Introduction
As a type of ligand-activated transcriptional factor
of nuclear receptor that is correlated with metabolic regulation,
peroxisome proliferator-activated receptor gamma (PPARγ) plays a
vital role in controlling the storage and release of fats, glucose
and lipid metabolism and energy metabolism, regulating insulin
resistance, blood glucose, cell growth and differentiation and
vascular inflammation reaction as well as influencing the
pathological processes of vascular sclerosis (1–3). Based
on the PPARγ expression in the brain neuron cells, PPARγ molecules
may be released to the blood circulation when the brain tissue
cells are injured (4, 5). However, whether the serum PPARγ level is
associated with the severity of arteriosclotic cerebral infarction
(ACI) and whether such an association is further closely related to
the nucleotide polymorphism of PPARγ gene still need to be
determined by more experiments.
As a result, this research aimed to provide a basis
and direction for assessing the risk of cerebrovascular disease
among the ACI patients by detecting their serum PPARγ level and
PPARγ gene polymorphism in clinical practices.
Materials and methods
Information
General information
In this study, 246 ACI patients presenting at the
Department of Neurology of Zengcheng District People's Hospital of
Guangzhou (Guangzhou, China) from April 2009 to July 2015 were
selected as the case group, and 382 subjects who were excluded of
cerebral infarction through health examination were enrolled as the
control group. The 246 patients in the case group were comprised of
182 males and 64 females, with an average age of (59.7±12.1) years.
Of the 382 participants in the control group, there were 226 males
and 156 females, with an average age of (56.1±10.8) years.
The study was approved by the Ethics Committee of
Zengcheng District People's Hospital of Guangzhou and written
informed consents were signed by the patients and/or guardians.
Experimental methods
General indexes
The general conditions of the participants were
recorded, including age, sex, body mass index and history of
hypertension, diabetes and smoking.
Blood routine tests
Fasting venous blood (5 ml) of all the participants
was withdrawn in the morning to measure the total cholesterol (TC),
triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and
fasting blood glucose. The levels and differences of indexes in the
case group and the control group were recorded and compared.
Hepatic function measurement
The body mass indexes as well as the levels of
indexes related to hepatic function, namely, alanine
aminotransferase (ALT) and aspartate aminotransferase (AST), in the
case group and the control group were measured and recorded, and
the differences in the data were compared.
Renal function measurement
The data of indexes related to the renal function
[blood urea nitrogen (BUN), serum creatinine (Scr) and uric acid
(UA)] in the case group and the control group were measured, and
the differences between the two groups were recorded.
Homocysteine (Hcy) level
measurement
Hcy is a vital metabolic intermediate of
sulfur-containing amino acid in the human body, which may be an
independent risk factor for the onset of atherosclerosis and other
cardiovascular diseases. The Hcy levels in both the case and
control groups were measured, and the differences were
compared.
PPARγ level measurement
Human PPARγ enzyme-linked immunosorbent assay
(ELISA) kit was applied to detect the serum PPARγ levels in ACI
patients three times, i.e., at acute stage (within 3–6 h), 48–72 h
after ACI attack and one week after attack.
Detection of PPARγ gene
The blood samples of the ACI patients were collected
to extract the genomic DNA (gDNA) of peripheral blood leucocyte and
detect the PPARγ gene. The peripheral venous blood of the
research subjects was withdrawn to extract the DNA. Later,
polymerase chain reaction and gel electrophoresis were performed to
detect the PPARγ gene. The upstream primer for the
PPARγ gene was 5′-TGAATGTGAAGCCCATTGAA-3′, and the
downstream primer was 5′-GAGCGGGTGAAGAAGACTCATGT-3′.
Statistical analysis
The experimental data were presented as mean ±
standard deviation (mean ± SD), and the experimental results were
analyzed using Statistical Product and Service Solutions (SPSS)
17.0 software. The analysis of variance or t-test was conducted for
data analysis and the post hoc test was LSD test. P<0.05
suggested that the difference between the two groups was
statistically significant.
