Introduction
Pulmonary thromboembolism (PTE), a blockage of the
main pulmonary artery or one of its branches, is a potentially
fatal disorder with a high mortality (1). Since the signs and symptoms of PTE are
diverse, nonspecific, and sometimes silent, PTE is difficult to be
diagnosed in a timely manner (2).
Currently, computed tomography pulmonary angiography (CTPA) is the
gold standard for the diagnosis of PTE (3). However, CTPA is associated with an
increased risk of radiation exposure and is especially
contraindicated in patients with renal insufficiency and in
pregnant women (2). It has been
reported that the prevalence of PTE in patients suspected of having
this disorder and undergoing CTPA is only 5 to 10% in the United
States and 20 to 30% in Europe (4,5). In
addition, the high cost greatly limits the application of CTPA
(6). Therefore, exploration of
simple and feasible tests, which are less invasive, well-priced,
and highly efficient in the diagnosis of PTE, has become a serious
consideration in clinical practice.
According to the European Society of Cardiology
(ESC) in 2014, assessing D-dimer is recommended as the first step
in excluding PTE among patients who have a low or moderate
likelihood of PTE. If the D-dimer result is positive, CTPA is then
performed to confirm the diagnosis of PTE (7). The level of D-dimer, a soluble
degradation product derived from cross-linked fibrin in the
fibrinolytic system, is increased in acute thromboembolic events
(8). It is known that a D-dimer
level lower than 500 ng/ml in the peripheral blood can exclude the
diagnosis of PTE (9). Although
D-dimer has a high sensitivity in the diagnosis of PTE, its
specificity (30 to 40%) is poor because it can be influenced by
various factors, such as increasing age, cardiovascular disease,
surgery, tumor, infection, and tissue necrosis (10,11).
Therefore, novel non-invasive biomarkers with high sensitivity and
specificity are urgently needed.
Microparticles are cellular vesicles of a
heterogeneous size ranging from 0.1 to 1 µm and located in multiple
cells, such as platelets, endothelial cells, and red cells
(12,13). Phosphatidylserine (PS) and tissue
factors on the surface of microparticles can promote the expression
of Xase and prothrombinase, thereby activating blood coagulation
and inducing thrombogenesis (14).
Elevated microparticles levels are associated with various
diseases, such as chronic rhinosinusitis (15), autoimmune diseases (16), acute myocardial infarction (17), endothelial injury (18), and chronic obstructive pulmonary
disease (COPD) (19).
Platelet-derived microparticles (PMPs) are a large population of
microparticles (70–90%) generated from the plasma membrane during
platelet activation (20). Recent
studies showed that PMPs are involved in the thrombin generation
via PS exposure and activation of both the intrinsic and extrinsic
pathway of coagulation, thus promoting blood coagulation (21–23). In
addition, accumulating evidence demonstrated that
phosphatidylserine (PS) positive PMPs can promote procoagulant
activity (24,25). It has been reported that PMP levels
are significantly elevated in patients with acute PTE compared with
those in healthy controls who have no history of venous
thromboembolism and/or cardiovascular risk factors (26). These PMPs are involved in the
occurrence and development of PTE and may serve as a biomarker of
PTE, as a new target for anti-platelet drugs, and as a new
indicator for antithrombotic activity (27). However, the diagnostic value of PMPs
in PTE still needs to be studied.
In the present study, the PMP levels of patients
with PTE were assessed. The diagnostic values of PMPs, D-dimer,
PMPs combined with D-dimer, and a combination of PMPs, platelet
distribution width, platelet count, P-selectin and D-dimer in PTE
were evaluated using a receiver operating characteristic (ROC)
analysis. Our findings may reveal a novel non-invasive biomarker in
the diagnosis of PTE with high sensitivity and specificity.
Materials and methods
Participants
A total of 102 patients with PTE were screened at
Shenzhen People's Hospital between August 2015 and August 2017. The
diagnosis of PTE was in accordance with the guidelines for the
diagnosis and management of acute pulmonary embolism (7). A positive CTPA result was reported for
these patients before admission or within 24 h after admission.
Forty patients with suspicious PTE but negative results of CTPA
were also included, they had similar symptom with PTE, such as
increased D-dimer level, dyspnea, chest pain, hemoptysis. Patients
who had histories of anticoagulant treatment, severe infectious
disease, malignant tumor, hepatic function deficiency,
transplantation, severe malnutrition, and hematological disorders
were excluded from this study (these factors can affect the
microparticles levels). A total of 102 healthy individuals (without
a history of venous thromboembolism or vascular risk) recruited
from the Physical Examination Department of the same hospital were
enrolled as the control group. The clinical characteristics of the
enrolled subjects were recorded, including sex, age, deep vein
thrombosis (DVT), platelet counts, mean platelet volume, platelet
distribution width, thrombophilia (protein C, protein S,
antithrombin III, fibrinogen degradation product, and lupus-like
anticoagulant) and baseline diseases. This study was approved by
the Institutional Review Board of Shenzhen People's Hospital, and
informed consents were obtained from all participants.
