Introduction
Coronary heart disease (CAD) is the most common type
of organ disease caused by atherosclerosis and a common disease
that is harmful to human health. With the improvement of people's
living standards and the arrival of an aging society, the incidence
of CAD increases year by year, and gradually becomes the first
cause of death (1,2). Studies worldwide have shown that the
incidence of premature CAD is increasing, and the course of disease
is developing rapidly. The condition is dangerous, and the rate of
sudden death is high (3). The
patients with CAD usually have no symptoms, and the clinical
manifestations are mainly myocardial infarction (MI). Patients with
CAD, especially when they have had MI, have more cardiovascular
risk factors, and comprehensive intensive interventions, including
treatment of lifestyle changes, are needed to reduce the risk of
future CAD and cardiovascular events (4,5).
CAD or MI patients often have a family history
(6). It is generally believed that
CAD is characterized in the context of genetic susceptibility
factors that are exposed to an atherosclerotic environment
throughout lifetime. In this context, the rapid evolution of
large-scale human research and genetic technology over the past few
years has revolutionized our understanding of the genetic basis of
CAD (7). In addition, coronary
morphological information may be an important additional feature of
disease prediction in patients with CAD or MI (8). The study suggests that vascular risk
factors explain only 90% of the risk of MI, suggesting that the
significance of genetic risk is low (9). However, this ignores the risk factors
such as hypertension, hypercholesterolemia, diabetes, and even
addictive behaviors (smoking), which are seriously affected by
genetic factors (10,11). Patients with CAD are always
accompanied by impairment of cardiac and endothelial function.
Combined with a previous study that risk factors, such as
hypertension, obesity, smoking and abnormal metabolism of serum
lipids, can mediate vascular endothelial injury by inflammation and
oxidative stress (12), resulting in
the change of structure and function of endothelial cells, which is
considered to be the initiating factor of atherosclerosis (13). Thus, variability in performance for
MI and CAD cannot be explained solely by frequency changes in
vascular risk factors (14,15), suggesting that racial or genetic
differences have important clinical implications in disease
pathogenesis.
Since 1980s, β-blockers have been used in patients
with CAD, especially when they have had MI (16). It is known that β-blockers improve
survival following MI. This has been established in multiple
randomized trials including CAPRICORN (17). β-blockers can slow down heart rate,
lower blood pressure, suppress the sympathetic nerve system, and
improve myocardial oxygen supply and demand imbalance, so as to
reduce the incidence of fatal arrhythmias after MI. For this
reason, the 1990 American College of Cardiology/American Heart
Association (ACC/AHA) guidelines (18) first recommended (class I or II a
recommendation) β-blocker therapy for essentially all post-MI
patients, except those with contraindications.
Although several studies have documented under
dosing of β-blockers, these studies showed that the majority of
patients delivered with β-blockers did not achieve the target-doses
demonstrated to be effective in the randomized trials (19,20). In
fact, most of the patients received <50% of the target-dose. The
randomized clinical trials did not assess the effects of different
doses of β-blockers. Furthermore, there have been no large-scale
studies that have addressed this topic. Thus, a hypothesis was made
that a lower dose of β-blockers may present a similar curative
effect with lower toxicity as the target-dose which has been
demonstrated in previous studies.
Our study evaluated the effects of different doses
of β-blockers on ventricular electrophysiological characteristics
and induced ventricular arrhythmias in canines after MI to explore
whether the lower doses may result in equivalent outcomes compared
with the target-dose. These data support the need for further
testing to determine optimal dosing of β-blockers after MI.
Materials and methods
Experimental models
Thirty-two mongrel dogs (16 males and 16 females)
were randomly divided into the control group (n=8), the low-dose
group (n=12) and the target-dose group (n=12). The dogs were
anesthetized with sodium pentobarbital (30 mg/kg, i.v.), intubated,
and ventilated with room air supplemented with oxygen from a
respirator (LTV-1000, Pulmonic Systems, USA). Additional
maintenance doses of 2 mg/kg sodium pentobarbital were administered
at the end of each hour during the procedure. Standard surface
electrocardiogram (ECG) was continuously monitored by using a
computer-based Lab System (TOP 2001; Hongtong Biology Technology
Company, Shanghai, China). Animal handling was performed in
accordance with the Shanghai Directive for Animal Research, and the
present Guidelines for the Care and Use of Laboratory Animals
published by the National Institutes of Health (NIH publication no.