Results
Measurement results of general
indexes
The general conditions of the participants were
recorded; it was known from Table I
that in the case group, the proportion of hypertension patients was
41.82%, the proportion of diabetes patients was 26.83%, and that
the proportion of smoking people was 30.08%; the proportions of
hypertension patients, diabetes patients and smoking people were
21.47, 14.14 and 14.92%, respectively, in the control group. It
could be seen that cerebral infarction had a certain correlation
with hypertension, diabetes and smoking.
 | Table I.Comparison of general information
between the case group and the control group. |
Table I.
Comparison of general information
between the case group and the control group.
| Group | Hypertension
patient/case (%) | Diabetes patient/case
(%) | Smoking people/case
(%) |
|---|
| Case group | 103 (41.82%) | 66 (26.83%) | 74 (30.08%) |
| Control group | 82 (21.47%) | 54 (14.14%) | 57 (14.92%) |
Results of blood routine tests
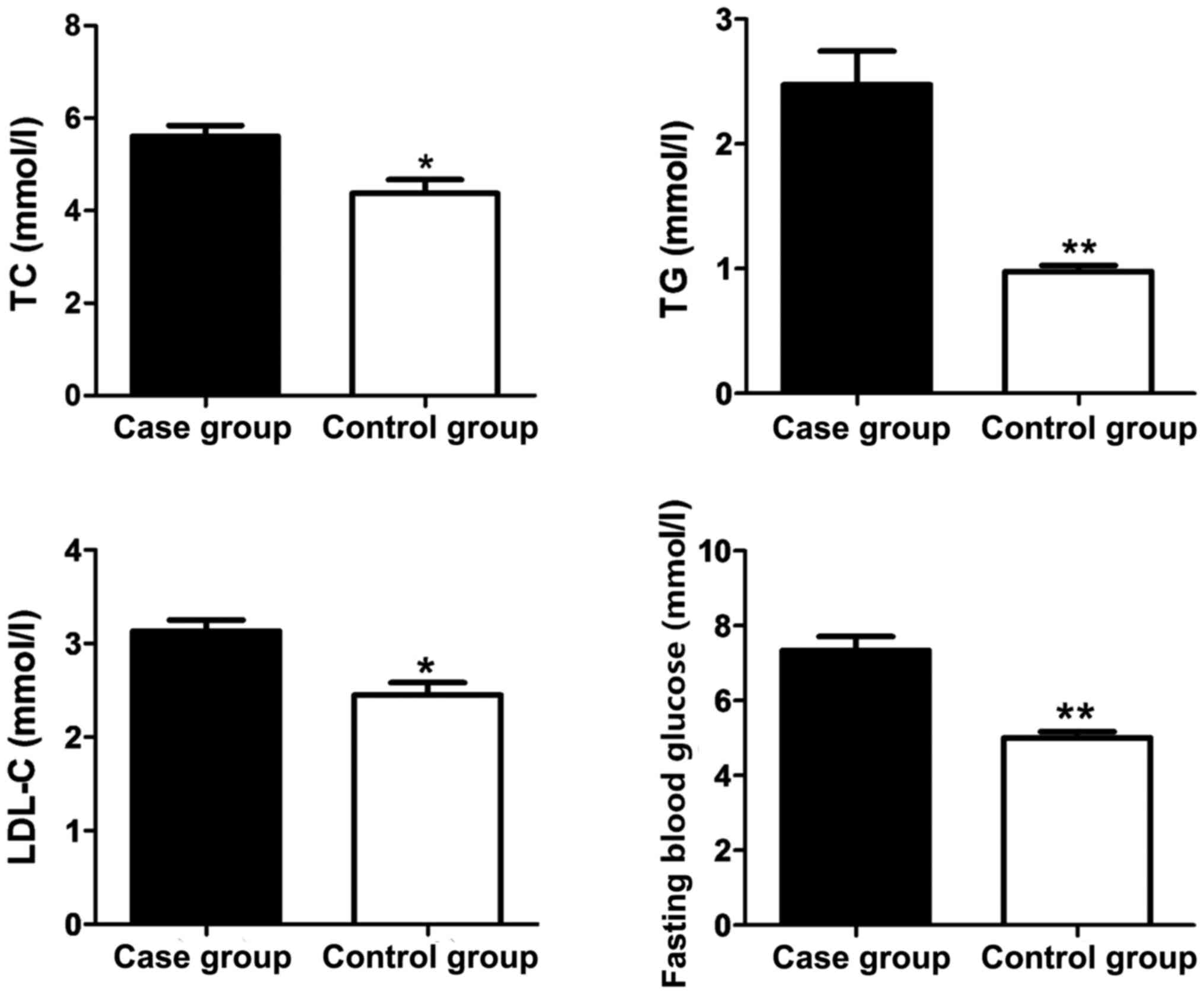
As shown in Fig. 1,
the fasting venous blood of all the participants was withdrawn in
the morning to measure the TC, TG, LDL-C and fasting blood glucose.