Assessment of the platelet count, mean
platelet volume, and platelet distribution width and
P-selectin
Ethylenediamine tetraacetic acid (EDTA)
anticoagulated blood samples were collected from all the
participants. Briefly, vacutainer was used to draw peripheral
venous blood with a 0.7 × 25 TWLB venepuncture needle. The
tourniquet was routinely used during the blood collection. The
platelet count, mean platelet volume, and platelet distribution
width were assessed using an automated hematology analyzer
(XS-800i, Sysmex Corporation, Kobe, Japan). Platelet-free plasma
supernatant was rapidly collected after the blood sampling (within
30 min) followed by 2,500 × g centrifugation for 15 min at room
temperature; the plasma supernatant is then again rapidly
centrifuged by 2,500 × g for 15 min at room temperature.
Platelet-free plasma is obtained by collecting the supernatant,
avoiding any contact with the platelet pellet. Plasma was stored
frozen at −80°C just before use (28). The level of P-selectin in the plasma
was assessed using an enzyme linked immunosorbent assay kit
(R&D Systems, Minneapolis, MN, USA) according to the
manufacturer's instruction. Optical density was determined at 450
nm and corrected at 620 nm.
Flow cytometry analysis of PMPs
Phycoerythrin-conjugated antibodies to PMPs, CD41a,
and Annexin V were used to label the PMPs. A total of 30 µl
platelet-free plasma was incubated using 10 µl Mouse anti-Human
CD41a (1:2, BD Pharmingen; BD Biosciences, San Jose, CA, USA) and
10 µl FITC-Annexin V (1:2, BD Pharmingen; BD Biosciences)
antibodies in Annexin V-binding buffer at 37°C for 30 min.
Thereafter, the samples were diluted in 290 µl Annexin V-binding
buffer and transferred to BD Trucount tubes (BD Pharmingen; BD
Biosciences) containing counting beads. For flow cytometry (FACS
LSRII, BD Pharmingen; BD Biosciences) analysis, the Megamix plus
SSC beads (BioCytex, Marseille, France) were backgated in a forward
scatter-side scatter plot (Fig. 1A),
and a gate was defined for identifying large MPs sized 0.5 µm (P10)
and small MPs sized 0.24 µm (P13) (Fig.
1A). The PMPs with double positive Annexin V and CD41a staining
were divided into large PMPs (P9) (Fig.
1B) and small PMPs (P14) (Fig.
1C). Counting beads were analyzed using a free fluorescence
channel of PerCP to avoid falling off-scale in the MP-optimized
settings (P11) (Fig. 1D). The PMP
level was calculated as follows: [(P9 events + P14 events)/P11
events] × (total bead counts/test volume). Isotype controls (PE
Mouse IgG1, κ Isotype control) were used to distinguish true
positive events from noise and increase the specificity of MP
detection. Sample values of a patient with PTE are shown in
Fig. 1E (PMPs=594.41/µl).
Statistical analysis
Statistical analysis was performed using SPSS
version 22.0 (SPSS Inc., Chicago, IL, USA). Quantitative data with
a normal distribution were expressed as means ± standard deviations
and compared using the Student's t-test. Quantitative data with a
non-normal distribution were expressed as medians (inter-quartile
ranges) and compared using the Mann-Whitney test. Qualitative data
were expressed as numbers (percentages) and compared using the
χ2 test. ROC curves were established to evaluate the
diagnostic values of PMPs, D-dimer, PMPs combined with D-dimer, and
a combination of PMPs, platelet distribution width, platelet count,
P-selectin, and D-dimer in PTE. The diagnostic values were compared
using MedCalc (version 11.4.2.0; MedCalc, Mariakerke, Belgium). A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Clinical characteristics of the
patients with PTE
The clinical characteristics of the patients with
PTE are presented in Table I. No
significant differences were revealed regarding sex and age among
the patients with (without) PTE and healthy controls. The platelet
count of the patients with (without) PTE were not significantly
different from those of the healthy controls, while the mean
platelet volume of patients with suspicious PTE were significantly
higher than those of patients with PTE and healthy controls
(P<0.01), the platelet distribution width of the patients with
(without) PTE were significantly lower than that of the healthy
controls (P<0.01). We observed DVT in 20 (19.61%) patients with
PTE and 2 (5.00%) in patients with suspicious PTE. A total of 27
(26.47), 16 (15.69), and 17 (16.67%) patients with PTE exhibited
decreased protein C (<65%), protein S (<63.5%), and
antithrombin III (<83%) levels, respectively. A total of 48
(47.06%) patients with PTE exhibited increased fibrinogen
degradation product (<80%) levels, and 27 (26.47%) patients had
positive findings for lupus-like anticoagulant. Furthermore, 77
patients with PTE had at least one underlying disease, such as
hypertension, diabetes, COPD, cerebral infarction, coronary heart
disease, among others.
 | Table I.Clinical characteristics of the
participants enrolled in this study. |
Table I.
Clinical characteristics of the
participants enrolled in this study.
| Variable | Patients
(n=102) | Suspicious patients
(n=40) | Healthy controls
(n=102) | P-value |
|---|
| Male sex, no.
