Introduction
Aging (physiological and pathological) refers to the
gradual decline of various organ functions of the body. Aging is
mainly regulated by the body's genetic information. However,
external factors, such as bad living habits and environmental
factors, are also associated with the aging of the body. In
contrast, good habits and healthy practices, such as moderate
exercise, can effectively delay aging and improve quality of life
(1,2).
Aerobic exercise refers to the physical exercise
performed by the human body with a full supply of oxygen. During
aerobic exercise, oxygen uptake and oxygen demand in the body are
equal (3). As the body obtains
energy via the aerobic oxidation of energy substances and that the
energy required for exercise must be provided by oxidizing starch
fat and protein in the body, aerobic exercise requires a duration
of at least 30 min (3,4). In addition, the exercise intensity is
in the middle (120–150 beats/min) or upper middle level
(>151-180 beats/min), the athlete's heart rate is maintained at
60–80% of the maximum heart rate (4). According to the exercise method,
aerobic exercise is divided into continuous aerobic exercise (CAE)
and intermittent aerobic exercise (IAE). Aerobic exercise is
important for improving body functions and quality of life
(3).
Klotho is an anti-aging gene that encodes a type I
transmembrane protein. It is highly expressed in the cerebral
choroid plexus and renal tubular epithelia (5). As a membrane-bound protein, Klotho can
be cleaved; Klotho has also been identified as a soluble protein in
the blood, urine and cerebrospinal fluid (6,7). Klotho
protein participates in many pathways that govern aging, such as
regulation of phosphate homeostasis, insulin signaling and Wnt
signaling (8). In mammals, the body
gradually undergoes an aging process over time. The expression of
Klotho also gradually declines (9).
In animal experiments, overexpression of Klotho protein delays
aging and prolongs life span (10,11). In
addition, it has previously been demonstrated that Klotho protein
increases the ability of cells to remove reactive oxygen species
(ROS) (12). In cell culture
experiments and transgenic mice, it was also demonstrated that
Klotho protein enhances the resistance of cells to ROS and prolongs
the life span (13). However, it
remains to be elucidated whether, under conditions of aerobic
exercise, Klotho-mediated signaling pathways also contribute to the
enhanced resistance of cells to ROS and to a prolonged life
span.
In the present study, two aerobic exercise models
were used in Sprague Dawley (SD) rats to investigate whether
aerobic exercise influenced body functions and prolonged life span
by regulating Klotho-mediated signaling pathways. The aim of the
study was to elucidate the association among the Klotho gene,
aerobic exercise, and aging.
Materials and methods
Experimental animals and aerobic
exercise models
Male 3-month-old (weight, 320–350 g) SD rats were
purchased from the Animal Experimental Center at the Medical School
of Xi'an Jiaotong University (Xi'an, China) and maintained in
microisolator cages in a specific-pathogen-free facility. All rat
experiments were performed using protocols approved by the
Institutional Ethics Committee on Animal Use of Xi'an Medical
University (Xi'an, China). The temperature of the breeding
environment was 20–25°C, the relative humidity was 50–70%, and the
light/dark cycle was set at 12-h/day. Experimental rats were fed
with national standard rodent feed and autoclaved water ad
libitum.
The total number of rats used in the present study
was 101. After they had adapted to the environment for one week, 30
rats were selected randomly as two control groups (one group
included 22 rats for survival analysis and the other one included 8
rats for tissue detection) where these rats were not subjected to
any type of exercise regime. The other rats received adaptive
training as follows: 10–15 m/min for 30 min/day for 5 days. A total
of 8 rats that failed to qualify for the exercise training were
removed, and the remaining 63 rats were randomly divided into four
groups, including two IAE groups (one group included 22 rats for
survival analysis and the other one included 9 rats for tissue
detection) and two CAE groups (one group included 22 rats for
survival analysis and the other one included 10 rats for tissue
detection). The CAE model was established following a previously
published protocol (14). The
specific steps were as follows: The treadmill slope was 0°; the
training speed was 16 m/min [50–60% maximal oxygen uptake
(VO2 max)]; and the training time was 60 min/day for 5
days/week for a total of 48 weeks (15,16). The
IAE model was established following a previously published protocol
(17). The specific steps were as
follows: The treadmill slope was 0°; the training program was 10
m/min (40–50% VO2 max) exercise for 10 min, 25 m/min
(80–90% VO2 max) exercise for 7 min, and 15 m/min
(50–60% VO2 max) exercise for 3 min; and the training
time was 60 min/day alternated with the above three protocols for 5
days/week for a total of 48 weeks.
Survival analysis
Following aerobic exercise for 48 weeks, 3 groups of
rats, including the control group, an IAE group, and a CAE group,
continued to be fed routinely and were observed daily until the end
of their lives. The survival time of the rats was then
analyzed.
Animal tissues
Following the 48-week aerobic exercise program, rats
in the other three groups (control, IAE and CAE) were sacrificed
with barbiturate (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany)
overdose (150 mg/kg) via intraperitoneal injection. Rat kidney and
brain tissues were quickly removed and stored at −80°C for later
use.