The levels of TC, TG, LDL-C and fasting blood glucose in the case
group were obviously higher than those in the control group.
Results of hepatic function
measurement
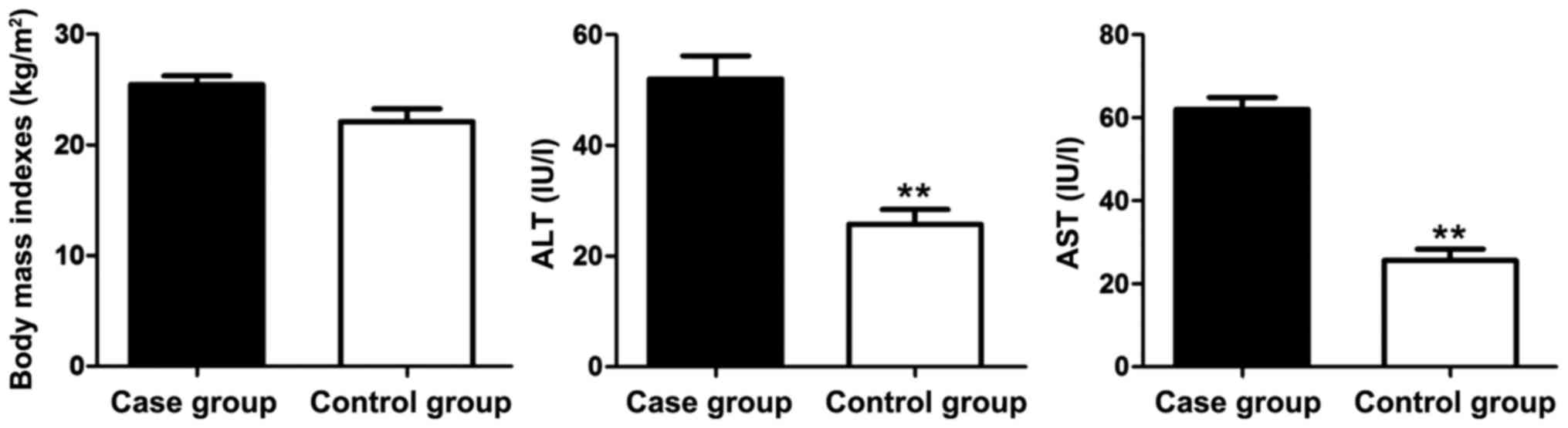
The measurement results of body mass indexes as well
as the levels of indexes related to the hepatic function (ALT and
AST) in the case group and the control group are shown in Fig. 2. The body mass index in the case
group was slightly higher than that in the control group but
without significant difference. The levels of hepatic function
indexes ALT and AST in the case group were significantly elevated
compared with those in the control group. Thus, liver injury of the
ACI patients was severe.