(%) | 54 (52.94) | 17 (42.50) | 43 (42.16) | 0.256 |
| Age, years | 60.23±16.73 | 64.22±13.59 | 53.01±40.88 | 0.089 |
| PLC
(×109/l) | 257.4±101.40 | 217.6±140.6 | 251.6±45.31 | 0.086 |
| MPV (fl) | 10.23±0.94 | 11.08±1.83 | 10.22±0.93 | <0.01 |
| PDW (fl) | 11.36±2.06 | 11.37±1.77 | 13.52±2.09 | <0.01 |
| P-selectin | 68.15±38.16 | 47.14±18.20 | 40.45±12.28 | <0.01 |
| Thrombophilia |
| PC
decrease, no. (%) | 27 (26.47) | 1 (2.50) | – | – |
| PS
decrease, no. (%) | 16 (15.69) | 0 (0) | – | – |
| AT- III
decrease, no. (%) | 17 (16.67) | 1 (2.50) |
| – |
| FDP
increase, no. (%) | 48 (47.06) | 1 (2.50) | – | – |
| LA (+),
no. (%) | 27 (26.47) | 2 (5.00) | – | – |
| Baseline
diseases |
|
Hypertension, no. (%) | 25 (24.51) | 10 (25.00) | 0 (0) | – |
| DVT,
no. (%) | 20 (19.61) | 2 (5.00) | 0 (0) | – |
|
Diabetes, no. (%) | 14 (13.73) | 6 (15.00) | 0 (0) | – |
| COPD,
no. (%) | 6 (5.88) | 6 (15.00) | 0 (0) | – |
|
Cerebral infarction, no.
(%) | 7 (6.86) | 3 (7.50) | 0 (0) | – |
|
Coronary heart disease, no.
(%) | 7 (6.86) | 3 (7.50) | 0 (0) | – |
|
Pulmonary hypertension, no.
(%) | 9 (8.82) | 5 (12.50) | 0 (0) | – |
| Other diseases, no.
(%) | 13 (12.75) | 4 (10.00) | 4 (3.92) | 0.066 |
Diagnostic value of PMPs and D-dimer
in PTE
Flow cytometry analysis showed that the PMP level
was significantly higher in the patients with PTE (609.10/µl;
163.80–8501.00/µl) than in the healthy controls (230.60/µl;
20.91–790.00/µl) and patients with suspicious PTE (166.70/µl;
52.07–722.70/µl (P<0.01) (Fig.
2A). The ROC analysis, using patients with PTE as experimental
group and healthy control as control group, showed that the
sensitivity and specificity of the PMPs in the diagnosis of PTE
were 93.14 and 51.96%, respectively [area under the curve (AUC),
0.822; cut-off point, 236.97/µl] (Fig.
2B). The PMPs had the following values in the diagnosis of PTE:
Positive likelihood ratio (+LR) of 1.94, negative likelihood ratio
(-LR) of 0.13, positive predictive value (PPV) of 65.52%, negative
predictive value (NPV) of 88.14%, and Youden's index of 45.10%. The
accuracy rate of PMP values in the diagnosis of PTE was 72.06%
(Table II).
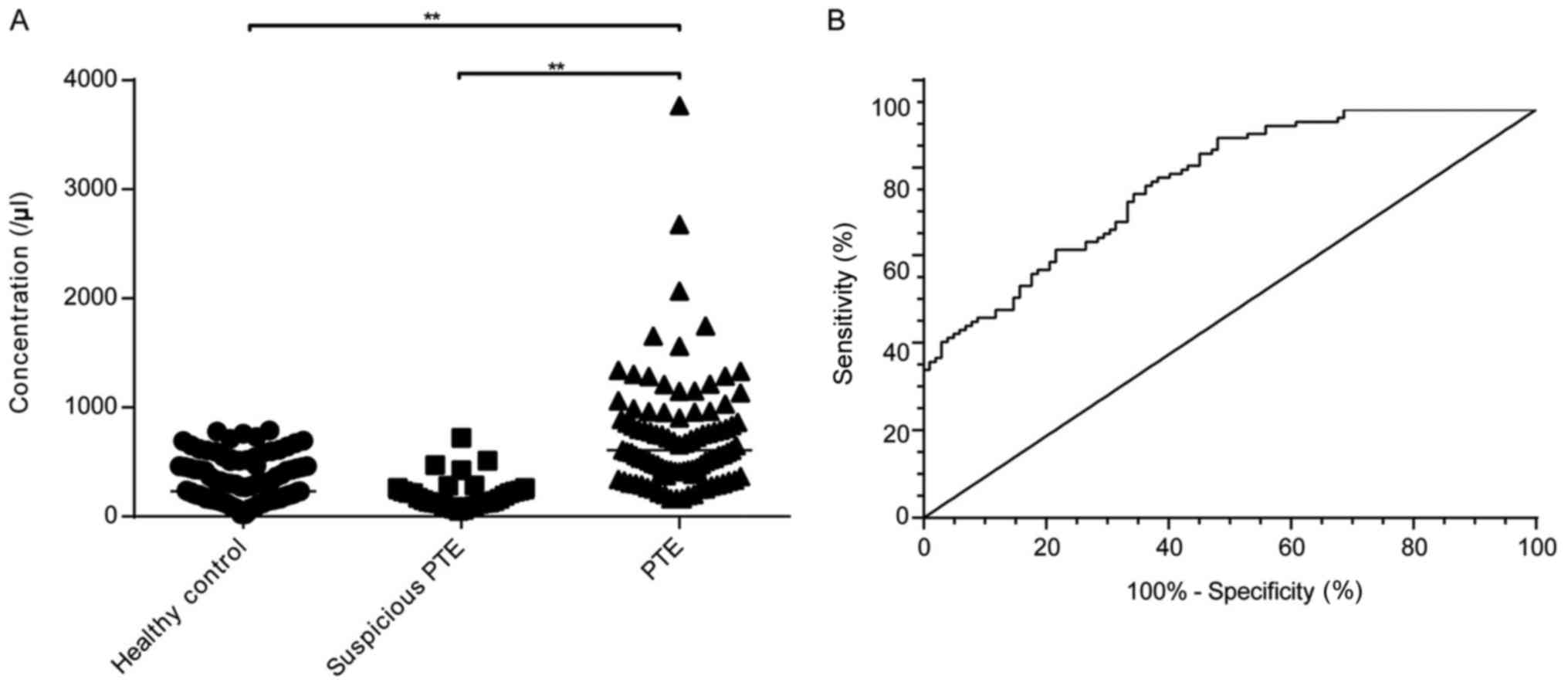 | Figure 2.Diagnostic values of PMPs in PTE.
**P<0.01. (A) PMP levels of the patients with PTE (n=102,
median=607.10/µl; range: 163.80–3769.63/µl), healthy controls
(n=102, median=230.60/µl; range: 20.91–790.00/µl) and suspicious