Determination of ROS in renal and
brain tissues
ROS levels were measured using a high quality green
fluorescence assay kit (GMS10016.3; GenMed Scientifics Inc.,
Shanghai, China). Quantitative detection of tissue homogenate was
performed according to the manufacturer's protocol. Briefly, 50 mg
kidney and brain tissues from each rat was collected and
homogenized. Subsequently, 5 µl of final tissue homogenate was used
for quantitative protein detection. Protein quantification was
performed using the GENMED Bradford kit (GMS30030.1; GenMed
Scientifics Inc.) according to the manufacturer's protocol. Based
on those results, the volume of final homogenate containing 100 µg
protein was calculated. The corresponding volume of the final
homogenate was then mixed with the detection reagent. The mixture
was incubated at 37°C in the dark for 20 min and tested
immediately. The relative fluorescence unit (RFU) values were
measured at 490/520 nm using a multifunction fluorescence
microplate reader, and the result was expressed as the RFU value of
the assay well minus the RFU value of the blank well.
mRNA expression and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
A 50 mg quantity of rat kidney and brain tissue was
collected, and total RNA was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The purity and
concentration of the extracted total RNA were determined via an
ultraviolet spectrophotometer (UV1800; Shimadzu Corporation, Kyoto,
Japan). A PrimeScript™ RT reageant kit (Takara Biomedical
Technology Co., Ltd., Beijing, China) and a PCR amplification
apparatus (Eppendorf, Hamburg, Germany) were used to reverse
transcribe the RNA into cDNA. The RT temperature protocol was 42°C
for 15 min, 85°C for 5 sec and standby at 4°C; then, the mRNA
expression levels of Klotho were measured using a thermal cycler
(ABI Veriti FAST thermal cycler; Thermo Fisher Scientific, Inc.).
SYBR Green Real-time PCR Master Mix was purchased from Takara
Biomedical Technology Co., Ltd.
The upstream primer sequence of the Klotho gene was
5′-ATCCGGCCTCAGATAACCTT-3′. The downstream primer sequence of the
Klotho gene was 5′-CCACCACTGGAGTGATGTTG-3′. The product length was
175 bp. The reference gene was rat GAPDH. The upstream primer
sequence of rat GAPDH gene was 5′-GGTGGACCTCATGGCCTACA-3′. The
downstream primer sequence of GAPDH gene was
5′-CTCTCTTGCTCTCAGTATCCTTGCT-3′. The product length was 200 bp. The
Applied Biosystems® 7500 SDS v2.0.6 Software (Thermo
Fisher Scientific, Inc.) that comes with the PCR instrument was
used to read and analyze the target gene quantitative CT values.
The reaction system was as follows: 2×SYBR Green Real-time PCR
Master Mix (Modified DNA polymerase, SYBR Green I, Optimized PCR
buffer, 5 mM MgCI2, dNTP mix including dUTP; Toyobo Life
Science, Osaka, Japan) 10 µl, DEPC water 8 µl, upstream primer 0.5
µl, downstream primer 0.5 µl, cDNA 1 µl. The thermocycling
conditions were as follows: 95°C for 3 min, 95°C for 30 sec and
60°C for 30 sec for 35 cycles, followed by 72°C for 5 min ad
holding at 4°C for further use.
Western blot analysis
A 100-mg sample of kidney tissue and brain tissue
was taken from each rat, minced, and ground with liquid nitrogen.
Then, 1 ml radioimmunoprecipitation assay protein lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) was added, and the samples were kept on ice for 10 min. The
samples were subsequently centrifuged at 12,000 × g for 5 min at
4°C and the total protein concentration in the clear supernatant
was evaluated using a bicinchoninic acid assay kit (Thermo Fisher
Scientific, Inc.). Aliquots containing 20 µg protein were subjected
to 10% SDS-PAGE. The proteins were then transferred to a
polyvinylidene difluoride membrane. Membranes were blocked for 1 h
at room temperature with a 5% skim milk in Tris buffered saline.
The membranes were washed with TBS buffer three times for 5 min per
wash. The primary antibodies [murine anti-GAPDH (ab8245; 1:10,000,
Abcam, Cambridge, UK) and rabbit-derived anti-Klotho (ab203576;
1:2,000, Abcam)] were blocked in 10 ml of 5% skim milk and
incubated at 4°C overnight. The membranes were washed twice for 10
min/wash with Tween-20 TBS buffer. The horseradish
peroxidase-labeled secondary antibodies (cat. no. ab6728 for Klotho
and ab6721 for GAPDH; 1:10,000, Abcam) were added to 5 ml of 5%
skim milk and incubated for 2 h at 37°C. The membranes were washed
twice for 10 min/wash with Tween-20 TBS buffer. Specific signals
were detected with enhanced chemiluminescence solution (Thermo
Fisher Scientific Inc., Waltham, US). Densitometry was performed
using Image Lab 4.1 software (Bio-Rad Laboratories, Inc., Hercules,
CA, USA), and the density of each band was normalized against that
of GAPDH. All experiments were performed in triplicate.
Statistical analysis
SPSS 19.0 statistical software (IBM Corp., Armonk,
NY, USA) was used for data analysis. All data are expressed as the
mean + standard deviation. Log-rank test was used in the survival
analysis of the rats in Fig. 1A.