Results of renal function
measurement
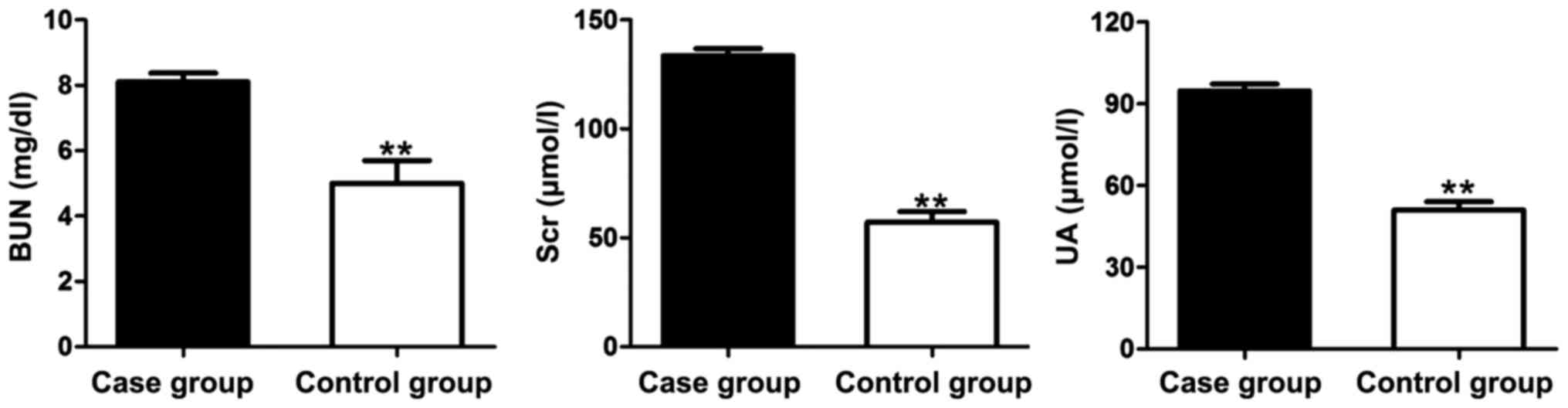
The levels of indexes related to the renal function,
i.e., BUN, Scr and UA, in the case group and the control group were
measured and recorded (Fig. 3). It
was indicated that the levels of BUN, Scr and UA in the case group
were significantly higher than those in the control group.
Therefore, the kidney injury of the ACI patients was severe.
Results of Hcy level measurement
Compared with that in the control group, the Hcy
level in the case group was increased significantly (Fig. 4). Patients with a high Hcy level were
vulnerable to thrombosis and cardiovascular diseases; thus, there
was a close relationship between the ACI patients and
cardiovascular diseases.
Results of PPARγ level
measurement
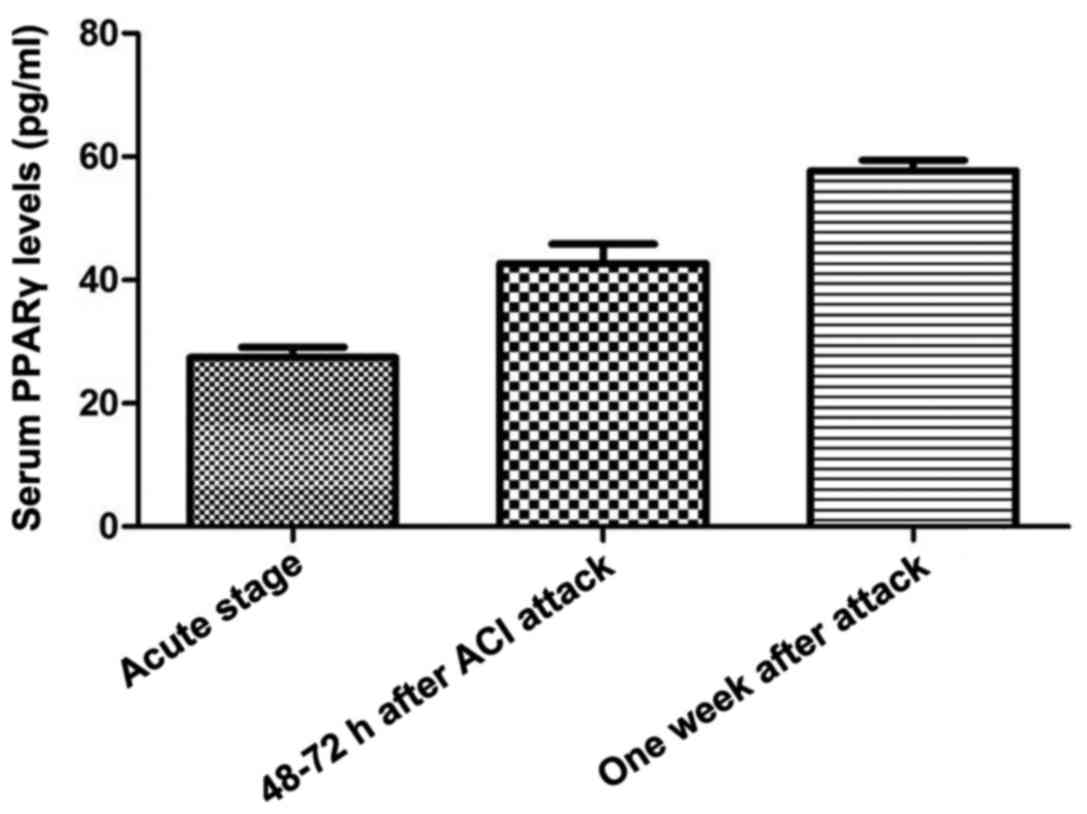
Human PPARγ ELISA kit was used to detect the serum
PPARγ levels of the ACI patients at acute stage (within 3–6 h),
48–72 h after ACI attack and one week after attack. As shown in
Fig. 5, the serum PPARγ levels were
increased progressively at the acute stage (3–6 h), 48–72 h after
ACI attack and one week after attack.
Results of PPARγ gene detection
The results of genotype distribution allele
frequencies in the case group and the control group are shown in
Table II. In the case group, the
distribution frequencies of PPARγ genotypes CC, CT and TT were
74.6, 22.3 and 2.6%, respectively, the C allele frequency was
87.3%, and the Tallele frequency was 14.1%. In the control group,
the distribution frequencies of genotypes CC, CT and TT were 61.1,
37.6 and 3.1%, respectively, of which the C allele frequency was
78.6%, and the T allele frequency was 20.6%.