85-23, revised 1996). The Ethics Committee of the Second Military
Medical University (Shanghai, China) approved the study
protocol.
Acute ischemia protocol
After anesthetized, the first diagonal artery was
isolated in all groups, and then occluded by ligature (3-0 silk)
for 1 h until the ischemic part turned dark red so as to make sure
that the ligation was successful. ECG was recorded and analyzed
continuously before and after MI. To achieve a stable status, we
gave the animals a 90 min-pause to make sure the ECG would not
change any further before proceeding.
Treatment
After ligation, the low-dose group was given
metoprolol at the dose of 0.6 mg/kg immediately by intravenous
injection, the target-dose group was given 1.6 mg/kg, while the
control group was injected with normal saline at the same dose as
the target-dose group.
Measurement of plasma norepinephrine
(NE) and epinephrine (E)
An hour after the ligation, blood samples were drawn
into heparinized tubes through a modified Morawitz cannula, which
was introduced into the coronary sinus (CS) through the azygos
vein. All samples were placed immediately on ice after collection
and centrifuged at 4°C within 30 min. Plasma was collected and
stored at −20°C for further analysis. NE and E levels in plasma
were measured by high-performance liquid chromatography (HPLC).
Electrophysiological measurements
Two multi-electrode catheters with 1 cm inter
electrode distance were sutured to evaluate effective refractory
periods (ERP) at six epicardial sites from the apex to the base of
the left ventricular free walls. The ventricular ERP in each site
was determined by programmed pacing that consisted of eight drive
stimuli (S1) followed by an extra-stimulus (S2) at twice threshold
pacing current with a 2 ms pulse duration. The interval between S1
and S2 was progressively decreased until refractoriness was
achieved. The ERP was defined as the longest S1S2 interval that
failed to capture the ventricles as described previously. ERPs were
measured at the baseline and an hour after ligation. ERP dispersion
was defined as the coefficient of variation (CV) of the ERP at all
six sites.
Measurement of ventricular arrhythmia
occurrence
Electrocardiogram was continuously monitored for 1 h
to record the occurrence and duration of ventricular arrhythmias
including ventricular premature contraction (VPC, identifiable
premature QRS complexes), ventricular tachycardia (VT, three or
more consecutive VPCs at a rate faster than the resting sinus rate)
and ventricular fibrillation (VF, unidentifiable and low voltage
QRS complexes). Especially, if VT progresses within a few beats to
VF (there are no sinus beats between VT and VF), we classified
these VTs as VF.
Statistical analysis
Data are expressed as mean ± SD. NE and E changes
were tested by the independent-sample t-test. ERP changes were
tested by the paired t-test. Pairwise comparisons between different
regions were calculated by the LSD method. Data were analyzed by
using SPSS21.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Metoprolol decreases the NE and E
levels in CS blood after MI
Since NE and E is a major cardiac stimulator and
contributes to ventricular arrhythmias induction, we investigated
whether low and target-dose of β-blocker, metoprolol, affects the
NE and E content differently in blood. As expected, NE and E levels
in CS blood dramatically increased in all three groups after MI
compared with that before MI (Fig. 1A
and B). Moreover, the NE and E levels in CS blood were
significantly decreased in both the low- and target-dose groups
compared with the control group after MI (p<0.01), but no
difference was found among the three groups in the NE and E levels
before MI. The NE and E levels in CS blood with the low-dose of
metoprolol treatment after MI was decreased by 32.7 and 11.1%
compared with the control group, respectively (p<0.01). The NE
and E levels in CS blood with the target-dose of metoprolol
treatment after MI was decreased by 28.3 and 14.5% compared with
the control group, respectively (p<0.01). However, there was no
difference between the low- and target-dose of metoprolol treatment
in the levels of NE and E in CS blood after MI. These results
suggest that metoprolol decreases the NE and E levels in CS blood
after MI in a dose-independent manner.