One-way analysis of variance was used for data in Figs. 1B and 2–4 followed
by a Student-Newman-Keul's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
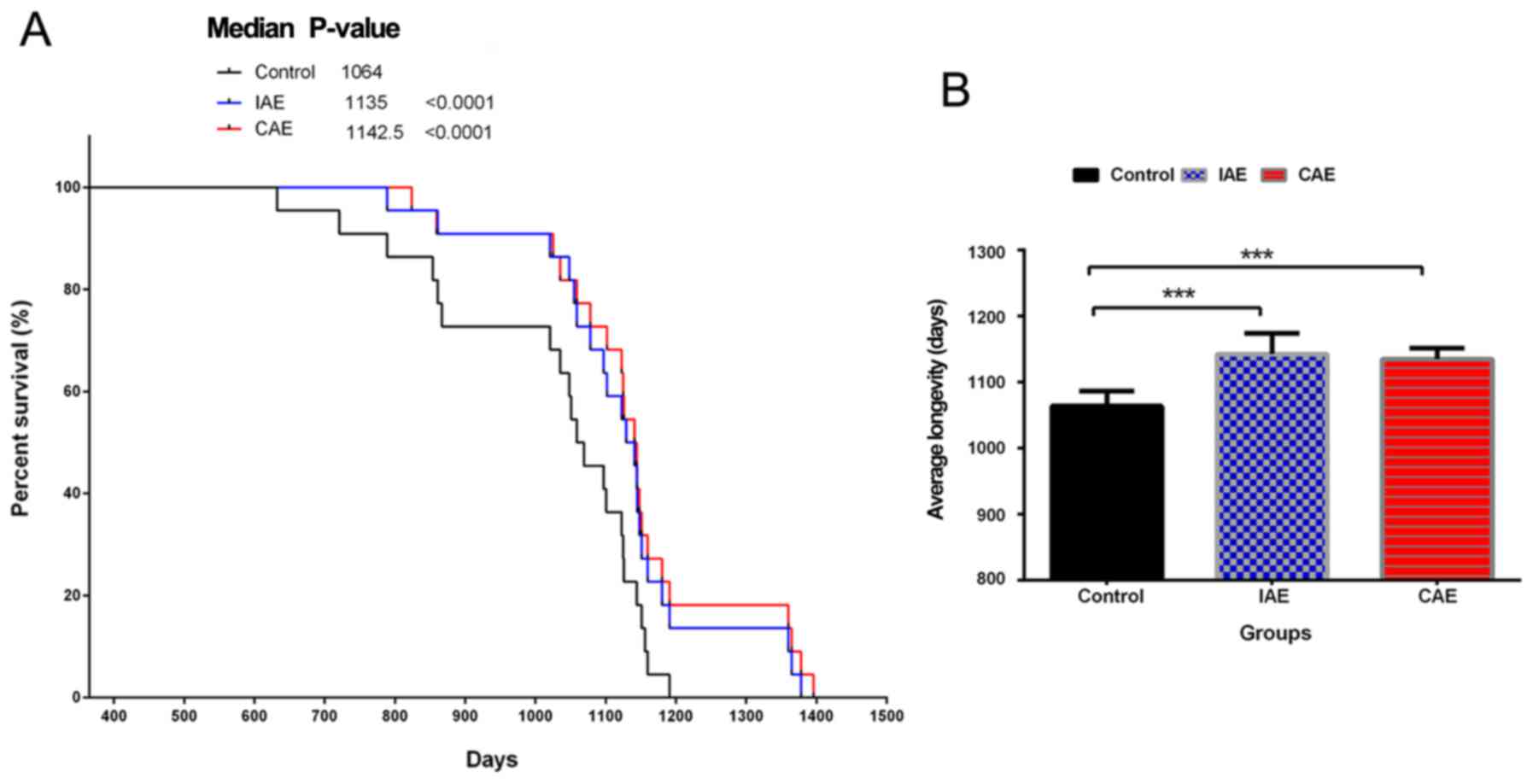
Aerobics exercise prolongs the
survival time of rats
To determine whether aerobics exercise could
influence physical quality and prolong the survival time, the rats
in different groups were observed daily until the end of their
lives (Fig. 1). Kaplan-Meier curve
analysis demonstrated that in both the IAE and CAE groups, the
survival time of rats was significantly prolonged compared with
that in the control group (P<0.0001; Fig. 1A). The mean survival time of rats in
the IAE and CAE groups was 1,142.5±32.0 and 1,135±17.0 days,
respectively. The mean survival time in the control group was only
1,064±23 days. Aerobic exercise increased the mean survival time of
rats by >70 days compared with that in the control group
(P<0.0001; Fig. 1B). There was no
significant difference between the IAE and CAE groups. The above
results demonstrated that aerobic exercise could improve physical
quality and extend life expectancy.
