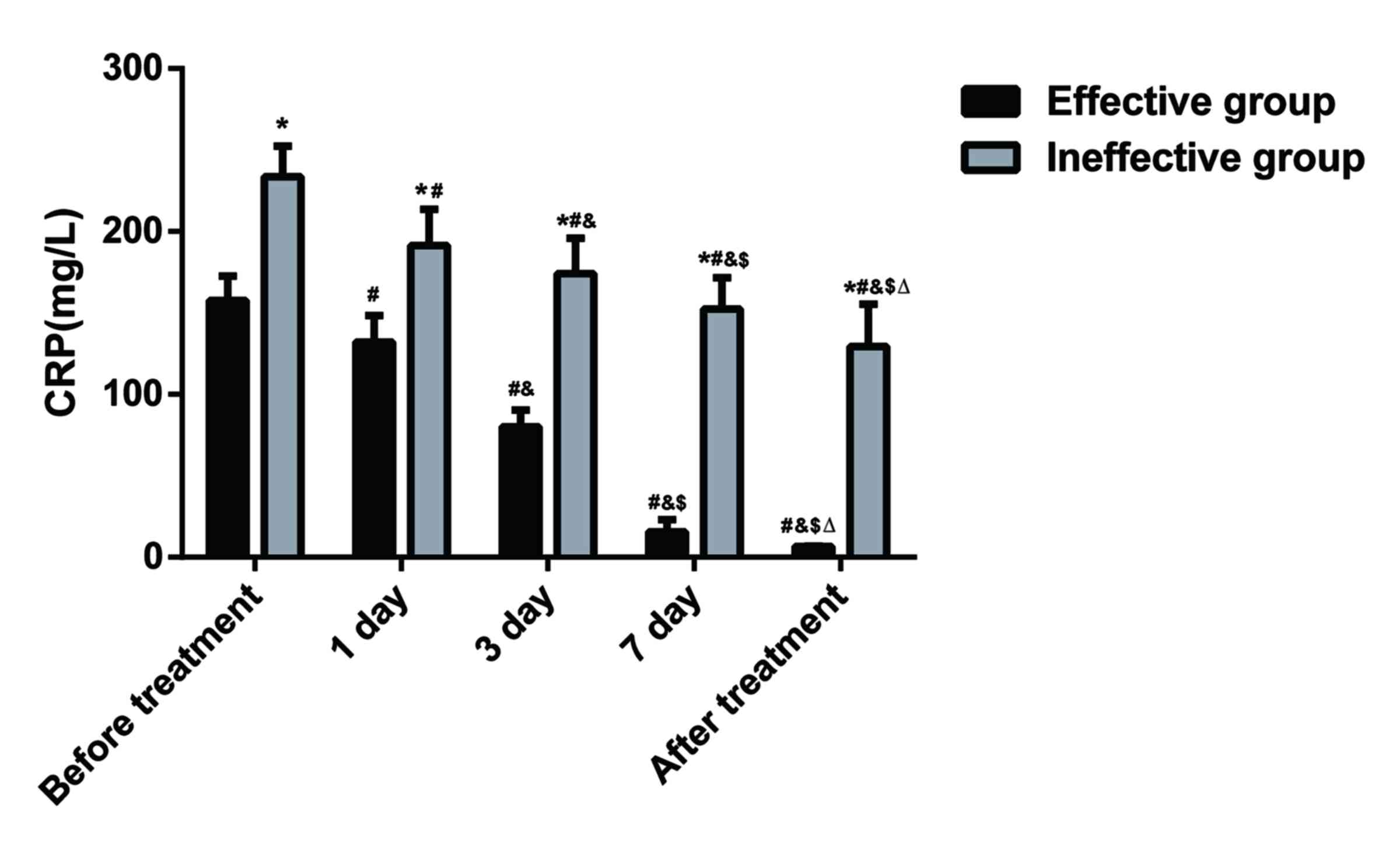
Lower serum CRP levels are identified
in SAP patients with effective treatment by ulinastatin combined
with somatostatin
Immunoturbidimetry was performed to determine the
serum CRP levels of patients. As presented in Fig. 1, prior to treatment, serum CRP levels
in the patients of the effective group were 157.54±15.02 mg/l, and
serum CRP levels in the patients of the ineffective group were
233.58±18.85 mg/l. The difference between the two groups was
statistically significant (P<0.05). After treatment, serum CRP
levels in patients of the two groups exhibited a downward trend,
but serum CRP levels were more significantly decreased in the
effective group. The CRP levels between the two groups were
significantly different at days 1, 3 and 7 of treatment
(P<0.05). The serum CRP levels in patients of the effective
group were 6.56±0.22 mg/l after treatment, and those in patients of
the ineffective group were 129.28±36.21 mg/l after treatment; the
difference was significant between the two groups (P<0.05).