Metoprolol inhibits ventricular ERP
shortening after MI
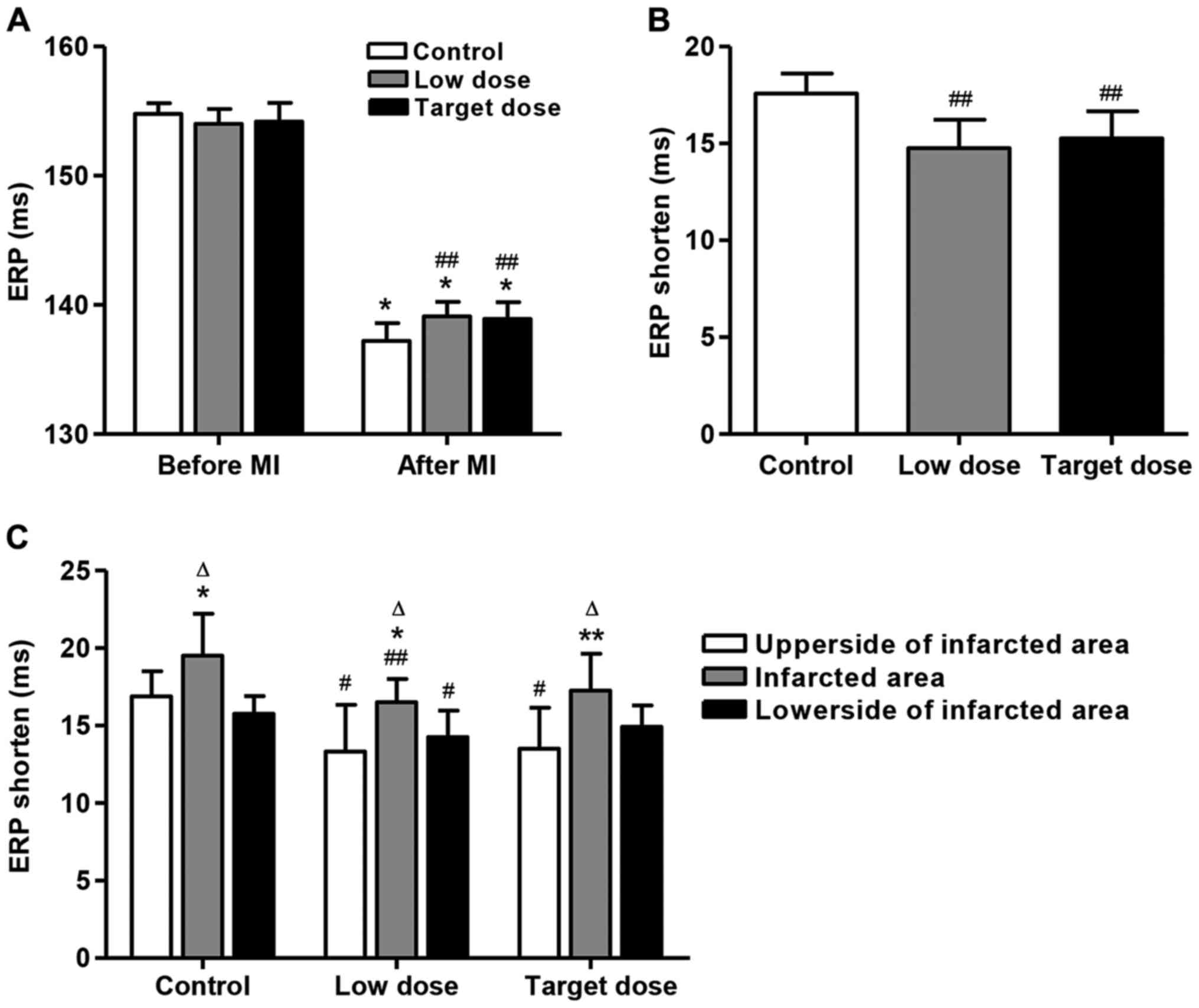
Fig. 2A summarizes
the ventricular ERP changes at 6 epicardial sites in all groups
before and after MI. As a result, ventricular ERP was significantly
decreased in all three groups after MI compared with that before MI
(p<0.01), but no difference was found among the three groups in
ventricular ERP before MI. After MI, low- and target-dose of
metoprolol increased the ventricular ERP by 1.38 and 1.24% compared
with the control group, respectively (p<0.01). However,
ventricular ERP in the low-dose group did not change significantly
compared with that in the target-dose group. Moreover, after MI,
low- and target-dose of metoprolol decreased the ventricular ERP
shortening by 16.1 and 13.1% compared with the control group,
respectively (Fig. 2B; p<0.01).
