Introduction
The pathogenesis of Type 1 diabetes mellitus (T1DM)
is mainly due to the lack of insulin production secreted by the
pancreas (1,2). Bone metabolism disorder is one of the
complications of T1DM, and it leads to a change of microstructure
(3,4), a reduction of bone mass, and an
increase in the probability of fracture (5), finally resulting in metabolic bone
diseases (6).
As early as 1927, Morrison stated that participants
who had metabolic syndrome as children were about 13 times more
likely to have cardiovascular disease and 6.5 times more likely to
have type 2 diabetes than the participants who did not have
metabolic syndrome as children (7).
There is growing evidence showing that diabetes mellitus influences
skeletal metabolism. The optimal management of glycemic control
reduces long-term complications (8).
More and more research has determined that physical activity is
associated with greater longevity and lower frequency and severity
of diabetes complications in individuals with T1MD (9,10);
however, the mechanism is not very clear.
The Wnt/β-catenin pathway is a bone metabolic
pathway and canonical Wnt signaling relies on β-catenin activity
(11). More and more studies have
suggested that Wnt signaling enhances bone formation by regulating
osteoblasts (12,13). Activin type IIB (ActRIIB), one of the
receptors of myostatin (MSTN), is expressed in osteoblasts in the
tibiae of neonatal rats (14). MSTN,
a member of the transforming growth factor-beta (TGF-β) superfamily
of proteins, is a negative regulator of muscle mass (15). Furthermore, it plays a role in
regulating bone mass (16). Our
previous studies showed that ladder-climbing training could prevent
bone loss in diet-induced obese rats by inhibiting MSTN expression
(17,18); meanwhile, MSTN is not expressed and
secreted in osteoblasts. Active MSTN binds to ActRIIB and could
lead to the subsequent phosphorylation of Smad proteins and the
initiation of gene expression, so we wondered if exercise could
modulate bone metabolism in streptozocin (STZ)-induced diabetes
through the inhibition of MSTN.
In the present study, we examined the effects of
weight-bearing treadmill running on bone metabolism in STZ-induced
diabetes rats, and we explored the molecular mechanism of
weight-bearing treadmill running and how it affects bone metabolism
in diabetic rats.
Materials and methods
Animals and experimental design
Healthy male SD rats (200–220 g) were obtained from
the Laboratory Animal Breeding and Research Center of Xi'an
Jiaotong University (Xi'an, China), and they were housed under a
controlled standard temperature (22±2°C), relative humidity
(60±5%), and 12-h light/dark cycle. After 5 days of acclimation,
the rats were randomly divided into the normal control group (NC,
n=10) and the T1D model group. Experimental T1DM was induced by a
peritoneal injection of STZ (60 mg/kg, 0.1 mol/l sodium citrate
buffer, pH=4.5; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). An
equal volume of buffer was injected into the control rats. Then,
the blood glucose levels in vein blood samples were measured on
days 1, 3, 7, and 10. The rats with blood glucose levels that were
greater or equal to 16.7 mmol/l (300 mg/dl) were considered to be
diabetic. Then, the diabetic rats were randomly assigned to the DM
group (n=10) and the DM + WTR group (n=10, trained with
weight-bearing running, on a motor-driven treadmill at a speed of
15 m/min (0 incline) bearing 35% of their body weight mass, 5 min
running and 2-min intervals between each 35-min cycles, 6 days/week
for 6 weeks; Fig. 1). All of the
experiments were conducted with the approval of the Ethics
Committee of Shaanxi Normal University and in accordance with the
Guide for the Care and Use of Laboratory Animals published by the
US National Institutes of Health (NIH Publication no. 85-23,
revised 1996).
Grip strength
At last week, fore-limb grip strength was measured
as the maximum tensile force using a rat grip strength meter
(YLS-13A; Huaibei Zhenghua Bioinstrumentation Co., Ltd., Anhui,
China). Rats were tested 3 times in succession without rest and the
results of the three tests were averaged for each rat.
Weight and sample preparation
After 6 weeks of treatment, the final body weight
was recorded, and then the rats were killed with pentobarbital
sodium at dose of 40 mg/kg wt. Blood was collected and centrifuged
in order to obtain the serum fractions. Serum was stored at −80°C
for further analysis. After killing the animals, the femurs and
quadriceps femoris were harvested and weighed, then immediately
stored in liquid nitrogen and stored at −80°C for RT-PCR and
western blot analysis.