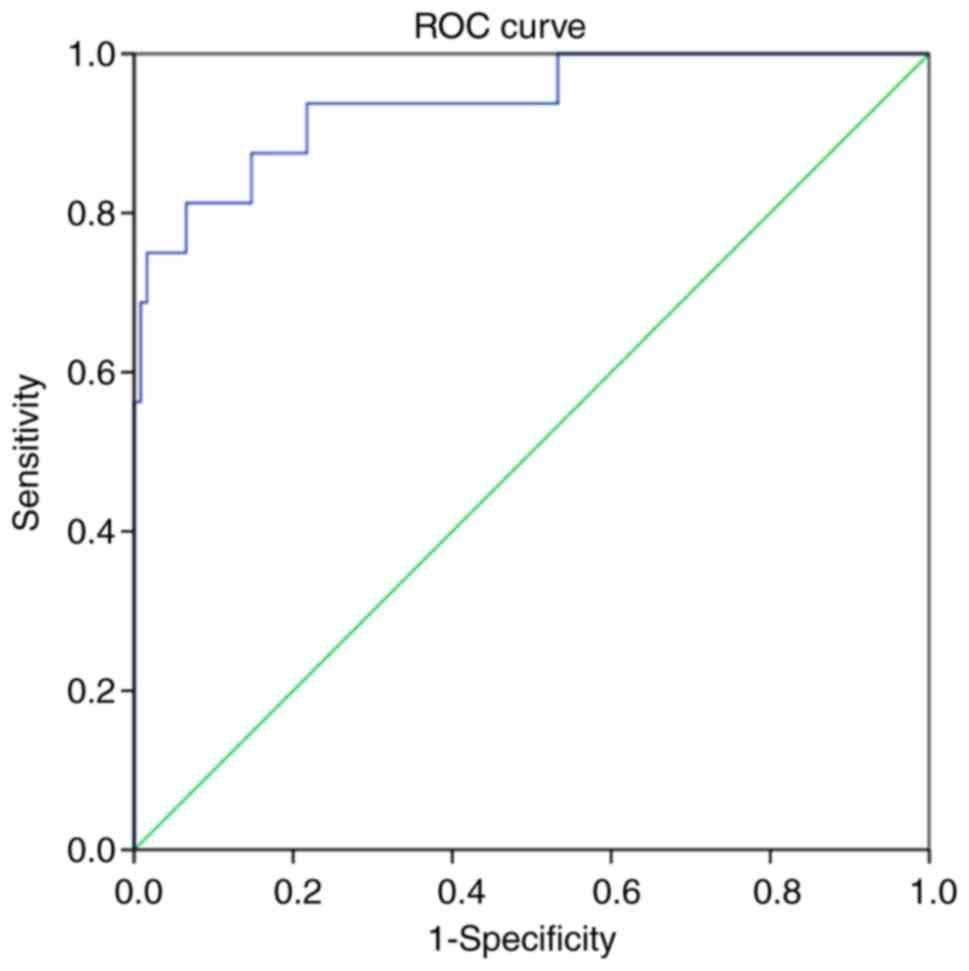
Serum CRP levels have the predictive
value for the therapeutic effects of ulinastatin combined with
somatostatin in SAP
Analysis of the ROC curve for the prediction of the
therapeutic effects of ulinastatin combined with somatostatin based
on the CRP provided an area under the curve of 0.938 [P<0.001;
95% confidence interval, 0.870–1.000]. With the optimal threshold
of 177.17 mg/l, the sensitivity and specificity were 0.813 and
0.934, respectively. The results indicated that serum CRP levels
had a high predictive value for the therapeutic effects of
ulinastatin combined with somatostatin in SAP (Fig. 2).