PTE (n=40, median=166.70/µl; range: 52.07–722.70/µl. (B) Receiver
operating characteristic curve of the PMPs. AUC=0.822; cut-off
point, 236.97/µl, sensitivity=93.14%, specificity=51.96%. PMPs,
platelet-derived microparticles; PTE, pulmonary thromboembolism;
AUC, area under the curve. |
 | Table II.Diagnostic value of PMPs, D-dimer,
PMPs combined with D-dimer, and combination of multiple parameters
in PTE. |
Table II.
Diagnostic value of PMPs, D-dimer,
PMPs combined with D-dimer, and combination of multiple parameters
in PTE.
| Groups | AUC | Sensitivity
(%) | Specificity
(%) | Accuracy (%) | PPV (%) | NPV (%) | Youden's index
(%) | +LR | -LR |
|---|
| PMPs | 0.822 | 93.14 | 51.96 | 72.06 | 65.52 | 88.14 | 45.10 |
1.94 | 0.13 |
| D-dimer | 0.773 | 56.86 | 74.51 | 65.69 | 69.05 | 63.33 | 31.37 |
2.23 | 0.58 |
| PMPs &
D-dimer | 0.875 | 86.27 | 71.57 | 78.43 | 74.58 | 83.72 | 57.84 |
3.03 | 0.19 |
| Multiple
parameters | 0.957 | 88.24 | 91.18 | 89.71 | 90.91 | 88.57 | 79.42 | 10.00 | 0.13 |
| χ2
value | – | 53.96 | 41.21 | 35.69 | 21.33 | 27.85 | – | – | – |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | – | – | – |
The diagnostic value of D-dimer in PTE was also
evaluated. The sensitivity and specificity of D-dimer in the
diagnosis of PTE were 56.86%, 74.51%, respectively (AUC, 0.773;
cut-off point, 500 ng/ml) when using patients with PTE as
experimental group and healthy control as control group. The
sensitivity was significantly lower than that of PMPs (P<0.01)
(Fig. 3 and Table II). In addition, D-dimer showed +LR,
-LR, PPV, NPV, and Youden's index of 2.23, 0.58, 69.05, 63.33 and
31.37%, respectively, with an accuracy rate of 65.69% (Table II).
Diagnostic value of the PMPs combined
with D-dimer in PTE
The diagnostic value of the PMPs combined with
D-dimer in PTE was further evaluated. A logit equation,
logit(z)=−3.0452 + 0.001153 × D-dimer + 0.004932 × PMPs, was
obtained, exhibiting a significant overall model fit
(χ2=99.349, P<0.01). The sensitivity and specificity
of the PMPs combined with D-dimer in the diagnosis of PTE were
86.27 and 71.57%, respectively (AUC, 0.875). The PMPs combined with
D-dimer showed the following values in the diagnosis of PTE: +LR of
3.03, -LR of 0.19, PPV of 74.58%, NPV of 83.72%, and Youden's index
of 57.84%. The accuracy rate of the PMPs combined with D-dimer in
the diagnosis of PTE was 78.43% (Fig.