However, ventricular ERP in the low-dose group did not change
significantly compared with that in the target-dose group.
In addition, to make sure whether there was any
difference in ERP prolongation among the sites which were above, in
and below the infracted area, we calculated and compared the
average ERPs of the two sites above the infracted area (upper
sides), two sites in the area (infarcted area) and two sites below
the area (lower sides). Then, we calculated the difference between
these 3 areas. We found that ERP shorten in the infarcted area was
significantly increased compared to those in the other two areas in
all three groups (Fig. 2C;
p<0.05). However, no significant difference between the upper
and lower sides of the infarcted area was detected. The same trend
was found in the low- and target-dose groups. Moreover, the low and
target-dose of metoprolol significantly decreased the ERP shorten
by 21.0 and 19.9% in the upper side of infracted area compared with
the control groups, respectively (Fig.
2C; p<0.05). However, only the low-dose of metoprolol
treatment could decrease the ERP shortening by 15.4 and 9.5% in the
infracted area and in the lower side of the infracted area compared
with the control groups, respectively (p<0.05). These results
indicate that the low-dose of metoprolol treatment ERP shortening
was found above, in and below the infracted area.
Effect of low- and target-dose of
metoprolol on ventricular arrhythmia occurrence
The episodes of PVCs and VT as well as the mean
duration of VT in the low-dose group have no significant changes
when compared to the high-dose group. 5 of 12 (41.7%) animals in
the low-dose group had spontaneous VF compared to 4 of 12 (33.3%)
animals in the target-dose group (p>0.05).
Discussion
In this study, we investigated the effects of early
intravenous low-doses of metoprolol on ventricular
electrophysiological properties by using an acute MI canine model.
The results indicated that the low-dose of metoprolol exhibited the
same effects on the level of NE and E as well as the ERP in animals
with MI. These results suggest that the low-dose of β-blockers may
exert a similar protective effect against ventricular arrhythmias
after MI compared with the target-dose of β-blockers.
At present, the common measures we take for the
prevention of arrhythmias after MI include β-blocker therapy,
implantable cardioverter-defibrillator (ICD) and other
antiarrhythmic medications. β-blockers have been proven to be
effective in slowing heart rate, decreasing myocardial
contractility and lowering blood pressure. Since the prevalence of
β-blockers in patients after MI, β-blockers were used at a
lower-dose than in clinical trials (21). In the RIMA (Registre des infarctus du
Maine-Anjou) study of 1,461 MI patients, only 34.8% patients
achieved the target-dose of β-blockers at discharge, and after one
year follow-up, still nearly 60% of patients had not achieved the
target-dose (22). A study of 208
post-MI patients, 154 of whom were treated with a mean β-blocker
dose of 34% of the target-dose, demonstrated a 60% reduction in
all-cause mortality at a mean follow-up of 58.5 months (23). Patients who had an acute MI and were
treated with a lower dose of β-blockers than used in clinical
trials may show similar or better survival than those given higher
doses. The multicenter study enrolled 7,057 consecutive patients
with acute MI at 26 centers in the United States and Canada
(24). Researchers found no
increased survival in patients treated with β-blocker doses
approximating those used in previous randomized clinical trials
compared with lower doses. These findings suggested that β-blocker
may have a similar therapeutic effect at a lower dosage in
comparison to the target-dose.
A previous study demonstrated that heterogeneous
cardiac nerve sprouting and sympathetic hyperinnervation plays an
important role in arrhythmogenesis and sudden cardiac death in both
human patients and animal models of MI (25). Multiple randomized control trials
have proven that β-blocker therapy could significantly reduce the
incidence of sudden cardiac death (SCD) after MI. Part of the
reason is that β-blockers reduce the activity of sympathetic system
so as to decrease the level of plasma catecholamine. In the present
study, we investigated the effects of early intravenous low- and
target-doses of metoprolol on ventricular electrophysiological
properties by using an acute MI canine model. Although significant
difference was identified between the low- and target-dose groups,
similar tendency was observed in the therapeutic effect of both
groups, indicating that the low-dose of metoprolol exhibited a
similar effect on the ERP as well as the level of NE and E in
animals with MI. These results suggested that low-dose β-blockers
may exert a similar protective effect against ventricular
arrhythmias after MI compared with the target-dose β-blockers. It
indicated that low-dose practice maybe more reasonable in the
β-blocker therapy against ventricular arrhythmias after MI due to
lower toxicity and more safety. However, these data support the
need for further testing. A further study will be conducted to
determine optimal dosing of β-blockers after MI.