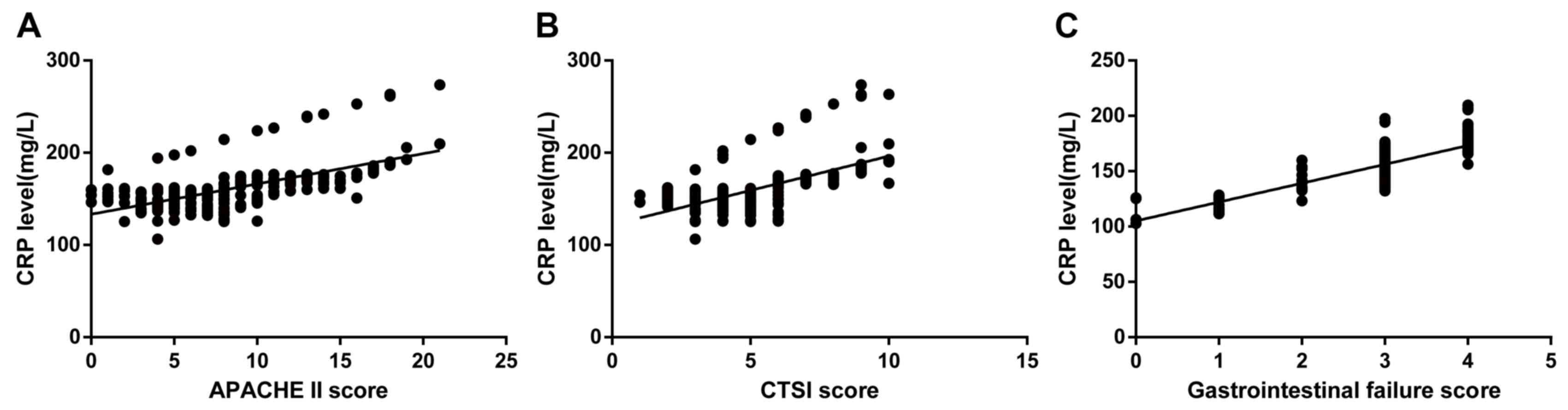
Correlation between serum CRP levels
and gastrointestinal failure in SAP patients
Correlation analyses of serum CRP levels, CTSI
score, APACHE II score, and gastrointestinal failure score in
patients on admission are presented in Fig. 3. The analysis revealed that serum CRP
levels were significantly and positively correlated with the APACHE
II score (Spearman's rank correlation coefficient=0.674;
P<0.001; Fig. 3A). The analysis
of serum CRP levels and the CTSI score demonstrated that higher
serum CRP levels corresponded with a more severe condition
expressed as a higher CTSI score (Spearman's rank correlation
coefficient=0.707; P<0.001; Fig.
3B). In addition, the correlation analysis indicated that serum
CRP levels directly reflected the gastrointestinal failure of SAP
patients (Spearman's rank correlation coefficient=0.736;
P<0.001; Fig. 3C).
Serum albumin, creatinine and CRP
levels are factors affecting the therapeutic effects of ulinastatin
combined with somatostatin in SAP patients
For the logistic regression analysis (Table III), the therapeutic effect of
ulinastatin combined with somatostatin was regarded as the
dependent variable, and gender, CTSI score, APACHE II score,
gastrointestinal failure score, serum albumin levels, BUN, serum
creatinine and serum CRP levels on admission were considered as
independent variables. The results indicated that serum albumin,
serum creatinine and serum CRP levels on admission were
significantly associated with the therapeutic effects of
ulinastatin combined with somatostatin in SAP patients (P<0.05).
However, gender, CTSI score, APACHE II score, gastrointestinal
failure score and BUN were not correlated with the therapeutic
effects of ulinastatin combined with somatostatin in SAP patients
(P>0.05).
 | Table III.Logistic regression analysis of
factors associated with the therapeutic efficacy in patients with
severe acute pancreatitis. |
Table III.
Logistic regression analysis of
factors associated with the therapeutic efficacy in patients with
severe acute pancreatitis.
| Factor | B | S.E. | Wald | df | P-value | OR | 95% CI |
|---|
| Age | 0.001 | 0.028 | 0 | 1 | 0.982 | 1.001 | 0.948–1.056 |
| CTSI score | −0.066 | 0.155 | 0.179 | 1 | 0.672 | 0.936 | 0.691–1.269 |
| APACHEII score | 0.027 | 0.077 | 0.124 | 1 | 0.725 | 1.027 | 0.884–1.194 |
| Gastrointestinal
failure score | 0.375 | 0.403 | 0.896 | 1 | 0.354 | 1.294 | 0.721–2.322 |
| Serum albumin | −0.643 | 0.200 | 10.316 | 1 | 0.001 | 0.526 | 0.355–0.778 |
| Blood urea
nitrogen | 0.187 | 0.191 | 0.961 | 1 | 0.327 | 1.206 | 0.830–1.752 |
| Serum
creatinine | −0.099 | 0.023 | 18.018 | 1 | <0.001 | 0.906 | 0.866–0.948 |
| Serum CRP levels on
admission | 0.053 | 0.027 | 3.952 | 1 | 0.047 | 1.055 | 1.001–1.112 |
Discussion
SAP is an acute disease of the gastrointestinal
tract that leads to intense systemic inflammatory responses and
progresses quickly from local pancreatic impairment to multiple
organ failure (5). Ulinastatin and
somatostatin are widely used in the treatment of AP, but the
therapeutic effects and the mechanism of how they function have not
been clearly established (20,21). In
addition, multiple studies have reported on the diagnostic and
predictive role of CRP in pancreatitis (22,23).