3 and Table II). When compared
with the D-dimder alone, the sensitivity of the PMPs combined with
D-dimer for the diagnosis of PTE significantly increased
(P<0.01), while the specificity significantly increased when
compaired with PMP (P<0.01).
Diagnostic value of the combination of
multiple parameters in PTE
Since the specificity of the PMPs combined with
D-dimer in the diagnosis of PTE was relatively low, the combination
of PMPs, platelet distribution width, P-selectin, and D-dimer was
used to diagnose PTE. A logit equation, logit (z)=−3.4068 +
0.001317 × D-dimer + 0.006114 × PMPs-0.3751 × platelet distribution
width + 0.09183 × P-selectin, was obtained, exhibiting a
significant overall model fit (χ2=169.47, P<0.01).
The ROC analysis showed that the sensitivity and specificity of the
combination of multiple parameters in the diagnosis of PTE were
88.24 and 91.18%, respectively (AUC: 0.957, cut-off point:
P=0.4537), and such a combination showed the following values: +LR
of 10.00, -LR of 0.13, PPV of 90.91%, NPV of 88.57%, and Youden's
index of 79.42%. The accuracy rate of the combination of multiple
parameters in the diagnosis of PTE was 89.71% (Fig. 3 and Table
II). All these indices showed that this combination had a
better diagnostic value in PTE than the PMPs, D-dimer, and PMPs
combined with D-dimer (P<0.01).
Validation of the diagnostic value of
multiple parameters in patients with suspicious PTE
In this study, a total of 40 patients with
suspicious PTE were included. To investigated the potential
application of the diagnostic value of the multiple parameters
method, we applied the established logistic model in the patients
with suspicious PTE and evaluated its performance in distinguishing
clinical suspicious as PTE but negative patients with those
positive patients. The possibility of being predicted as PTE was
calculated following the equation: P=ez/(1+
ez), if P-value was higher than the cut off point
(0.4537), the patient would be identified as PTE patients,
otherwise, as non-PTE patients. Thirty-two (80%) of suspicious
patients were correctly clarified as non-PTE patients.
Discussion
The PMPs are a large heterogeneous population of
circulating microparticles released from the platelet as a result
of membrane phospholipid reconstruction or cytoskleton hydrolysis
(20). Accumulating evidence has
suggested that PMPs play an important role in thromboembolism
through direct cell-to-cell contact interactions or release of
active components (29). In this
study, we found that the PMP level was significantly higher in the
patients with PTE than in the healthy controls. Our finding is
consistent with those of previous studies (26,29,30), and
further illustrates the association between elevated PMP levels and
PTE. When the blood vessels are injured, the release of PMPs can
lead to the exposure of collagen and von Willebrand factor, thereby
leading to the adhesion, aggregation, and activation of platelets
(31). Thereafter, thromboxane A2
and endothelin produced by platelets can lead to contraction of the
blood vessels, thus promoting thrombogenesis (32). Meanwhile, the membrane proteins of
PMPs gathering on an anion phospholipid surface can enhance the
assembly and catalytic activity of tissue factors, thereby
exacerbating blood clotting responses (33). Since PMPs are associated with a
strong procoagulant activity, elevated PMP levels may be considered
as a potential indicator of PTE in clinical practice.
Microparticles are known as a biomarker of DVT
(34,35). It has been reported that the
sensitivity, specificity, and accuracy of total circulating
microparticles in the diagnosis of DVT were 59, 62, and 61%,
respectively (34). In addition,
microparticles > P95 increased the venous thromboembolism risk
from 1.63 (0.60–4.50) to 6.09 (1.03–36.1), and high levels of
circulating microparticles may be a possible independent risk
factor for venous thromboembolism (29). To date, related studies regarding
PMPs are still limited, and the diagnostic role of PMPs in PTE has
not been revealed. To reveal the diagnostic value of PMPs in PTE, a
ROC analysis was performed in this study. The result showed that
the sensitivity of PMPs in the diagnosis of PTE was 93.14%, higher
than that of D-dimer in the diagnosis of PTE, suggesting a
potential value of combination of these two makers in clinical
practice.
Although the sensitivity of PMPs in the diagnosis of
PTE was higher, the specificity and accuracy were still limited.
Thus, we combined PMPs and D-dimer to diagnose PTE. The ROC
analysis showed that the combination of PMPs and D-dimer
significantly increased the specificity in the diagnosis of PTE.
The findings indicate that using PMPs combined with D-dimer is
useful for the diagnosis of PTE. However, the specificity of PMPs
combined with D-dimer for the diagnosis of PTE was still low. This
phenomenon may be attributed to the low specificity of PMPs and
D-dimer. A previous study has shown that the guidelines
recommending clinical probability and D-dimer assessment as the
initial screening tests for venous thromboembolism diagnosis in
low-risk patients are underused (36). Many risk factors of venous
thromboembolism also increase the D-dimer level, including old age,
cardiovascular disease, surgery, tumor, infections, and tissue
necrosis (10,11). Therefore, we suspect that the
combination of D-dimer and PMPs may not improve the diagnostic
efficiency of PTE in clinical practice.