Biochemical analysis
Blood glucose was measured using an eBsensor Blood
Glucose Monitor (Visgeneer Inc., Hsinchu, Taiwan). Serum insulin
levels were measured using a commercial ELISA (EMD Millipore,
Billerica, MA, USA). Serum calcium (Ca2+), phosphorus
(P), alkaline phosphatase (ALP), and tartrate-resistant acid
phosphatase (TRAP) concentrations were measured by using a
commercially available test kit according to the manufacturer's
guidelines (Nanjing Jiancheng Bioengineering Institute, Jiangsu,
China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagents
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions, and then it was
reverse-transcribed using a PrimeScript™ II 1st Strand
cDNA Synthesis kit (Takara Shuzo, Shiga, Japan) (19). Briefly, the RNA with 2 µg was added
to 12 µl reaction system of reverse transcription (including 1 µl
oligo(dT)18) and then placed in PCR amplifier at 70°C
for 5 min. Then the above system was added in order 4 µl 5×buffer,
2 µl 10 mM dNTPs, 1 µl RNA inhibitor and 1 µl reverse transcriptase
and placed in PCR amplifier at 42°C for 60 min, the reaction was
ended at 80°C for 5 min. Gene expression was analyzed by the CFX96
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The reverse transcription products with 2.5 µl
was added to reaction system of PCR. And then, the products were
denatured at 95°C for 10 min followed by amplification for 40
cycles (95°C for 15 sec and 60°C for 60 sec). The solubility curve
was 75 to 95°C, warming 1°C per 20 sec. All of the real-time PCR
reactions were performed in triplicate, glyceraldehyde-3-phosphate
dehydrogenase (GAPDH)/beta-actin (β-actin) was used as an internal
control to normalize the data to determine the relative expression
of the target genes. The relative change in gene expression was
analyzed by the 2−ΔΔCT method. The PCR primers used in
this study are described in Table
I.
 | Table I.Nucleotide sequences of rat primers
used for reverse transcription-quantitative polymerase chain
reaction. |
Table I.
Nucleotide sequences of rat primers
used for reverse transcription-quantitative polymerase chain
reaction.
| Gene | Primer sequences
(5′-3′) | Product size
(bp) | Annealing
temperature (°C) |
|---|
| MSTN |
| F |
TCTCAGACCCGTCAAGACTCCT | 260 | 60 |
| R |
CTCCTGGTCCTGGGAAGGTTAC |
|
|
| ActRIIB |
| F |
GCAGTCGTGGCAGAGTGAGCG | 127 | 60 |
| R |
CTTGAGGTAATCCGTGAGGGAGC |
|
|
| Smad3 |
| F |
CTGGCTACCTGAGTGAAGATGG- | 212 | 60 |
| R |
CTGTGAGGCGTGGAATGTCT |
|
|
| Wnt |
| F |
GCGTTCATCTTCGCAATCAC | 282 | 60 |
| R |
GCACTCTTGGCGCATCTCAG |
|
|
| β-catenin |
| F |
GGACCCCAAGCCTTAGTAAACA | 286 | 60 |
| R |
TTATATCATCGGAACCCAGAAGC |
|
|
| GSK-3β |
| F |
GCGTGAGGAGGGATAAGG- | 322 | 59 |
| R |
CACCAACAAGGGAGCAAAT |
|
|
| GAPDH |
| F |
AGGAGCGAGACCCCACTAACA | 247 | 60 |
| R |
AGGGGGGCTAAGCAGTTGGTC |
|
|
| β-actin |
| F |
GTGACGTTGACATCCGTAAAGA | 287 | 60 |
| R |
GTAACAGTCCGCCTAGAAGCAC |
|
|
Western blotting analysis
To study the protein expression in the femur
tissues, the femurs were dissected from the animals and immediately
stored in liquid nitrogen. Protein concentrations were determined
and equal amounts of the sample were loaded on SDS-polyacrylamide
gel electrophoresis (PAGE). Samples were transferred onto
nitrocellulose membranes after electrophoresis and separation.
Membranes were blocked for 1 h in Tween 20 Tris-base sodium (TBST)
containing 5% milk followed by incubation with the appropriate
primary antibody overnight at 4°C. After washing 3 times in TBST,
the membranes were incubated with goat anti-rabbit horse radish
peroxidase- conjugated secondary antibodies (1:3,000; Wuhan
Servicebio Technology. Co., Ltd. Wuhan, China) for 30 min at room
temperature. Then, the membranes were washed 3 times in TBST at
room temperature. All of the protein expression data were
normalized by β-actin or GAPDH. The blots were visualized with
ECL-plus reagent and the results were quantified by Lab Image
v.2.7.1. The primary antibodies were used as follows: MSTN
(EPR4567(2), ab124721), ActRIIB (EPR10739, ab180185) and Wnt
(ab85060) were purchased from Abcam company. Smad2/3 (5678S),
β-catenin (8480S), phospho-Smad2 (3108L), phospho-Smad3 (9520S),
GSK-3β (27C10, 93158), and phospho-GSK-3β (D2Y9Y, 14630S) were
purchased from Cell Signaling Technology, Inc., (Danvers, MA, USA).
Dilution ratio of all the primary antibodies were 1:1,000.
Statistical analysis
Data are expressed as mean ± SD. Statistical
analyses were performed using SPSS v20.0 (SPSS, Inc., Chicago, IL,
USA). The levels of statistical significance between the two groups
were calculated using an unpaired Student's t test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Weight-bearing running increases the
body weight, muscle mass, bone mass, and grip strength of diabetic
rats
The effects of weight-bearing running on body
weight, muscle mass, bone mass, and grip strength in diabetic rats
are depicted in Table II. Compared
with the NC group, the body weight, muscle mass, bone mass, and
grip strength of the DM group all decreased significantly
(P<0.01). After six weeks of weight-bearing running, the body
mass and bone mass were increased significantly compared with the
DM group (P<0.05). Furthermore, weight-bearing running increased
the muscle mass and grip strength significantly compared with the
DM group of rats (P<0.01).
 | Table II.Effects of weight-bearing running on
blood glucose, serum insulin, body weight, muscle mass, bone mass
and grip strength |
Table II.