 | Table II.PPARγ genotypes and allele
distribution in the case group and the control group. |
Table II.
PPARγ genotypes and allele
distribution in the case group and the control group.
|
| Genotype frequency
(%) | Diabetes patient
(%) |
|---|
|
|
|
|
|---|
| Group | CC | CT | TT | C | T |
|---|
| Case group | 74.6% | 22.3% | 2.6% | 87.3% | 14.1% |
| Control group | 61.1% | 37.6% | 3.1% | 78.6% | 20.6% |
Discussion
ACI is also known as atherothrombotic brain
infarction (6). It may occur in all
ages, particularly in the elderly; many lifestyle habits and
diseases, such as high-fat diet, diabetes and smoking, can induce
and accelerate ACI (7–9). With the development of the society,
improvement of living standards and huge changes in lifestyle in
recent years, ACI has become a disease that poses a serious threat
to both life and health, and its incidence rate is on the increase
annually (10–13). However, the pathogenesis of ACI also
remains unclear due to currently imperfect diagnosis and treatment
of ACI. Multiple factors, including inflammation, oxidative stress
and apoptosis, influence the occurrence and progression of the
disease. Therefore, it is imminent to identify safe and effective
ACI treatment methods (14,15).
There are three types of PPAR family, namely, PPARα,
PPARβ and PPARγ, which have different histologic manifestations and
reflect varying physiological functions (16). PPAR is praised as the ‘the ultimate
mystery of human health’, whose amazing effects on promoting human
health, preventing diseases and treating various difficult and
miscellaneous diseases are discovered by more and more scientific
research and clinical trials (17).