To eliminate the limitation of D-dimer, combinations
of other biomarkers are used to diagnose thromboembolism. A
sensitivity of 73%, specificity of 81%, and accuracy of 77% have
been reported for the identification of DVT by combining D-dimer,
soluble P-selectin, and total microparticles (34). The combination of D-dimer and MPV
results in an increase in the AUC (0.799) in the diagnosis of PE
(37). The combined measurement of
D-dimer and FXIII helps to distinguish PE from serious diseases
with similar symptoms (38). In this
study, the combination of PMPs, D-dimer, platelet distribution
width, and P-selectin was applied in the diagnosis of PTE. The ROC
analysis showed high sensitivity (88.24%), specificity (91.18%),
and accuracy (89.71%), indicating promising prospects in clinical
practice.
The pro-coagulant properties of PMPs have been
extensively studied (22–25). Zhao et al recently found that
PMPs platelets and MPs from the colon cancer patients significantly
enhanced intrinsic/extrinsic FXa and thrombin generation, greatly
shortened coagulation time, and increased fibrin formation
(24). Similarly, the study of Wang
et al also suggested that PMPs formed in sepsis are a potent
inducer of thrombin generation via PS exposure and activation of
both the intrinsic and extrinsic pathway of coagulation (21). P-selectin, on the other hand, was
another known marker of platelet procoagulant activity that exposed
on the platelet membrane when the platelet was activated (39). Besides, P-selectin can mediate the
adhesion of activated platelet with other cells, leading to the
hypercoagulant state of the blood (40). More recently, Prakash et al
found that P-selectin can even promote thrombus propagation
independently of both von Willebrand factor and thrombospondin-1 in
mice (41). A single assessment of
P-Selectin, at baseline in prospective epidemiological studies is
also suggested to be appropriate to investigate associations
between platelet activation and risks of chronic diseases (42). In the current study, both PMPs and
P-selectin were increased in PTE patients, indicating that they
were involved in the procoagulant activity of platelets.
Currently, D-dimer is the major exclusion of PTE
(9). Nevertheless, there are still a
certain part of D-dimer positive patients found to be PTE negative
after undergoing CTPA confirmation. Unnecessary CTPA will put the
patients into radiation expose and may cause potential
complication, and increase the expense of the patients. The
findings in this study showed higher sensitivity of multiple
parameters than D-dimer, and achieved 80% of accuracy in
distinguishing patients suspected with PTE from positive PTE
patients. In addition, some patients may be allergic to iodine or
too severe to undergo CTPA, in which case, the method in this study
might be an alternative option for the diagnosis of PTE in the
clinical practice.
In conclusion, an elevated PMP level was an
effective biomarker of PTE. The diagnostic value of PMPs was
similar to that of D-dimer. The combination of D-dimer and PMPs
significantly increased the sensitivity of D-dimer in the diagnosis
of PTE. The combination of PMPs, D-dimer, platelet distribution
width, and P-selectin presents a novel non-invasive strategy for
the diagnosis of PTE with high sensitivity and specificity.
However, this study was limited by its small population, further
studies about the application of PMPs for the diagnosis of PTE
based on larger populations are still needed.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shenzhen Science and
Technology Project, China (grant nos. 20150314104846179 and
JCYJ20170413093032806), Shenzhen Key Laboratory of Respiratory
Disease (grant no. ZDSYS201504301616234) and Guangdong Science and
Technology Project, China (grant no. 2017A020214016).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
YF conceived and designed the study. LXu, MW, LXi,
QH, SLiu and YL recruited subjects and performed the experiments.
MW and SLi analyzed the data. MW, YF and YY wrote the paper. YY and
YL were assisted with flow cytometry, the adjustment of the
scientific design of the study and the revision of the
manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of
Institutional Review Board of Shenzhen People's Hospital. Informed
consent were considered and obtained from all participants after
approval by the research ethical review board.
Patient consent for publication
All the study participants provided informed consent
for the publication of data.
Competeing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chung WS, Lin CL, Ho FM, Li RY, Sung FC,
Kao CH and Yeh JJ: Asthma increases pulmonary thromboembolism risk:
A nationwide population cohort study. Eur Respir J. 43:801–817.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shokoufeh H, Kerman SR, Mojtaba K, Vaferi
H, Ramezani R, Jourshari NM, Mousavi SA and Pouraliakbar H:
Accuracy of D-dimer: Fibrinogen ratio to diagnose pulmonary
thromboembolism in patients admitted to intensive care units.