Therefore, the present study was performed with the central aim of
exploring the role of serum CRP levels in predicting the
therapeutic effects of ulinastatin combined with somatostatin in
the treatment of SAP and determining the severity of
gastrointestinal failure.
Prior to and after treatment, serum CRP levels were
significantly lower in patients with effective and ineffective
treatment outcomes; however, those with an effective treatment
outcome had a more obvious reduction after treatment. The
sensitivity and specificity of serum CRP levels in predicting the
therapeutic effects of ulinastatin combined with somatostatin in
SAP patients upon hospital admission were 0.813 and 0.934,
respectively. These results indicated that serum levels of CRP may
serve as a predictive indicator for the therapeutic effects of
ulinastatin combined with somatostatin in SAP. The results of a
previous study corroborated with the present results, suggesting
that the level of CRP is a predictor of the severity of SAP
(9). In the study of Schütte et
al (24), CRP was proposed as a
single reference parameter of pancreatic necrosis, as it may be
determined via a readily available and inexpensive laboratory test
and has good prognostic value in the clinical setting. In the
treatment of AP, ulinastatin suppresses pancreatic activity or
ameliorates the severity of pancreatic injury by exerting
anti-inflammatory effects through reducing serum levels of
inflammatory cytokines, including CRP, IL-6 and TNF-α (25). Furthermore, CRP, as a well-documented
biomarker for predicting SAP, has a crucial pathophysiological role
in pancreatitis, is linked with the associated morbidity and
mortality, and is positively correlated with clinical end-points in
AP patients (22,26). Consistent with the present study,
Cardoso et al (27) performed
a population-based cohort study to determine clinical features
based on the prescription of prophylactic antibiotics for AP
patients. They revealed that the elevation of serum CRP levels
contributed to clinicians' decisions to administer prophylactic
antibiotics. The present study further substantiated the results
that CRP has a predictive role regarding the therapeutic effects of
ulinastatin combined with somatostatin in SAP and may be an
indicator of gastrointestinal failure severity in SAP.
In the present study, serum CRP levels were
positively correlated with APACHE II, CTSI and gastrointestinal
failure scores of SAP patients. The logistic regression analysis
demonstrated that serum albumin, creatinine and CRP levels on
admission were factors influencing the therapeutic effects of
ulinastatin combined with somatostatin in SAP patients. Ulinastatin
enables the stabilization of lysosomal membranes, and suppresses
trypsin and the release of inflammatory cytokines, thereby
repressing organ failure and other serious illnesses (28). SAP compromises immune function,
whereas ulinastatin restores immune function by enhancing the
cytokine release of splenocytes and proliferation responses
(8). Based on the results of the
present correlation analysis, it may be concluded that serum CRP
levels directly reflect the gastrointestinal function of SAP
patients. The major cause of intestinal mucosal injury during AP
has been reported to be the excessive secretion of inflammatory
mediators (29). A possible
mechanism involved is that of somatostatin, which suppresses the
production of pancreatic enzymes and represses the motor activity
of Oddi's sphincter and Oddi basal pressure, while protecting
gastrointestinal mucosal cells and stimulating the
reticulo-endothelial system (30).
Furthermore, patients with Crohn's disease, a chronic inflammatory
bowel disease, who have increased serum CRP levels, demonstrate
more active inflammation are more susceptible to a high clinical
efficacy of infliximab (anti-TNF-α monoclonal antibody) when
compared with those patients with low serum CRP levels (31). According to a study of Tachyla et
al (15), the elevated CRP
levels indicate the progression of systemic inflammatory responses,
which are correlated with the progression of multiple organ
failure. According to the logistic regression analysis, serum
albumin, creatinine and CRP levels are factors affecting the
therapeutic effects of ulinastatin combined with somatostatin in
SAP patients, which is consistent with previous studies (32,33).