In conclusion, our study demonstrated that low-dose
β-blockers exhibit similar effects to the target-dose on the
improvement of ventricular electrophysiological properties through
shortened ERP and decreased NE and E levels after MI. Thus,
low-dose of metoprolol in β-blocker therapy may be reasonable due
to similar effect and lower toxicity.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (General Program, 81270244; Beijing,
China).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DNW, LW and DNL designed the study. DNW, LW and YH
were responsible for the animal experiments and tests. LH, HMC and
PFC were responsible for the electrophysiological experiments. DNW,
XL and JYZ were responsible for statistic analysis. DNW, LW and DNL
wrote and finalized the study. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shanghai Changzheng Hospital (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al:
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee: Heart disease and stroke statistics - 2014
update: A report from the American Heart Association. Circulation.
129:399–410. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dawber TR, Moore FE and Mann GV: Coronary
heart disease in the Framingham study. Am J Public Health Nations
Health. 47:4–24. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sivapalaratnam S, Boekholdt SM, Trip MD,
Sandhu MS, Luben R, Kastelein JJ, Wareham NJ and Khaw KT: Family
history of premature coronary heart disease and risk prediction in
the EPIC-Norfolk prospective population study. Heart. 96:1985–1989.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bolland MJ, Avenell A, Baron JA, Grey A,
MacLennan GS, Gamble GD and Reid IR: Effect of calcium supplements
on risk of myocardial infarction and cardiovascular events:
Meta-analysis. BMJ. 341:c36912010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Canto JG, Kiefe CI, Rogers WJ, Peterson
ED, Frederick PD, French WJ, Gibson CM, Pollack CV Jr, Ornato JP,
Zalenski RJ, et al: NRMI Investigators: Number of coronary heart
disease risk factors and mortality in patients with first
myocardial infarction. JAMA. 306:2120–2127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Horne BD, Camp NJ, Muhlestein JB and
Cannon-Albright LA: Identification of excess clustering of coronary
heart diseases among extended pedigrees in a genealogical
population database. Am Heart J. 152:305–311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hartiala J, Schwartzman WS, Gabbay J,
Ghazalpour A, Bennett BJ and Allayee H: The genetic architecture of
coronary artery disease: Current knowledge and future
opportunities. Curr Atheroscler Rep. 19:62017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Assmann G, Cullen P and Schulte H: Simple
scoring scheme for calculating the risk of acute coronary events
based on the 10-year follow-up of the prospective cardiovascular
Münster (PROCAM) study. Circulation. 105:310–315. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gyárfás I, Keltai M and Salim Y: Effect of
potentially modifiable risk factors associated with myocardial
infarction in 52 countries in a case-control study based on the
INTERHEART study. Orv Hetil. 147:675–686. 2006.(In Hungarian).
PubMed/NCBI
|
|
10
|
Basat O, Ucak S, Seber S, Oztekin E and
Altuntas Y: After myocardial infarction carvedilol improves insulin
resistance compared to metoprolol. Clin Res Cardiol. 95:99–104.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tölg R, Witt M, Schwarz B, Kurz T,
Kurowski V, Hartmann F, Geist V and Richardt G: Comparison of
carvedilol and metoprolol in patients with acute myocardial
infarction undergoing primary coronary intervention - the PASSAT
Study. Clin Res Cardiol. 95:31–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ilic Deljanin M, Pavlovic RF, Kocic G,
Simonovic D and Lazarevic G: Effects of music therapy on
endothelial function in patients with coronary artery disease
participating in aerobic exercise therapy. Altern Ther Health Med.