Effects of weight-bearing running on
blood glucose, serum insulin, body weight, muscle mass, bone mass
and grip strength
| Group | NC | DM | DM-WTR |
|---|
| Blood glucose
(mmol/l) | 4.88±1.01 |
17.37±3.68a |
8.72±3.42a,b |
| Serum insulin
(mlU/l) | 21.33±4.06 |
7.25±2.42a |
12.08±2.18a,b |
| Body weight
(g) | 371.32±24.23 |
229.77±27.22a |
282.55±49.9a,b |
| Bone mass (g) | 1.29±0.07 |
0.72±0.15a |
0.90±0.12a,b |
| Muscle mass
(g) | 2.83±0.11 |
1.44±0.14a |
2.18±0.63a,c |
| Grip strength
(N) | 1143.30±31.74 |
800.79±141.29a |
1083.84±32.16c |
Weight-bearing running regulates the
blood glucose and serum insulin of diabetic rats
The DM group had higher blood glucose compared with
the NC group, and weight-bearing running showed significant
improvements in this outcome (P<0.05). With respect to serum
insulin levels, the DM group presented impaired serum insulin
compared with nondiabetic rats (P<0.01). Weight-bearing running
improved the insulin levels (P<0.05). These data are shown in
Table II.
Weight-bearing running increases level
of serum Ca2+ and reduces levels of serum ALP and TRAP
of diabetic rats
As shown in Fig. 1,
diabetes resulted in a significant decrease in serum
Ca2+ (P<0.01). In comparison with the DM group, 6
weeks of weight-bearing running significantly increased the
secretions of serum Ca2+ (P<0.05). There was no
significant difference in serum P level between the rats in the NC
group, the DM group, and the DM-WTR group. Serum ALP levels were
significantly increased in diabetic non-treated animals (P<0.01)
as compared to the NC group. Increased ALP levels confirm that
diabetes induces bone damage. Serum ALP levels increased
significantly in the DM-WTR groups (P<0.05) as compared to the
DM group. This result shows that weight-bearing running treatment
enhanced the bone formation and mineralization process in
hyperglycemic conditions. When compared with the NC group, the
serum levels of TRAP were significantly increased in the diabetic
rats (P<0.01). The weight-bearing running treatment
significantly decreased the serum levels of TRAP compared to the DM
group (P<0.01).
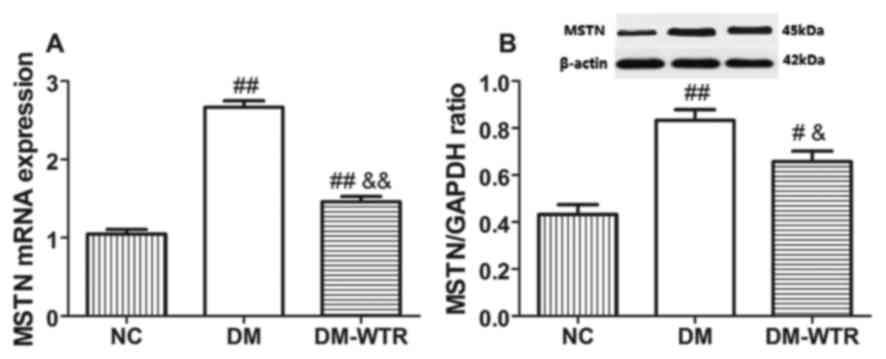
Weight-bearing running inhibits MSTN
expression in diabetic rats
We detected the MSTN expression in the quadriceps.
The mRNA expression (Fig. 2A) and
protein expression (Fig. 2B) of MSTN
(P<0.01) in the DM group was significantly higher than that in
the NC group. However, 6 weeks of weight-bearing running
significantly reduced the mRNA and protein expressions of MSTN as
compared to the DM rats (P<0.05 and P<0.01,
respectively).