In conclusion, the results of the present study
indicated that lower serum CRP levels prior to and after treatment
led to better therapeutic effects of ulinastatin combined with
somatostatin in SAP patients. Furthermore, a positive correlation
was determined between serum CRP levels and the severity of
gastrointestinal failure. Accordingly, serum CRP levels may serve
as a predictive indicator for the therapeutic effects of
ulinastatin combined with somatostatin and the severity of
gastrointestinal failure in SAP. In addition, a better
understanding of the association between serum CRP levels and the
efficacy of ulinastatin combined with somatostatin in SAP may
provide a basis for the development of novel treatment regimens to
reduce pancreatic activity and ameliorate its symptoms and multiple
organ failure. Due to a limited sample size and the experimental
time window, an extended investigation is recommended in the
future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
LL conceived, designed and developed all
methodology. YW Acquired data (acquired and managed patients and
provided facilities), analyzed results and interpreted data. YW and
LL wrote, reviewed and revised the manuscript. The final version of
the manuscript has been read and approved by all authors, and each
author believes that the manuscript represents honest work.
Ethical approval and consent to
participate
The study was approved by the Institutional Review
Board of The First People's Hospital of Kunming. All participants
signed a document of informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Türkvatan A, Erden A, Türkoğlu MA, Seçil M
and Yener Ö: Imaging of acute pancreatitis and its complications.
Part 1 Acute pancreatitis. Diagn Interv Imaging. 96:151–160. 2015.
View Article : Google Scholar
|
|
2
|
Kuramatsu JB, Huttner HB and Schwab S:
Advances in the management of intracerebral hemorrhage. J Neural
Transm (Vienna). 120 Suppl 1:S35–S41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yadav D and Lowenfels AB: The epidemiology
of pancreatitis and pancreatic cancer. Gastroenterology.
144:1252–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maheshwari R and Subramanian RM: Severe
acute pancreatitis and necrotizing pancreatitis. Crit Care Clin.
32:279–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang ZW, Meng XX and Xu P: Central role of
neutrophil in the pathogenesis of severe acute pancreatitis. J Cell
Mol Med. 19:2513–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sternby H, Hartman H, Johansen D,
Thorlacius H and Regner S: Predictive capacity of biomarkers for
severe acute pancreatitis. Eur Surg Res. 56:154–163. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu R, Qi H, Wang J, Wang Y, Cui L, Wen Y
and Yin C: Ulinastatin activates the renin-angiotensin system to
ameliorate the pathophysiology of severe acute pancreatitis. J
Gastroenterol Hepatol. 29:1328–1337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen S, Shi H, Zou X and Luo H: Role of
ulinastatin in preventing post-endoscopic retrograde
cholangiopancreatography pancreatitis: The Emperor's new clothes or
aladdin's magic lamp? Pancreas. 39:1231–1237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia R, Tang M, Qiu L, Sun R, Cheng L, Ma
X, Yin G, Hu G, Wang X and Zhao Y: Increased interleukin-23/17 axis
and C-reactive protein are associated with severity of acute
pancreatitis in patients. Pancreas. 44:321–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gurleyik G, Emir S, Kilicoglu G, Arman A
and Saglam A: Computed tomography severity index, APACHE II score,
and serum CRP concentration for predicting the severity of acute
pancreatitis. JOP. 6:562–567. 2005.PubMed/NCBI
|
|
11
|
Ansar W and Ghosh S: C-reactive protein
and the biology of disease. Immunol Res. 56:131–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Allin KH and Nordestgaard BG: Elevated
C-reactive protein in the diagnosis, prognosis, and cause of
cancer. Crit Rev Clin Lab Sci. 48:155–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh PP, Zeng IS, Srinivasa S, Lemanu DP,
Connolly AB and Hill AG: Systematic review and meta-analysis of use
of serum C-reactive protein levels to predict anastomotic leak
after colorectal surgery. Br J Surg. 101:339–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato T, Ohno K, Tamamoto T, Oishi M,
Kanemoto H, Fukushima K, Goto-Koshino Y, Takahashi M and Tsujimoto
H: Assesment of severity and changes in C-reactive protein
concentration and various biomarkers in dogs with pancreatitis. J
Vet Med Sci. 79:35–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tachyla SA, Marochkov AV, Lipnitski AL and
Nikiforova YG: The prognostic value of procalcitonin, C-reactive
protein and cholesterol in patients with an infection and multiple
organ dysfunction. Korean J Anesthesiol. 70:305–310. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pongprasobchai S, Jianjaroonwong V,
Charatcharoenwitthaya P, Komoltri C, Tanwandee T, Leelakusolvong S,
Pausawasdi N, Srikureja W, Chainuvati S, Prachayakul V, et al:
Erythrocyte sedimentation rate and C-reactive protein for the
prediction of severity of acute pancreatitis. Pancreas.