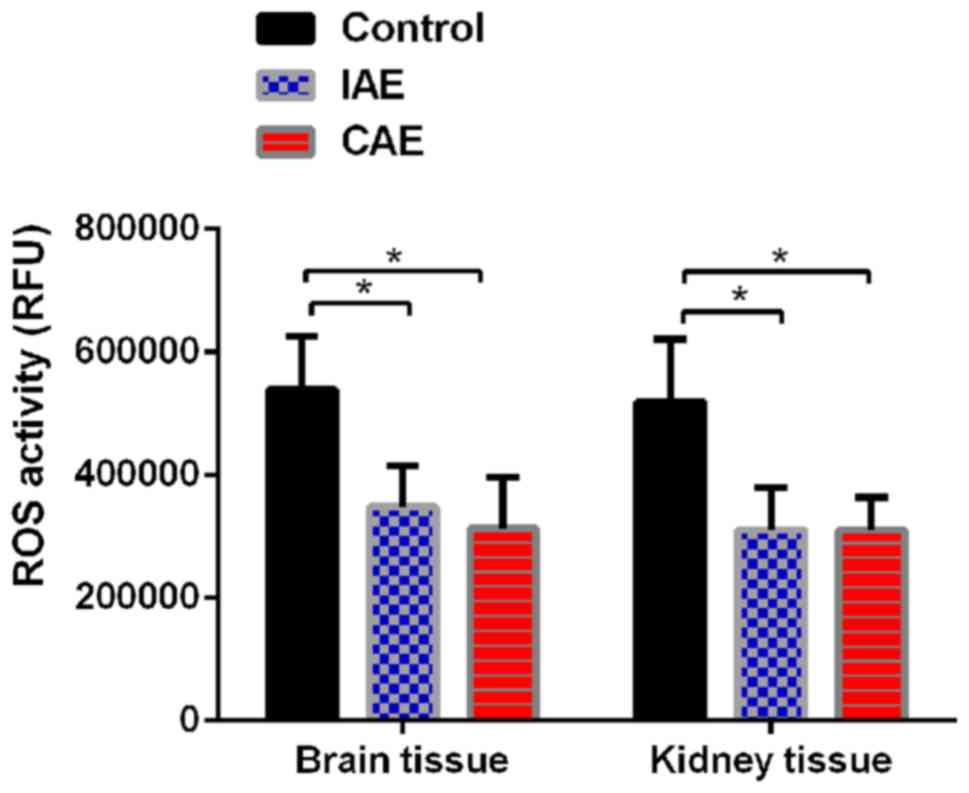
Aerobic exercise decreases ROS levels
in rat kidney and brain tissues
To determine whether aerobic exercise could increase
the clearance rate of ROS, the ROS levels in rat kidney and brain
tissues were evaluated. As presented in Fig. 2, following aerobic exercise for 48
weeks, ROS levels were significantly decreased in brain and kidney
tissues of IAE and CAE rats compared with those of control rats
(P<0.05). The ROS levels in the brain tissues of the IAE rats
were reduced to 64% of those of the control rats, and the ROS
levels in the brain tissues of the CAE rats were reduced to 58% of
those of the control rats. Similarly, the ROS levels in the kidney
tissues of the IAE rats were decreased to 60% of those of the
control rats, and the ROS levels in the kidney tissues of the CAE
rats were decreased to 59% of those of the control rats. There was
no statistically significant difference between IAE and CAE rats
(P>0.05).
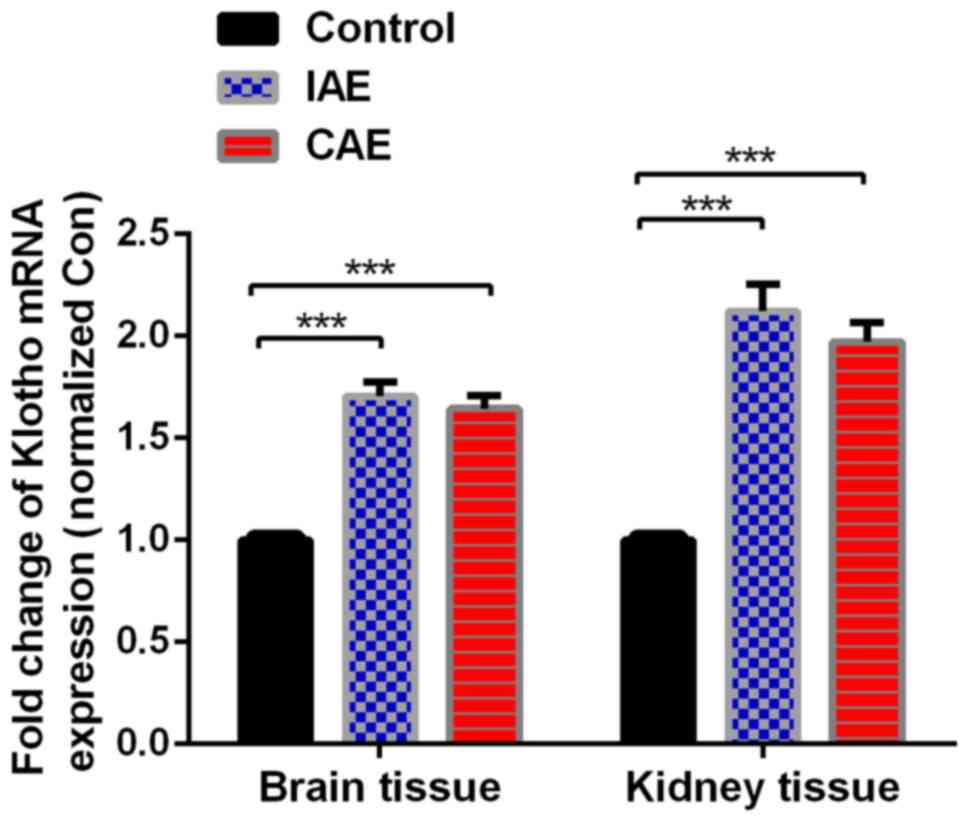
Aerobic exercise upregulates the mRNA
expression levels of Klotho in rat brain and kidney tissues
Following aerobic exercise for 48 weeks, mRNA levels
of Klotho in the rat brain and kidney tissues, as detected by
RT-qPCR, were significantly increased in the IAE and CAE groups
compared with the control group (P<0.05; Fig. 3). The mRNA levels of Klotho were
increased by 1.71-fold in the brain tissues of IAE rats compared
with those of control rats, and ROS levels were increased by
1.64-fold in the brain tissues of CAE rats compared with those of
control rats. Similarly, ROS levels were decreased by 2.12-fold in
the kidney tissues of IAE rats compared with those of control rats,
and ROS levels were decreased by 1.97-fold in the kidney tissues of
CAE rats compared with those of control rats. There was no
statistically significant difference between the IAE and CAE groups
(P>0.05).