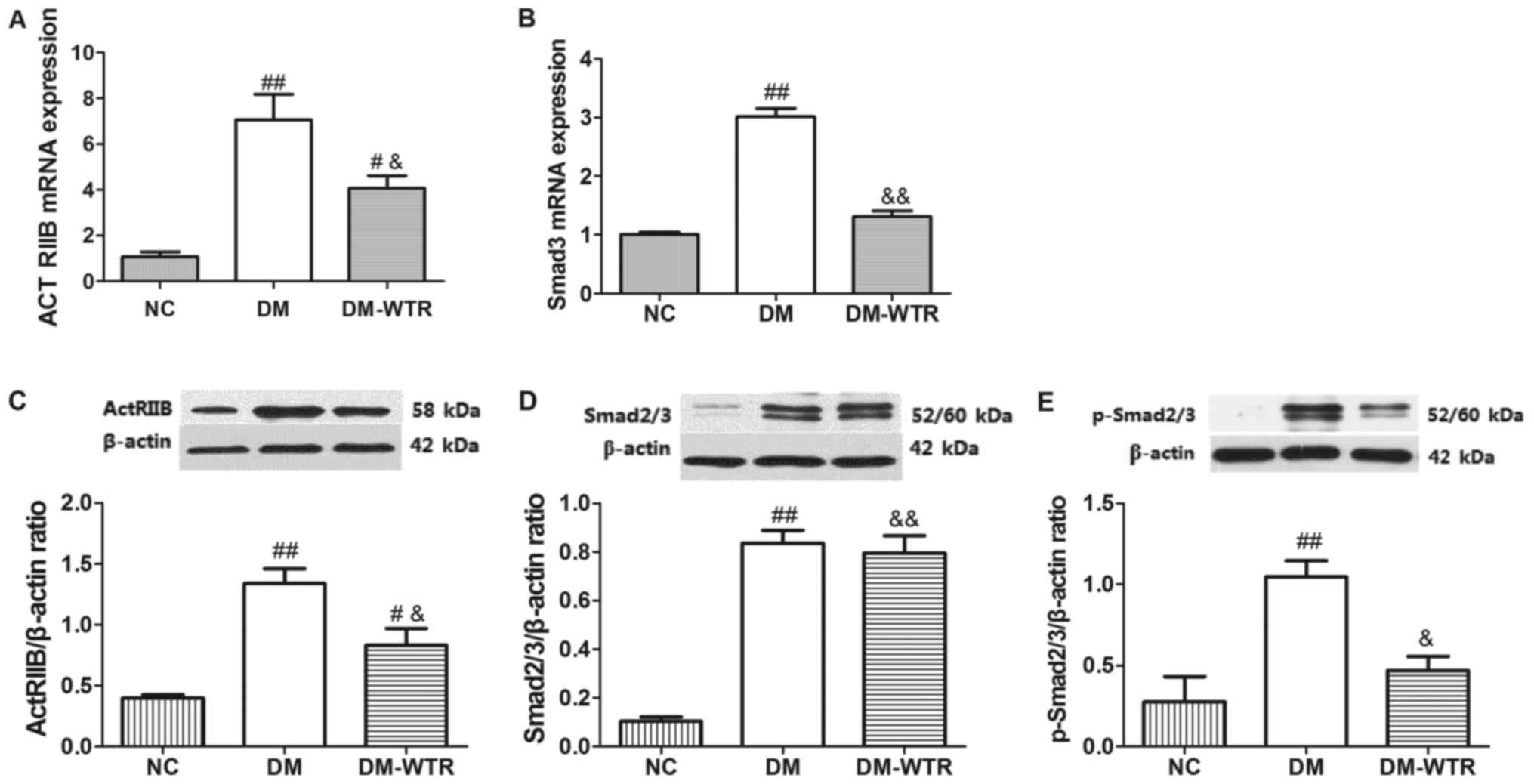
Weight-bearing running regulates the
ActRIIB/Smad2/3 pathway in diabetic rats
The expression of ActRIIB and Smad2/3 and its
phosphorylation were measured in the femurs. As shown in Fig. 3, the mRNA and protein expressions of
ActRIIB (P<0.01 and P<0.01, respectively) in the DM group
were all significantly higher than that in the NC group. After 6
weeks of weight-bearing running treatment, the mRNA and protein
expressions of ActRIIB (P<0.05 and P<0.05, respectively) in
the DM-WTR group were all significantly downregulated compared with
that in the DM group (Fig. 3A and
C). Similar to the above-mentioned results, the mRNA and
protein expressions of Smad2/3 (P<0.01 and P<0.01,
respectively) in the DM group were all significantly higher than
that in the NC group. After 6 weeks of weight-bearing running
treatment, the mRNA and protein expressions of Smad2/3 (P<0.01
and P<0.01, respectively) in the DM-WTR group were all
significantly downregulated compared with that in the DM group
(Fig. 3B and D). Additionally,
weight-bearing running significant inhibited (P<0.05) the
STZ-induced increase of expression of Smad2/3 phosphorylation
(Fig. 3E). These results indicated
that weight-bearing running maybe downregulated in the
ActRIIB/Smad2/3 pathway.
Weight-bearing running modulates the
Wnt/GSK-3β/β-catenin pathway in diabetic rats
The expression of Wnt, GSK-3β and its
phosphorylation, and β-catenin and its phosphorylation were
measured in the femurs. The mRNA and protein expressions of Wnt
(P<0.01 and P<0.01, respectively) in the DM group were all
significantly lower than that in the NC group. After 6 weeks of
weight-bearing running treatment, the mRNA and protein expressions
of Wnt (P<0.01 and P<0.05, respectively) were significantly
increased compared with the DM group (Fig. 4). The mRNA and protein expressions of
GSK-3β (P<0.01 and P<0.01, respectively) in the DM group were
all significantly higher than that in the NC group. After 6 weeks
of weight-bearing running treatment, the mRNA expression of GSK-3β
(P<0.05) was significantly inhibited compared with the DM group,
and there was no significant difference between that in the DM
group and the DM-WTR group (Fig. 4B and
E). Additionally, weight-bearing running significant
downregulated the STZ-induced increase of GSK-3β phosphorylation
expression (P<0.05; Fig. 4F). The
mRNA and protein expressions of β-catenin (P<0.01 and P<0.01,
respectively) in the DM group were all significantly lower than
that in the NC group. After 6 weeks of weight-bearing running
treatment, the mRNA and protein expressions of β-catenin (P<0.01
and P<0.05, respectively) were significantly increased compared
with the DM group (Fig. 4C and G).