The PPAR molecules in the human body are a group of nucleoprotein
receptors with transcription-regulating function, which consist of
the subtypes PPARα, PPARβ and PPARγ; they are widely present in the
nuclei of liver, fat, vessel wall and skeletal muscle (18). The activated PPARs can affect the
manifestations of different genes in the body, act on the
mechanisms of adjusting metabolism of glucose and lipid,
ameliorating resistance, guarding against inflammation and
preventing cell malignant transformation, and operate for a
‘stable’ state in the human body (19). PPARγ is a superfamily of nuclear
receptors, which is involved in many regulation processes for cell
functions and pathophysiology at the transcription level, including
adipocyte differentiation, glucose and lipid metabolism,
inflammatory reaction, atherosclerosis as well as cancer cell
differentiation and formation; in addition to the treatment of
diabetes, the PPARγ agonist may be applied to treat
atherosclerosis, tumors and inflammatory diseases in the future
(20).
In this study, 246 ACI patients presenting at the
Department of Neurology of our hospital between April 2009 and July
2015 were selected as the case group, and 382 control subjects, who
were excluded of cerebral infarction through health examination,
were enrolled as the control group. The general information and
blood routine of the two groups were recorded. The hepatic and
renal functions and Hcy expression level were measured, and ELISA
was utilized to detect the serum PPARγ levels of the ACI patients
at acute stage, 48–72 h after ACI attack and one week after attack.
Polymerase chain reaction-restriction fragment length polymorphism
method was applied to measure the PPARγ gene polymorphism, and the
results indicated that the proportion of hypertension patients,
diabetes patients and smoking people in the case group were
significantly higher than those in the control group. The results
of blood routine tests showed that the levels of TC, TG, LDL-C and
fasting blood glucose in the case group were evidently elevated
compared with those in the control group. The levels of the hepatic
function-related indexes ALT and AST as well as renal
function-related indexes BUN, Scr and UA in the case group were
significantly higher than those in the control group. Moreover,
compared with that in the control group, the Hcy level in the case
group was increased notably. It was revealed in the ELISA results
that the serum PPARγ levels were increased progressively at acute
stage, 48–72 h after ACI attack and one week after attack. The
distribution frequencies of PPARγ genotypes CC, CT and TT in the
case group were higher than those in the control group. Compared
with that in the control group, the proportion of C allele in the
case group was significangtly increased, while that of T allele was
lowered significantly. In conclusion, the serum PPARγ level has a
close association with the PPARγ gene polymorphism in ACI
patients, and PPARγ is also markedly related to the severity of
brain injury. Thus, PPARγ can play a crucial role in the diagnosis
and treatment of ACI in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and LZ contributed to the detection of PPARγ
gene. XC and XL collected and analyzed the general information of
the patients. FS helped with routine blood test. SL and QS were
responsible for Hcy level measurement. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zengcheng District People's Hospital of Guangzhou (Guangzhou,
China) and written informed consents were signed by the patients
and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Not applicable.
References
|
1
|
Semple RK, Chatterjee VK and O'Rahilly S:
PPAR gamma and human metabolic disease. J Clin Invest. 116:581–589.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forman BM, Tontonoz P, Chen J, Brun RP,
Spiegelman BM and Evans RM: 15-Deoxy-delta 12, 14-prostaglandin J2
is a ligand for the adipocyte determination factor PPAR γ. Cell.
83:803–812. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kliewer SA, Lenhard JM, Willson TM, Patel
I, Morris DC and Lehmann JM: A prostaglandin J2 metabolite binds
peroxisome proliferator-activated receptor γ and promotes adipocyte
differentiation. Cell. 83:813–819. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yue Tl TL, Chen J, Bao W, Narayanan PK,
Bril A, Jiang W, Lysko PG, Gu JL, Boyce R, Zimmerman DM, et al: In
vivo myocardial protection from ischemia/reperfusion injury by the
peroxisome proliferator-activated receptor-γ agonist rosiglitazone.