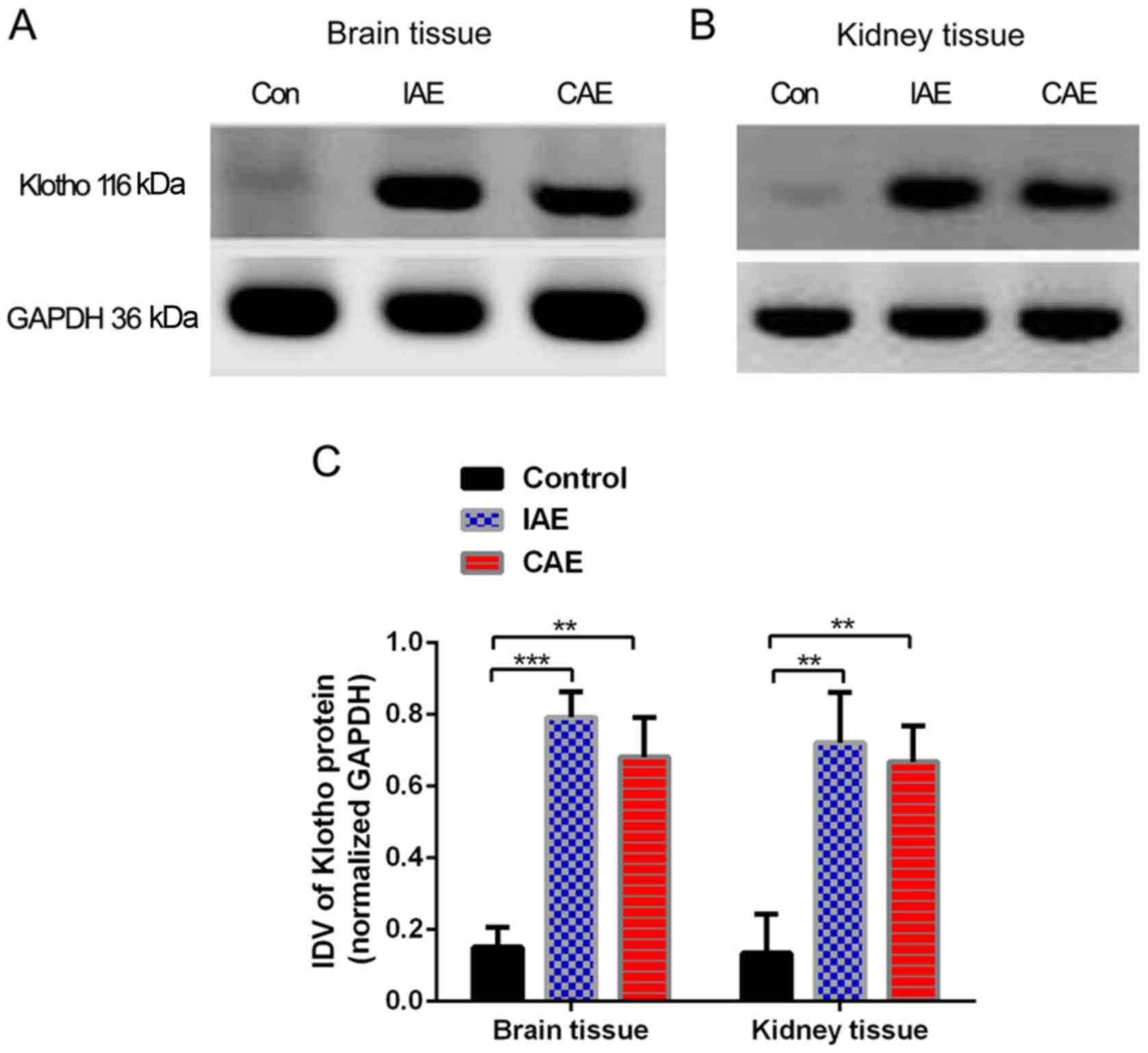
Aerobic exercise increases the Klotho
protein levels in rat brain and kidney tissues
As presented in Fig.