Cardiovasc J Afr. 23:446–456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah N, Shah D, Shah S, Gohil Y, Vasani B
and Patel A: Computed tomography angiography in chronic pulmonary
thromboembolism. Apollo Med. 8:44–52. 2011. View Article : Google Scholar
|
|
4
|
Perrier A, Roy PM, Aujesky D, Chagnon I,
Howarth N, Gourdier AL, Leftheriotis G, Barghouth G, Cornuz J,
Hayoz D and Bounameaux H: Diagnosing pulmonary embolism in
outpatients with clinical assessment, D-dimer measurement, venous
ultrasound, and helical computed tomography: A multicenter
management study. Am J Med. 116:291–299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Righini M, Le Gal G, Aujesky D, Roy PM,
Sanchez O, Verschuren F, Rutschmann O, Nonent M, Cornuz J, Thys F,
et al: Diagnosis of pulmonary embolism by multidetector CT alone or
combined with venous ultrasonography of the leg: A randomised
non-inferiority trial. Lancet. 371:1343–1352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schluger N, Henschke C, King T, Russo R,
Binkert B, Rackson M and Hayt D: Diagnosis of pulmonary embolism at
a large teaching hospital. J Thorac Imaging. 9:180–184. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konstantinides SV, Torbicki A, Agnelli G,
Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M,
Kucher N, et al: 2014 ESC guidelines on the diagnosis and
management of acute pulmonary embolism. Eur Heart J. 35:3033–3069,
3069a-3069k. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adam SS, Key NS and Greenberg CS: D-dimer
antigen: Current concepts and future prospects. Blood.
113:2878–2887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirsh J and Lee AY: How we diagnose and
treat deep vein thrombosis. Blood. 99:3102–3110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lippi G, Cervellin G, Franchini M and
Favaloro EJ: Biochemical markers for the diagnosis of venous
thromboembolism: The past, present and future. J Thromb
Thrombolysis. 30:459–471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rafee A, Herlikar D, Gilbert R, Stockwell
RC and Mclauchlan GJ: D-Dimer in the diagnosis of deep vein
thrombosis following total hip and knee replacement: A prospective
study. Ann R Coll Surg Engl. 90:123–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arraud N, Linares R, Tan S, Gounou C,
Pasquet JM, Mornet S and Brisson AR: Extracellular vesicles from
blood plasma: Determination of their morphology, size, phenotype
and concentration. J Thromb Haemost. 12:614–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu ZH, Ji CL, Li H, Qiu GX, Gao CJ and
Weng XS: Membrane microparticles and diseases. Eur Rev Med
Pharmacol Sci. 17:2420–2427. 2013.PubMed/NCBI
|
|
14
|
Liu ML, Reilly MP, Casasanto P, Mckenzie
SE and Williams KJ: Cholesterol enrichment of human
monocyte/macrophages induces surface exposure of phosphatidylserine
and the release of biologically-active tissue factor-positive
microvesicles. Arterioscler Thromb Vasc Biol. 27:430–435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takahashi T, Kato A, Berdnikovs S, Stevens
WW, Suh LA, Norton JE, Carter RG, Harris KE, Peters AT, Hulse KE,
et al: Microparticles in nasal lavage fluids in chronic
rhinosinusitis: Potential biomarkers for diagnosis of
aspirin-exacerbated respiratory disease. J Allergy Clin Immunol.
140:720–729. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu YJJ, Hua CC, Chen JY, Chang YW and
Tseng JC: The role of endothelial microparticles in autoimmune
disease patients with Raynaud's phenomenon. J Microbiol Immunol
Infect. 50:857–862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Christersson C, Thulin à and Siegbahn A:
Microparticles during long-term follow-up after acute myocardial
infarction. Association to atherosclerotic burden and risk of
cardiovascular events. Thromb Haemost. 117:1571–1581. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Awad HA, Tantawy AA, Elfarrash RA, Ismail
EA and Youssif NM: CD144+ endothelial microparticles as a marker of
endothelial injury in neonatal ABO blood group incompatibility.
Blood Transfus. 12:250–259. 2014.PubMed/NCBI
|
|
19
|
Takahashi T, Kobayashi S, Fujino N, Suzuki
T, Ota C, Tando Y, Yamada M, Yanai M, Yamaya M, Kurosawa S, et al:
Annual FEV1 changes and numbers of circulating endothelial
microparticles in patients with COPD: A prospective study. BMJ
Open. 4:e0045712014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
György B, Szabó TG, Pásztói M, Pál Z,
Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, et al:
Membrane vesicles, current state-of-the-art: Emerging role of
extracellular vesicles. Cell Mol Life Sci. 68:2667–2688. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Zhang S, Luo L, Norström E, Braun
OÖ, Mörgelin M and Thorlacius H: Platelet-derived microparticles
regulates thrombin generation via phophatidylserine in abdominal
sepsis. J Cell Physiol. 233:1051–1060. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Horn P, Erkilet G, Veulemans V, Kröpil P,
Schurgers L, Zeus T, Heiss C, Kelm M and Westenfeld R:
Microparticle-induced coagulation relates to coronary artery
atherosclerosis in severe aortic valve stenosis. PLoS One.