These results indicated that weight-bearing running may be
upregulated in the Wnt/GSK-3β/β-catenin pathway.
 | Figure 4.Effects of weight-bearing running on
expressions of Wnt, GSK-3β, p-GSK-3β and β-catnin in femora (A) Wnt
mRNA expression, (B) GSK-3β mRNA expression, (C) β-catnin mRNA
expression, (D) Wnt1 protein expression, (E) GSK-3β protein
expression, (F) GSK-3β phosphorylation protein expression, (G)
β-catnin protein expression. Data are expressed as mean SD (n=8 per
group) #P<0.05, ##P<0.01 vs. NC;
&P<0.05, &&P<0.01 vs.
DM. |
Discussion
T1DM, also called insulin-dependent diabetes
mellitus, is an autoimmune disease. It is associated with
irreversible autoimmune destruction of pancreatic islet β-cells
(13). T1DM is characterized by
insulin deficiency and hyperglycemia, and it is one of the most
common metabolic disorders in children and adolescents. Patients
depend entirely on daily insulin injection replacement therapy to
regulate their blood glucose levels (20). The etiology of T1DM is not completely
understood, but genetic and environmental factors have been
recognized as contributors to the development and progression of
the disease (21).
Individuals with insufficient insulin therapy could
influence the development of muscle function in T1DM patients.
Furthermore, glycemic control is directly related to muscle
metabolism and it could be an important determinant of muscle force
and power in T1DM (22,23). Skeletal disorders are common in
diabetic patients, and many researchers have found that the
patients had reduced bone mineral content (24,25),
deranged calcium and phosphate levels, and altered bone metabolism
(26,27). While it has been proven that aerobic
and resistance training improves bone health (28,29),
there are only a few studies that have reported the effects of
weight-bearing treadmill running on diabetes-induced bone loss. In
accordance with the previous studies (30–32), we
found that weight-bearing treadmill treatment reduced STZ-induced
blood glucose, and increased STZ-induced blood insulin, weight
mass, muscle mass, bone mass, and grip strength. Our results
indicated that weight-bearing treadmill running treatment showed
protective effects on rats with diabetic disease.
It has been reported that calcium concentration has
been associated with diabetes (33).
Calcium influx to β cells can regulate the insulin secretion
process, which is a calcium-dependent process (34,35). It
is possible that persistent alterations of calcium concentration
could affect the insulin secretory response. In our study,
weight-bearing treadmill running increased STZ-induced calcium
excretion in serum, which is consistent with other findings in
diabetic patients (36). Reports are
variable about the high phosphorus level in T1DM (37). Diabetes may lead to kidney damage and
the reduction of the renal excretion of phosphorus, which then
causes a high phosphorus level. Our findings confirm earlier
studies, but they did not show a statistically significant
difference between the three groups; this might be because of the
differences of the model animal. TRAP is a lysosomal hydrolyser,
and it has been shown to be released from osteoclasts during bone
resorption (38); this increase
indicated excessive bone resorption in diabetic rats. In our study,
weight-bearing treadmill running reduced STZ-induced TRAP levels in
serum; these results were consistent with previous findings
(39,40). However, normal TRAP activity was also
observed in the experimental diabetic animals (41). Even in another study, TRAP activity
was found to be low in patients with T1DM (2). The reason lies in the differences of
the study object and dosages of STZ.
Muscle and bone have a close relationship in not
only anatomy but also in function. However, the mechanisms of their
synergistic action are not completely known. The role of MSTN in
muscle growth regulation and the disruption of its gene have been
demonstrated to cause muscle hypertrophy and increase bone mass.
Our previous work showed that blocking MSTN with a polyclonal
anti-MSTN antibody preparation improves the trabecular bone
microstructure (42), suggesting
that therapeutic modulation of MSTN in vivo may be an
effective strategy for preserving muscle mass and bone metabolism
with diabetes. In the present study, weight-bearing running
significantly inhibited STZ-induced MSTN expression, indicating
that the inhibition of MSTN may alleviate STZ-induced diabetic
muscle atrophy. However, the mechanism remains to be fully
elucidated.
MSTN binds ActRIIB to activate signaling, ultimately
resulting in Smad2/3 phosphorylation and translocation to the
nucleus to modulate the transcription of numerous genes (43). ActRIIB and Smad3 are the downstream
signaling molecules of MSTN, and they have important roles in the
regulation of bone metabolism (44,45). Guo
et al (46), found that MSTN
inhibited adipogenesis in human bone marrow-derived mesenchymal
stem cells and mediated preadipocytes by activating Smad3, and
cross-communication of the TGFβ/Smad signaling to the
Wnt/β-catenin/TCF4 pathway partly, leading to the downregulation of
PPARγ. Our present study demonstrated that weight-bearing running
reduced the STZ-induced expressions of ActRIIB and Smad2/3 in the
femur. Moreover, weight-bearing running downregulated the
expression of p-Smad2/3, indicating that weight-bearing running
inactivated Smad2/3 by possibly inhibiting ActRIIB expression,
which was associated with MSTN downregulation.