4, following 48 weeks of aerobic training, Klotho protein
levels in the rat brain and kidney tissues increased significantly
(P<0.05) in the IAE and CAE groups compared with the control
group. The protein levels of Klotho in the brain tissues of the IAE
rats were 5.2 times more than those of the control rats, and
protein levels of Klotho in the brain tissues of the CAE rats were
4.5 times more than those of the control rats. Similarly, the
protein levels of Klotho in the kidney tissues of IAE rats were 5.3
times greater than those of control rats, and protein levels of
Klotho in the kidney tissues of the CAE rats were 4.9-times greater
than those of control rats. There was no statistically significant
difference between the IAE and CAE groups (P>0.05).
Discussion
Aerobic exercise is important for improving body
functions and quality of life. Previous studies have demonstrated
that aerobic exercise delays aging, the retardation of cognitive
functions and the decline of neurological functions (18,19). The
Klotho gene is closely associated with anti-aging functions in
mammals. A previous study has demonstrated that the phenotype of
Klotho gene knockout mice is very similar to the features of human
aging, both of which exhibit pathological manifestations such as
shortened life span, atherosclerosis, osteoporosis, emphysema and
reduced immune function (20).
The Klotho gene is mainly expressed in brain and
kidney tissues (21,22). In animal models of kidney diseases,
it has been demonstrated that the expression level of Klotho
protein is reduced (23).
Administration of exogenous Klotho protein attenuated renal disease
and restored the function of renal tubular resorption (13). In conditional brain Klotho knockout
mice, the phenotypes are also similar to the features of human
aging, exhibiting neurodegeneration and reduced synapses in the
hippocampus (24). Reduced
expression of the Klotho gene increased the speed of nerve cell
senescence and caused the degeneration of glial cells (25). In addition, it was previously
demonstrated that the expression level of the Klotho gene in the
brain of aged macaque monkeys was reduced. These aged macaque
monkeys exhibited traits similar to those of human patients with
Alzheimer's disease (26,27). It was recently demonstrated that gene
therapy via Clustered Regularly Interspaced Short Palindromic
Repeats technology increased the expression of Klotho, which
improved the cognitive ability of experimental mice (28,29).
ROS lead to accumulation of oxidative damage to DNA,
lipids and proteins in cells, which further lead to the degradation
of cell functions, ultimately causing senescence. Therefore, the
body needs to improve the ability to remove or resist ROS to keep
healthy (30). One of the most
important functions of the Klotho gene is to increase the ability
of organisms to clear harmful ROS by increasing the cell functions
that detoxify harmful ROS (31,32). It
was previously reported that Klotho can enhance cardiac function by
inhibiting ROS (31). Furthermore,
previous studies have demonstrated that moderate exercise may
upregulate Klotho gene expression in muscle tissues and control the
production of ROS (33,34).
Based on the above findings, it was hypothesized
that if aerobic exercise could increase Klotho gene expression, it
would be a physiological and safe method to relieve aging and many
aging-associated diseases. Consistent with these previous findings,
the present experimental results also demonstrated that aerobic
exercise significantly prolonged the survival time of rats and that
aerobic exercise upregulated the expression of Klotho in brain and
kidney tissues. The effect of IAE on Klotho expression was slightly
higher than that of CAE, but the difference was not statistically
significant. Previous studies have demonstrated that the Klotho
protein has both membrane-bound and secretory forms in humans and
mice and the latter is significantly higher than the former
(35,36). However, in rats, the membrane-bound
form Klotho is more prevalent, whereas the secreted form is faintly
expressed (37). In the present
study, expression levels of the membrane-bound Klotho protein were
quantified using a rabbit polyclonal antibody specific to Klotho.
The predicted molecular weight of this protein is 116 kDa. It was
demonstrated that the expression of the membrane-bound Klotho
protein was significantly reduced following a long period of
aerobic exercise. This result suggests that the membrane-bound
Klotho protein may be the active form. In addition, aerobic
exercise significantly suppressed the levels of ROS in rat brain
and kidney tissues. Compared with that in the IAE group, the
reduction in ROS level was slightly lower in the CAE group, but the
difference was not statistically significant. It has been
previously reported that both types of aerobic exercise benefit the
improvement of heart function and myocardial structure in
myocardial infarction patients (38,39).
Therefore, the aim of the present study was to test whether the two
aerobic exercise methods are equally effective at delaying aging.
According to the present results, both types of exercise are
effective, and there is no statistically significant difference
between the two methods. Therefore, any aerobic exercise method can
be selected according to one's own conditions.
In conclusion, the present results results suggest
that exercise could delay aging and extend life span by increasing
the expression of the Klotho gene in rat brain and kidney tissues.
Increased Klotho protein expression is beneficial for the
elimination of ROS damage to the body, delaying aging and improving
various body functions. These findings support as association among
the Klotho gene, aerobic exercise and aging. As Klotho exhibits a
potential anti-aging effect, promoting Klotho expression through
aerobic exercise may be a novel approach for the prevention and
treatment of aging and aging-related diseases. In addition, these
results provide support for physical exercise being good for
health.
Acknowledgements
Not applicable.
Funding
The present study was supported by grant no.
17JK0666 from the Scientific Research Program funded by the
Education Department of Shaanxi Province, grant no. 17036 from
Common Projects by the Sports Bureau of Shaanxi Province, grant no.
2017GJFY36 from the National Natural Science Foundation Training
Program and grant no. 2017DOC03 from the Scientific Research
Foundation of Xi'an Medical University.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
NJ and KL conceived the project and designed the
present study. NJ, JL, FH, YZ, WW and BL performed the experiments
and data analysis, and wrote the manuscript. JL, MX and XZ
performed data analysis and revised the manuscript. KL supervised
the work, provided administrative support, performed data analysis
and proofread the paper.