23:at54912017.PubMed/NCBI
|
|
13
|
Cai X, Bao L, Ding Y, Dai X, Zhang Z and
Li Y: Quercetin alleviates cell apoptosis and inflammation via the
ER stress pathway in vascular endothelial cells cultured in high
concentrations of glucosamine. Mol Med Rep. 15:825–832. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bischoff B, Silber S, Richartz BM, Pieper
L, Klotsche J and Wittchen HU: DETECT Study-Group: Inadequate
medical treatment of patients with coronary artery disease by
primary care physicians in Germany. Clin Res Cardiol. 95:405–412.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koch KC, Schaefer WM, Liehn EA, Rammos C,
Mueller D, Schroeder J, Dimassi T, Stopinski T and Weber C: Effect
of catheter-based transendocardial delivery of stromal cell-derived
factor 1alpha on left ventricular function and perfusion in a
porcine model of myocardial infarction. Basic Res Cardiol.
101:69–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Looi KL, Chow KL, Looi JL, Lee M, Halliday
S, White H and Ellis C: Under-use of secondary prevention
medication in acute coronary syndrome patients treated with
in-hospital coronary artery bypass graft surgery. N Z Med J.
124:18–27. 2011.PubMed/NCBI
|
|
17
|
Costalunga A and Gavazzi A: Effect of
carvedilol on outcome after myocardial infarction in patients with
left ventricular dysfunction: The CAPRICORN randomized trial. Ital
Heart J Suppl. 2:1246–1247. 2001.(In Italian). PubMed/NCBI
|
|
18
|
Gunnar RM, Bourdillon PD, Dixon DW, Fuster
V, Karp RB, Kennedy JW, Klocke FJ, Passamani ER, Pitt B, Rapaport
E, et al: ACC/AHA guidelines for the early management of patients
with acute myocardial infarction. A report of the American College
of Cardiology/American Heart Association Task Force on Assessment
of Diagnostic and Therapeutic Cardiovascular Procedures
(subcommittee to develop guidelines for the early management of
patients with acute myocardial infarction). Circulation.
82:664–707. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gislason GH, Rasmussen JN, Abildstrøm SZ,
Gadsbøll N, Buch P, Friberg J, Rasmussen S, Køber L, Stender S,
Madsen M, et al: Long-term compliance with beta-blockers,
angiotensin-converting enzyme inhibitors, and statins after acute
myocardial infarction. Eur Heart J. 27:1153–1158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rochon PA and Gurwitz JH: Prescribing for
seniors: Neither too much nor too little. JAMA. 282:113–115. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ruta J, Ptaszyński P, Maciejewski M, Goch
JH and Chizyński K: Effect of low doses of metoprolol, bisoprolol
and carvedilol on mortality in patients with left ventricular
dysfunction after acute myocardial infarction. Wiad Lek.
59:649–653. 2006.(In Polish). PubMed/NCBI
|
|
22
|
Grall S, Biere L, Le Nezet M, Bouvier JM,
Lucas-Chauvelon P, Richard C, Abi-Khalil W, Delepine S, Prunier F
and Furber A: Relationship between beta-blocker and
angiotensin-converting enzyme inhibitor dose and clinical outcome
following acute myocardial infarction. Circ J. 79:632–640. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Siu CW, Pong V, Jim MH, Yue WS, Ho HH, Li
SW, Lau CP and Tse HF: Beta-blocker in post-myocardial infarct
survivors with preserved left ventricular systolic function. Pacing
Clin Electrophysiol. 33:675–680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goldberger JJ, Bonow RO, Cuffe M, Liu L,
Rosenberg Y, Shah PK, Smith SC Jr and Subačius H: OBTAIN
Investigators: Effect of beta-blocker dose on survival after acute
myocardial infarction. J Am Coll Cardiol. 66:1431–1441. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyauchi Y, Zhou S, Okuyama Y, Miyauchi M,
Hayashi H, Hamabe A, Fishbein MC, Mandel WJ, Chen LS, Chen PS, et
al: Altered atrial electrical restitution and heterogeneous
sympathetic hyperinnervation in hearts with chronic left
ventricular myocardial infarction: Implications for atrial
fibrillation. Circulation. 108:360–366. 2003. View Article : Google Scholar : PubMed/NCBI
|