11:e01514992016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bidot L, Jy W, Bidot C Jr, Jimenez JJ,
Fontana V, Horstman LL and Ahn YS: Microparticle-mediated thrombin
generation assay: Increased activity in patients with recurrent
thrombosis. J Thromb Haemost. 6:913–919. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao L, Bi Y, Kou J, Shi J and Piao D:
Phosphatidylserine exposing-platelets and microparticles promote
procoagulant activity in colon cancer patients. J Exp Clin Cancer
Res. 35:542016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nijiati M, Saidaming A and Guoqing L: In
vitro study of the thrombogenic activity of platelet-derived
microparticles from patients with acute coronary syndrome. Ann Clin
Lab Sci. 47:156–161. 2017.PubMed/NCBI
|
|
26
|
Bal L, Ederhy S, Di Angelantonio E, Toti
F, Zobairi F, Dufaitre G, Meuleman C, Mallat Z, Boccara F, Tedgui
A, et al: Factors influencing the level of circulating procoagulant
microparticles in acute pulmonary embolism. Arch Cardiovasc Dis.
103:394–403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu L and Fu YY: The role of platelet
microparticles in pulmonary thromboembolism. Clinical Research and
Practice. 11:187–189. 2017.(In Chinese).
|
|
28
|
Christersson C, Lindahl B and Siegbahn A:
The composition and daily variation of microparticles in whole
blood in stable coronary artery disease. Scand J Clin Lab Invest.
76:25–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bucciarelli P, Martinelli I, Artoni A,
Passamonti SM, Previtali E, Merati G, Tripodi A and Mannucci PM:
Circulating microparticles and risk of venous thromboembolism.
Thromb Res. 129:591–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramacciotti E, Hawley AE, Farris DM,
Ballard NE, Wrobleski SK, Myers DD Jr, Henke PK and Wakefield TW:
Leukocyte- and platelet-derived microparticles correlate with
thrombus weight and tissue factor activity in an experimental mouse
model of venous thrombosis. Thromb Haemost. 101:748–754.
2009.PubMed/NCBI
|
|
31
|
Freyssinet JM: Cellular microparticles:
what are they bad or good for? J Thromb Haemost. 1:1655–1662. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Biró E, Sturk-Maquelin KN, Vogel GMT,
Meuleman DG, Smit MJ, Hack CE, Sturk A and Nieuwland R: Human
cell-derived microparticles promote thrombus formation in vivo in a
tissue factor-dependent manner. J Thromb Haemost. 1:2561–2568.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qi H, Zhong Z, Zhou HX, Deng CY, Zhu H, Li
JF, Wang XL and Li FR: A rapid and highly sensitive protocol for
the detection of Escherichia coli O157:H7 based on
immunochromatography assay combined with the enrichment technique
of immunomagnetic nanoparticles. Int J Nanomedicine. 6:3033–3039.
2011.PubMed/NCBI
|
|
34
|
Rectenwald JE, Myers DD Jr, Hawley AE,
Longo C, Henke PK, Guire KE, Schmaier AH and Wakefield TW: D-dimer,
P-selectin, and microparticles: novel markers to predict deep
venous thrombosis. A pilot study. Thromb Haemost. 94:1312–1317.
2005.PubMed/NCBI
|
|
35
|
Floresnascimento MC, Beltrame MP, De Paula
EV, Montalvão SL, Pereira FG, Orsi FL, Lorand-Metze I and
Annichino-Bizzacchi JM: Microparticles in deep venous thrombosis,
antiphospholipid syndrome and Factor V Leiden. Platelets.
20:367–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee JA and Zierler BK: The current state
of practice in the diagnosis of venous thromboembolism at an
academic medical center. Vasc Endovascular Surg. 45:22–27. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang J, Chen Y, Cai Z and Chen P:
Diagnostic value of platelet indices for pulmonary embolism: The
authors respond. Am J Emerg Med. 33:10942015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tang N, Sun Z, Li D, Yang J, Yin S and
Guan Q: Combined measurement of factor XIII and D-dimer is helpful
for differential diagnosis in patients with suspected pulmonary
embolism. Clin Chem Lab Med. 55:1948–1953. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Napoleão P, Monteiro Mdo C, Cabral LB,
Criado MB, Ramos C, Selas M, Viegas-Crespo AM, Saldanha C, Carmo
MM, Ferreira RC and Pinheiro T: Changes of soluble CD40 ligand in
the progression of acute myocardial infarction associate to
endothelial nitric oxide synthase polymorphisms and vascular
endothelial growth factor but not to platelet CD62P expression.
Transl Res. 166:650–659. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
George R, Bhatt A, Narayani J,
Thulaseedharan JV, Sivadasanpillai H and Tharakan JA: Enhanced
P-selectin expression on platelet-a marker of platelet activation,
in young patients with angiographically proven coronary artery
disease. Mol Cell Biochem. 419:125–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prakash P, Nayak MK and Chauhan AK:
P-selectin can promote thrombus propagation independently of both
von Willebrand factor and thrombospondin-1 in mice. J Thromb
Haemost. 15:388–394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Graf ME, Sookthai D, Johnson T, Schübel R,
Katzke V, Bugert P, Hoffmeister M, Kaaks R and Kühn T: Biological
reproducibility of circulating P-Selectin, Thrombopoietin,
GPIIb/IIIa and Thrombomodulin over one year. Clin Biochem.
50:942–946. 2017. View Article : Google Scholar : PubMed/NCBI
|

