39:1226–1230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loveday BP, Srinivasa S, Vather R, Mittal
A, Petrov MS, Phillips AR and Windsor JA: High quantity and
variable quality of guidelines for acute pancreatitis: A systematic
review. Am J Gastroenterol. 105:1466–1476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuji Y, Takahashi N and Tsutomu C:
Pancreatic perfusion CT in early stage of severe acute
pancreatitis. Int J Inflam. 2012:4973862012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reintam A, Parm P, Kitus R, Starkopf J and
Kern H: Gastrointestinal failure score in critically ill patients:
A prospective observational study. Crit Care. 12:R902008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang G, Wen J, Wilbur RR, Wen P, Zhou SF
and Xiao X: The effect of somatostatin, ulinastatin and Salvia
miltiorrhiza on severe acute pancreatitis treatment. Am J Med Sci.
346:371–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoo YW, Cha SW, Kim A, Na SY, Lee YW, Kim
SH, Lee HIe, Lee YJ, Yang HW and Jung SH: The use of gabexate
mesylate and ulinastatin for the prevention of post-endoscopic
retrograde cholangiopancreatography pancreatitis. Gut Liver.
6:256–261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin G, Hu G, Cang X, Yu G, Hu Y, Xing M,
Chen C, Huang Y, Tang M, Zhao Y, et al: C-reactive protein:
Rethinking its role in evaluating the severity of hyperlipidemic
acute pancreatitis. Pancreas. 43:1323–1328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mroczko B, Groblewska M, Gryko M, Kedra B
and Szmitkowski M: Diagnostic usefulness of serum interleukin 6
(IL-6) and C-reactive protein (CRP) in the differentiation between
pancreatic cancer and chronic pancreatitis. J Clin Lab Anal.
24:256–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schütte K and Malfertheiner P: Markers for
predicting severity and progression of acute pancreatitis. Best
Pract Res Clin Gastroenterol. 22:75–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Wang Y, Fu W, Zhang W, Wang T and
Qin H: A Meta-analysis on the effect of ulinastatin on serum levels
of C-reactive protein, interleukin 6, and tumor necrosis factor
alpha in Asian patients with acute pancreatitis. Genet Test Mol
Biomarkers. 20:118–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vermeire S, Van Assche G and Rutgeerts P:
The role of C-reactive protein as an inflammatory marker in
gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol.
2:580–586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cardoso FS, Ricardo L, Gondar P, Deus JR
and Horta D: C-reactive protein may influence decisively the
prescription of prophylactic antibiotics in acute pancreatitis: A
population-based cohort study. Pancreas. 44:404–408.
2015.PubMed/NCBI
|
|
28
|
Wang X, Zhuang X, Wei R, Wang C, Xue X and
Mao L: Protective effects of Acanthopanax vs. Ulinastatin against
severe acute pancreatitis-induced brain injury in rats. Int
Immunopharmacol. 24:285–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen Y, Cui N, Miao B and Zhao E: Immune
dysregulation in patients with severe acute pancreatitis.
Inflammation. 34:36–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang G, Liu Y, Zhou SF, Qiu P, Xu L, Wen
P, Wen J and Xiao X: Effect of Somatostatin, Ulinastatin and
gabexate on the treatment of severe acute pancreatitis. Am J Med
Sci. 351:506–512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reinisch W, Wang Y, Oddens BJ and Link R:
C-reactive protein, an indicator for maintained response or
remission to infliximab in patients with Crohn's disease: A
post-hoc analysis from ACCENT I. Aliment Pharmacol Ther.
35:568–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muddana V, Whitcomb DC, Khalid A, Slivka A
and Papachristou GI: Elevated serum creatinine as a marker of
pancreatic necrosis in acute pancreatitis. Am J Gastroenterol.
104:164–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Zhang ZW, Wang B, Yin WH, Zuo YY,
Kang Y and Liu J: Relationship between early serum albumin
variation and prognosis in patients with severe acute pancreatitis
treated in ICU. Sichuan Da Xue Xue Bao Yi Xue Ban. 44:237–241.
2013.(In Chinese). PubMed/NCBI
|