Ethics approval and consent to
participate
All rat experiments were performed using protocols
approved by the Institutional Ethics Committee on Animal Use of
Xi'an Medical University (Xi'an, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Garatachea N, Pareja-Galeano H,
Sanchis-Gomar F, Santos-Lozano A, Fiuza-Luces C, Morán M, Emanuele
E, Joyner MJ and Lucia A: Exercise attenuates the major hallmarks
of aging. Rejuvenation Res. 18:57–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seals DR: Edward F. Adolph distinguished
lecture: The remarkable anti-aging effects of aerobic exercise on
systemic arteries. J Appl Physiol (1985). 117:425–439. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jadczak AD, Makwana N, Luscombe-Marsh N,
Visvanathan R and Schultz TJ: Effectiveness of exercise
interventions on physical function in community-dwelling frail
older people: An umbrella review of systematic reviews. JBI
Database Syst Rev Implement Rep. 16:752–775. 2018. View Article : Google Scholar
|
|
4
|
Matos N and Winsley RJ: Trainability of
young athletes and overtraining. J Sports Sci Med. 6:353–367.
2007.PubMed/NCBI
|
|
5
|
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi
H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et
al: Mutation of the mouse klotho gene leads to a syndrome
resembling ageing. Nature. 390:45–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu MC, Shi M, Zhang J, Quiñones H,
Griffith C, Kuro-o M and Moe OW: Klotho deficiency causes vascular
calcification in chronic kidney disease. J Am Soc Nephrol.
22:124–136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imura A, Iwano A, Tohyama O, Tsuji Y,
Nozaki K, Hashimoto N, Fujimori T and Nabeshima Y: Secreted Klotho
protein in sera and CSF: Implication for post-translational
cleavage in release of Klotho protein from cell membrane. FEBS
Lett. 565:143–147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mencke R and Hillebrands JL; NIGRAM
consortium, : The role of the anti-ageing protein klotho in
vascular physiology and pathophysiology. Ageing Res Rev.
35:124–146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zuo Z, Lei H, Wang X, Wang Y, Sonntag W
and Sun Z: Aging-related kidney damage is associated with a
decrease in klotho expression and an increase in superoxide
production. Age (Dordr). 33:261–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JH, Hwang KH, Park KS, Kong ID and Cha
SK: Biological role of anti-aging protein klotho. J Lifestyle Med.
5:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pako J, Barta I, Balogh Z, Kerti M,
Drozdovszky O, Bikov A, Antus B, Horvath I and Varga J: Assessment
of the anti-aging klotho protein in patients with COPD undergoing
pulmonary rehabilitation. COPD. 14:176–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baluchnejadmojarad T, Eftekhari SM,
Jamali-Raeufy N, Haghani S, Zeinali H and Roghani M: The anti-aging
protein klotho alleviates injury of nigrostriatal dopaminergic
pathway in 6-hydroxydopamine rat model of Parkinson's disease:
Involvement of PKA/CaMKII/CREB signaling. Exp Gerontol. 100:70–76.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsieh CC, Kuro-o M, Rosenblatt KP, Brobey
R and Papaconstantinou J: The ASK1-Signalosome regulates p38 MAPK
activity in response to levels of endogenous oxidative stress in
the klotho mouse models of aging. Aging (Albany NY). 2:597–611.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Wang S, Wier WG, Zhang Q, Jiang H,
Li Q, Chen S, Tian Z, Li Y, Yu X, et al: Exercise improves the
dilatation function of mesenteric arteries in postmyocardial
infarction rats via a PI3K/Akt/eNOS pathway-mediated mechanism. Am
J Physiol Heart Circ Physiol. 299:H2097–H2106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karvinen SM, Silvennoinen M, Ma H,
Törmäkangas T, Rantalainen T, Rinnankoski-Tuikka R, Lensu S, Koch
LG, Britton SL and Kainulainen H: Voluntary running Aids to
maintain high body temperature in rats bred for high aerobic
capacity. Front Physiol. 7:3112016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calabrese EJ: Pre- and post-conditioning
hormesis in elderly mice, rats, and humans: Its loss and
restoration. Biogerontology. 17:681–702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wisløff U, Støylen A, Loennechen JP,
Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA,
Lee SJ, et al: Superior cardiovascular effect of aerobic interval
training versus moderate continuous training in heart failure
patients: A randomized study. Circulation. 115:3086–3094. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colcombe SJ, Erickson KI, Scalf PE, Kim
JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L and Kramer
AF: Aerobic exercise training increases brain volume in aging
humans. J Gerontol A Biol Sci Med Sci. 61:1166–1170. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jonasson LS, Nyberg L, Kramer AF,
Lundquist A, Riklund K and Boraxbekk CJ: Aerobic exercise
intervention, cognitive performance, and brain structure: Results
from the physical influences on brain in aging (PHIBRA) study.
Front Aging Neurosci. 8:3362017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eren M, Boe AE, Murphy SB, Place AT,
Nagpal V, Morales-Nebreda L, Urich D, Quaggin SE, Budinger GR,
Mutlu GM, et al: PAI-1-regulated extracellular proteolysis governs
senescence and survival in Klotho mice. Proc Natl Acad Sci USA.