The Wnt/GSK3β/β-catenin signaling pathway controls a
variety of life processes, including organism growth, development,
diseases, aging, and death, as well as cell differentiation and the
maintenance of form and function, immune, stress, cell
carcinogenesis, and cell apoptosis (47). Study has shown that MSTN mediates
cross-communication between Smad3 and Wnt/β-catenin signaling
pathways (46). Similar to our
study, MSTN not only regulates Smad3 but also mediates
Wnt/GSK3β/β-catenin signaling pathway. However, the above-mentioned
study also demonstrated that β-Catenin interacts with Smad3 and
acts downstream of Smad3 to mediate the inhibitory effect of MSTN
on adipogenesis (46). But, MSTN can
inhibit directly expressions of Wnt and β-catenin to modulate femur
atrophy under our control conditions. It is speculated that MSTN
regulates Smad3 and Wnt/β-catenin signaling pathway, which have
organizational differences. Of course, the details also need
further confirmation. MSTN may act upstream of the Wnt pathway and
inhibit the expression of Wnt (48).
The downstream signaling molecules of MSTN, such as ActRIIB and
Smad3, are directly involved in the enhancement of β-catenin
levels. Researchers have demonstrated that the Wnt/GSK3β/β-catenin
signaling pathway is involved in bone formation and contributes to
osteoblastic differentiation (49).
Our results demonstrated that weight-bearing running enhanced the
STZ-induced Wnt and β-catenin expressions and reduced STZ-induced
GSK-3β expression in diabetic rats' femora. Combined with the
above-mentioned literature, we speculated that weight-bearing
running may have activated the Wnt/GSK3β/β-catenin signaling
pathway possibly by the MSTN downregulation in the femur of
diabetic rats.
In conclusion, the present study indicated that
weight-bearing running could partially ameliorate STZ-induced femur
atrophy by MSTN downregulation. and this may be associated with the
inactivation of the ActRIIB/Smad2/3 signaling pathways and the
activation of the Wnt/GSK3β/β-catenin signaling pathway. Further
studies are needed to confirm this.
Acknowledgements
The authors would like to thank the graduate
students of the Institute of Sports Biology, Shaanxi Normal
University (Shaanxi, China) for their cooperation, and College of
Life Sciences, Shaanxi Normal University (Shaanxi, China), and
Department of Physical Education, Xi'an University of Post and
Telecommunications (Shaanxi, China).
Funding
The authors received no financial support for the
research, authorship, and/or publication of the present study.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LT and SA conceived the present study. JY and LS
performed the western blot analysis. BY and YK performed exercise
training to all experimental animals, measured their body weight
and grip strength, and collected blood and tissue samples. XF and
LS performed the ELISA and RT-qPCR experiments, and collected and
analyzed all data. JY prepared the manuscript, LS revised it
critically for important intellectual content, and all authors were
involved in the writing and editing of the manuscript.
Ethics approval and consent to
participate
All of the experiments were conducted with the
approval of the Ethics Committee of Shaanxi Normal University and
in accordance with the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (NIH
Publication no. 85-23, revised 1996).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kemink SA, Hermus AR, Swinkels LM,
Lutterman JA and Smals AG: Osteopenia in insulin-dependent diabetes
mellitus; prevalence and aspects of pathophysiology. J Endocrinol
Invest. 23:295–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Campos Pastor MM, López-Ibarra PJ,
Escobar-Jiménez F, Serrano Pardo MD and García-Cervigón AG:
Intensive insulin therapy and bone mineral density in type 1
diabetes mellitus: A prospective study. Osteoporos Int. 11:455–459.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Erdal N, Gürgül S, Demirel C and Yildiz A:
The effect of insulin therapy on biomechanical deterioration of
bone in streptozotocin (STZ)-induced type 1 diabetes mellitus in
rats. Diabetes Res Clin Pract. 97:461–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
López-Ibarra PJ, Pastor MM,
Escobar-Jiménez F, Pardo MD, González AG, Luna JD, Requena ME and
Diosdado MA: Bone mineral density at time of clinical diagnosis of
adult-onset type 1 diabetes mellitus. Endocr Pract. 7:346–351.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roggen I, Gies I, Vanbesien J, Louis O and
De Schepper J: Trabecular bone mineral density and bone geometry of
the distal radius at completion of pubertal growth in childhood
type 1 diabetes. Horm Res Paediatr. 79:68–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saha MT, Sievänen H, Salo MK, Tulokas S
and Saha HH: Bone mass and structure in adolescents with type 1
diabetes compared to healthy peers. Osteoporos Int. 20:1401–1406.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Albright F: Bone development in diabetic
children: A roentgen study. Am J Med Sci. 174:313–319. 1948.
|
|
8
|
Diabetes Control and Complications Trial
Research Group, ; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford
O, Davis M, Rand L and Siebert C: The effect of intensive treatment
of diabetes on the development and progression of long-term
complications in insulin-dependent diabetes mellitus. N Engl J Med.