Circulation. 104:2588–2594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimazu T, Inoue I, Araki N, Asano Y,
Sawada M, Furuya D, Nagoya H and Greenberg JH: A peroxisome
proliferator-activated receptor-γ agonist reduces infarct size in
transient but not in permanent ischemia. Stroke. 36:353–359. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sundararajan S, Gamboa JL, Victor NA,
Wanderi EW, Lust WD and Landreth GE: Peroxisome
proliferator-activated receptor-gamma ligands reduce inflammation
and infarction size in transient focal ischemia. Neuroscience.
130:685–696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin TN, Cheung WM, Wu JS, Chen JJ, Lin H,
Chen JJ, Liou JY, Shyue SK and Wu KK: 15d-prostaglandin J2 protects
brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc
Biol. 26:481–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen ST, Hsu CY, Hogan EL, Maricq H and
Balentine JD: A model of focal ischemic stroke in the rat:
Reproducible extensive cortical infarction. Stroke. 17:738–743.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin TN, He YY, Wu G, Khan M and Hsu CY:
Effect of brain edema on infarct volume in a focal cerebral
ischemia model in rats. Stroke. 24:117–121. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsai YS, Kim HJ, Takahashi N, Kim HS,
Hagaman JR, Kim JK and Maeda N: Hypertension and abnormal fat
distribution but not insulin resistance in mice with P465L
PPARgamma. J Clin Invest. 114:240–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu X, Rea HC, Wiktorowicz JE and
Perez-Polo JR: Proteomic analysis of hypoxia/ischemia-induced
alteration of cortical development and dopamine neurotransmission
in neonatal rat. J Proteome Res. 5:2396–2404. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu K, Mori S, Takahashi HK, Tomono Y,
Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, et al:
Anti-high mobility group box 1 monoclonal antibody ameliorates
brain infarction induced by transient ischemia in rats. FASEB J.
21:3904–3916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liou JY, Ghelani D, Yeh S and Wu KK:
Nonsteroidal anti-inflammatory drugs induce colorectal cancer cell
apoptosis by suppressing 14-3-3epsilon. Cancer Res. 67:3185–3191.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawamoto Y, Akiguchi I, Tomimoto H,
Shirakashi Y, Honjo Y and Budka H: Upregulated expression of 14-3-3
proteins in astrocytes from human cerebrovascular ischemic lesions.
Stroke. 37:830–835. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Umahara T, Uchihara T, Tsuchiya K,
Nakamura A and Iwamoto T: Intranuclear localization and
isoform-dependent translocation of 14-3-3 proteins in human brain
with infarction. J Neurol Sci. 260:159–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lehmann JM, Moore LB, Smith-Oliver TA,
Wilkison WO, Willson TM and Kliewer SA: An antidiabetic
thiazolidinedione is a high affinity ligand for peroxisome
proliferator-activated receptor γ (PPAR γ). J Biol Chem.
270:12953–12956. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ricote M, Li AC, Willson TM, Kelly CJ and
Glass CK: The peroxisome proliferator-activated receptor-γ is a
negative regulator of macrophage activation. Nature. 391:79–82.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang C, Ting AT and Seed B: PPAR-γ
agonists inhibit production of monocyte inflammatory cytokines.
Nature. 391:82–86. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chawla A, Barak Y, Nagy L, Liao D,
Tontonoz P and Evans RM: PPAR-γ dependent and independent effects
on macrophage-gene expression in lipid metabolism and inflammation.
Nat Med. 7:48–52. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Welch JS, Ricote M, Akiyama TE, Gonzalez
FJ and Glass CK: PPARgamma and PPARdelta negatively regulate
specific subsets of lipopolysaccharide and IFN-γ target genes in
macrophages. Proc Natl Acad Sci USA. 100:pp. 6712–6717. 2003;
View Article : Google Scholar : PubMed/NCBI
|