111:7090–7095. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng G and Liu D: Klotho: A promising
biomarker closely related to kidney transplant. Exp Clin
Transplant. 16:253–258. 2018.PubMed/NCBI
|
|
22
|
Boksha IS, Prokhorova TA, Savushkina OK
and Tereshkina EB: Klotho protein: Its role in aging and central
nervous system pathology. Biochemistry (Mosc). 82:990–1005. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kooman JP, Dekker MJ, Usvyat LA, Kotanko
P, van der Sande FM, Schalkwijk CG, Shiels PG and Stenvinkel P:
Inflammation and premature aging in advanced chronic kidney
disease. Am J Physiol Renal Physiol. 313:F938–F950. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Teocchi MA, Ferreira AÉ, da Luz de
Oliveira EP, Tedeschi H and D'Souza-Li L: Hippocampal gene
expression dysregulation of klotho, nuclear factor kappa B and
tumor necrosis factor in temporal lobe epilepsy patients. J
Neuroinflammation. 10:532013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mecocci P, Boccardi V, Cecchetti R,
Bastiani P, Scamosci M, Ruggiero C and Baroni M: A long journey
into aging, brain aging, and Alzheimer's disease following the
oxidative stress tracks. J Alzheimers Dis. 62:1319–1335. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dubal DB, Zhu L, Sanchez PE, Worden K,
Broestl L, Johnson E, Ho K, Yu GQ, Kim D, Betourne A, et al: Life
extension factor klotho prevents mortality and enhances cognition
in hAPP transgenic mice. J Neurosci. 35:2358–2371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeldich E, Chen CD, Colvin TA,
Bove-Fenderson EA, Liang J, Tucker Zhou TB, Harris DA and Abraham
CR: The neuroprotective effect of Klotho is mediated via regulation
of members of the redox system. J Biol Chem. 289:24700–24715. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen CD, Zeldich E, Li Y, Yuste A and
Abraham CR: Activation of the anti-aging and cognition-enhancing
gene klotho by CRISPR-dCas9 transcriptional effector complex. J Mol
Neurosci. 64:175–184. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou HJ, Zeng CY, Yang TT, Long FY, Kuang
X and Du JR: Lentivirus-mediated klotho up-regulation improves
aging-related memory deficits and oxidative stress in
senescence-accelerated mouse prone-8 mice. Life Sci. 200:56–62.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ung L, Pattamatta U, Carnt N,
Wilkinson-Berka JL, Liew G and White AJR: Oxidative stress and
reactive oxygen species: A review of their role in ocular disease.
Clin Sci (Lond). 131:2865–2883. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu H, Gao Y, Zhu S, Cui Q and Du J:
Klotho improves cardiac function by suppressing reactive oxygen
species (ROS) mediated apoptosis by modulating Mapks/Nrf2 signaling
in doxorubicin-induced cardiotoxicity. Med Sci Monit. 23:5283–5293.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo Y, Zhuang X, Huang Z, Zou J, Yang D,
Hu X, Du Z, Wang L and Liao X: Klotho protects the heart from
hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated
inflammation both in vitro and in vivo. Biochim biophys acta.
1864:238–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li DJ, Fu H, Zhao T, Ni M and Shen FM:
Exercise-stimulated FGF23 promotes exercise performance via
controlling the excess reactive oxygen species production and
enhancing mitochondrial function in skeletal muscle. Metabolism.
65:747–756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Skulachev MV and Skulachev VP: Programmed
aging of mammals: Proof of concept and prospects of biochemical
approaches for anti-aging therapy. Biochemistry (Mosc).
82:1403–1422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsumura Y, Aizawa H, Shiraki-Iida T,
Nagai R, Kuro-o M and Nabeshima Y: Identification of the human
klotho gene and its two transcripts encoding membrane and secreted
klotho protein. Biochem Biophys Res Commun. 242:626–630. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shiraki-Iida T, Aizawa H, Matsumura Y,
Sekine S, Iida A, Anazawa H, Nagai R, Kuro-o M and Nabeshima Y:
Structure of the mouse klotho gene and its two transcripts encoding
membrane and secreted protein. FEBS Lett. 424:6–10. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ohyama Y, Kurabayashi M, Masuda H,
Nakamura T, Aihara Y, Kaname T, Suga T, Arai M, Aizawa H, Matsumura
Y, et al: Molecular cloning of rat klotho cDNA: Markedly decreased
expression of klotho by acute inflammatory stress. Biochem Biophys
Res Commun. 251:920–925. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haykowsky M, Scott J, Esch B, Schopflocher
D, Myers J, Paterson I, Warburton D, Jones L and Clark AM: A
meta-analysis of the effects of exercise training on left
ventricular remodeling following myocardial infarction: Start early
and go longer for greatest exercise benefits on remodeling. Trials.
12:922011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kraljevic J, Marinovic J, Pravdic D, Zubin
P, Dujic Z, Wisloff U and Ljubkovic M: Aerobic interval training
attenuates remodelling and mitochondrial dysfunction in the
post-infarction failing rat heart. Cardiovasc Res. 99:55–64. 2013.
View Article : Google Scholar : PubMed/NCBI
|