329:977–986. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moy CS, Songer TJ, LaPorte RE, Dorman JS,
Kriska AM, Orchard TJ, Becker DJ and Drash AL: Insulin-dependent
diabetes mellitus, physical activity, and death. Am J Epidemiol.
137:74–81. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kriska AM, LaPorte RE, Patrick SL, Kuller
LH and Orchard TJ: The association of physical activity and
diabetic complications in individuals with insulin-dependent
diabetes mellitus: The Epidemiology of Diabetes Complications
Study-VII. J Clin Epidemiol. 44:1207–1214. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yee CS, Xie L, Hatsell S, Hum N, Murugesh
D, Economides AN, Loots GG and Collette NM: Sclerostin antibody
treatment improves fracture outcomes in a Type I diabetic mouse
model. Bone. 82:122–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rodda SJ and McMahon AP: Distinct roles
for Hedgehog and canonical Wnt signaling in specification,
differentiation and maintenance of osteoblast progenitors.
Development. 133:3231–3244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Espe K, Galler A, Raila J, Kiess W and
Schweigert FJ: High-normal C-reactive protein levels do not affect
the vitamin A transport complex in serum of children and
adolescents with type 1 diabetes. Pediatr Res. 62:741–745. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Funaba M, Ogawa K and Abe M: Expression
and localization of activin receptors during endochondral bone
development. Eur J Endocrinol. 144:63–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amthor H, Macharia R, Navarrete R,
Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbóva G,
Partridge T, et al: Lack of myostatin results in excessive muscle
growth but impaired force generation. Proc Natl Acad Sci USA.
104:1835–1840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bialek P, Parkington J, Li X, Gavin D,
Wallace C, Zhang J, Root A, Yan G, Warner L, Seeherman HJ and
Yaworsky PJ: A myostatin and activin decoy receptor enhances bone
formation in mice. Bone. 60:162–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang L, Gao X, Yang X, Liu C, Wang X, Han
Y, Zhao X, Chi A and Sun L: Ladder-climbing training prevents bone
loss and microarchitecture deterioration in diet-induced obese
rats. Calcif Tissue Int. 98:85–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang L, Gao X, Yang X, Zhang D, Zhang X,
Du H, Han Y and Sun L: Combination of weight-bearing training and
anti-MSTN polyclonal antibody improve bone quality in rats. Int J
Sport Nutr Exerc Metab. 26:516–524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan CX, Xu M, Huang SH, Wu QQ, Yuan Y,
Deng W and Tang QZ: Baicalein protects against endothelial cell
injury by inhibiting the TLR4/NF-κB signaling pathway. Mol Med Rep.
17:3085–3091. 2018.PubMed/NCBI
|
|
20
|
Balamurugan AN, Bottino R, Giannoukakis N
and Smetanka C: Prospective and challenges of islet transplantation
for the therapy of autoimmune diabetes. Pancreas. 32:231–243. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hirschhorn JN: Genetic epidemiology of
type 1 diabetes. Pediatr Diabetes. 4:87–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aftab Guy D, Sandoval D, Richardson MA,
Tate D and Davis SN: Effects of glycemic control on target organ
responses to epinephrine in type 1 diabetes. Am J Physiol
Endocrinol Metab. 289:E258–E265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Corigliano G, Iazzetta N, Corigliano M and
Strollo F: Blood glucose changes in diabetic children and
adolescents engaged in most common sports activities. Acta Biomed.
77 Suppl 1:S26–S33. 2006.
|
|
24
|
Levin ME, Boisseau VC and Avioli LV:
Effects of diabetes mellitus on bone mass in juvenile and
adult-onset diabetes. N Engl J Med. 294:241–245. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Santiago JV, McAlister WH, Ratzan SK,
Bussman Y, Haymond MW, Shackelford G and Weldon VV: Decreased
cortical thickness & osteopenia in children with diabetes
mellitus. J Clin Endocrinol Metab. 45:845–848. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heath H III, Lambert PW, Service FJ and
Arnaud SB: Calcium homeostasis in diabetes mellitus. J Clin
Endocrinol Metab. 49:462–466. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McNair P, Madsbad S, Christiansen C, Faber
OK, Transbøl I and Binder C: Osteopenia in insulin treated diabetes
mellitus. Its relation to age at onset, sex and duration of
disease. Diabetologia. 15:87–90. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Almstedt HC, Grote S, Korte JR, Perez
Beaudion S, Shoepe TC, Strand S and Tarleton HP: Combined aerobic
and resistance training improves bone health of female cancer
survivors. Bone Rep. 5:274–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gomes TS, Aoike DT, Baria F, Graciolli FG,
Moyses RMA and Cuppari L: Effect of aerobic exercise on markers of
bone metabolism of overweight and obese patients with chronic
kidney disease. J Ren Nutr. 27:364–371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Junod A, Lambert AE, Stauffacher W and
Renold AE: Diabetogenic action of streptozotocin: Relationship of
dose to metabolic response. J Clin Invest. 48:2129–2139. 1969.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li RJ, Qiu SD, Tian H and Zhou SW:
Diabetes induced by multiple low doses of STZ can be spontaneously
recovered in adult mice. Dongwuxue Yanjiu. 34:238–243. 2013.(In
Chinese). PubMed/NCBI
|
|
32
|
Tsai CC, Chan P, Chen LJ, Chang CK, Liu Z
and Lin JW: Merit of ginseng in the treatment of heart failure in
type 1-like diabetic rats. Biomed Res Int. 2014:4841612014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jorde R, Schirmer H, Njolstad I, Løchen
ML, Bøgeberg Mathiesen E, Kamycheva E, Figenschau Y and Grimnes G:
Serum calcium and the calcium-sensing receptor polymorphism
rs17251221 in relation to coronary heart disease, type 2 diabetes,
cancer and mortality: The Tromsø Study. Eur J Epidemiol.
28:569–578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Henquin JC: Triggering and amplifying
pathways of regulation of insulin secretion by glucose. Diabetes.
49:1751–1760. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pittas AG, Lau J, Hu FB and Dawson-Hughes
B: The role of vitamin D and calcium in type 2 diabetes. A
systematic review and meta-analysis. J Clin Endocrinol Metab.
92:2017–2029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mosso C, Hodgson MI, Ortiz T and Reyes ML:
Bone mineral density in young Chilean patients with type 1 diabetes
mellitus. J Pediatr Endocrinol Metab. 29:731–736. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hamed EA, Faddan NH, Elhafeez HA and Sayed
D: Parathormone-25(OH)-vitamin D axis and bone status in children
and adolescents with type 1 diabetes mellitus. Pediatr Diabetes.
12:536–546. 2011.PubMed/NCBI
|
|
38
|
Halleen JM, Tiitinen SL, Ylipahkala H,
Fagerlund KM and Väänänen HK: Tartrate-resistant acid phosphatase
5b (TRACP 5b) as a marker of bone resorption. Clin Lab. 52:499–509.
2006.PubMed/NCBI
|
|
39
|
Rao Sirasanagandla S, Ranganath Pai
Karkala S, Potu BK and Bhat KM: Beneficial effect of cissus
quadrangularis Linn. on osteopenia associated with
streptozotocin-induced type 1 diabetes mellitus in male wistar
rats. Adv Pharmacol Sci. 2014:4830512014.PubMed/NCBI
|
|
40
|
Gopalakrishnan V, Arunakaran J, Aruldhas
MM and Srinivasan N: Effects of streptozotocin-induced diabetes
mellitus on some bone turnover markers in the vertebrae of
ovary-intact and ovariectomized adult rats. Biochem Cell Biol.
84:728–736. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Waud CE, Marks SC Jr, Lew R and Baran DT:
Bone mineral density in the femur and lumbar vertebrae decreases
after twelve weeks of diabetes in spontaneously diabetic-prone
BB/worcester rats. Calcif Tissue Int. 54:237–240. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang L, Yang X, Gao X, Du H, Han Y, Zhang
D, Wang Z and Sun L: Inhibiting myostatin signaling prevents
femoral trabecular bone loss and microarchitecture deterioration in
diet-induced obese rats. Exp Biol Med (Maywood). 241:308–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arounleut P, Bialek P, Liang LF, Upadhyay
S, Fulzele S, Johnson M, Elsalanty M, Isales CM and Hamrick MW: A
myostatin inhibitor (propeptide-Fc) increases muscle mass and
muscle fiber size in aged mice but does not increase bone density
or bone strength. Exp Gerontol. 48:898–904. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Inoue Y, Canaff L, Hendy GN, Hisa I,
Sugimoto T, Chihara K and Kaji H: Role of Smad3, acting
independently of transforming growth factor-beta, in the early
induction of Wnt-beta-catenin signaling by parathyroid hormone in
mouse osteoblastic cells. J Cell Biochem. 108:285–294. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lerner UH and Ohlsson C: The WNT system:
Background and its role in bone. J Intern Med. 277:630–649. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guo W, Flanagan J, Jasuja R, Kirkland J,
Jiang L and Bhasin S: The effects of myostatin on adipogenic
differentiation of human bone marrow-derived mesenchymal stem cells
are mediated through cross-communication between Smad3 and
Wnt/beta-catenin signaling pathways. J Biol Chem. 283:9136–9145.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu JD, Deng Q, Tian HH, Pang YT and Deng
GL: Wnt/glycogen synthase kinase 3β/β-catenin signaling activation
mediated sevoflurane preconditioning-induced cardioprotection. Chin
Med J (Engl). 128:2346–2353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Steelman CA, Recknor JC, Nettleton D and
Reecy JM: Transcriptional profiling of myostatin-knockout mice
implicates Wnt signaling in postnatal skeletal muscle growth and
hypertrophy. FASEB J. 20:580–582. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Day TF, Guo X, Garrett-Beal L and Yang Y:
Wnt/beta-catenin signaling in mesenchymal progenitors controls
osteoblast and chondrocyte differentiation during vertebrate
skeletogenesis. Dev Cell. 8:739–750. 2005. View Article : Google Scholar : PubMed/NCBI
|