Introduction
Intracerebral hemorrhage (ICH) has a high rate of
morbidity, disability and mortality, but lacks a specific or
efficient therapeutic method (1,2).
Pathological injuries of ICH include: i) Injury to cerebral tissue
caused by a large amount of bleeding; ii) mass effect causing
hypothalamus, epithalamus or brain-stem injury, and iii) secondary
nerve injury in the peripheral tissue of the foci (3–5). Thus,
nerve injury following ICH not only results from the mass effect
and the direct damage to the surrounding cerebral tissue caused by
hematoma, but also secondary nerve injury (6). Therefore, investigating the mechanism
of secondary cerebral tissue injury following ICH is an important
research theme.
Nuclear factor (NF)-κB is a key nuclear
transcription factor, associated with the regulation of gene
transcription and activated by environmental stimuli applied to the
whole body (7). NF-κB is widely
expressed in nerve cells, astrocytes and microglia (8,9). The
role of NF-κB in central nervous system diseases; particularly in
ischemic cerebrovascular disease, has become an increasingly
popular research topic (10).
Following cerebral ischemia, NF-κB is stimulated and brain injury
is exacerbated through mechanisms including the promotion of the
inflammatory reaction, cell apoptosis induction and free radical
injury mediation (11,12). As the pathogenesis of brain injury
subsequent to cerebral hemorrhage is similar to that of cerebral
infarction, an ICH model was established by injecting autogenous
non-heparin anticoagulant arterial blood into the caudate putamen
of rats in the present study, to preliminarily investigate the
pathogenesis of brain injury following ICH.
Materials and methods
Animals and experimental protocol
A total of 80 healthy male Wistar rats, aged 3–4
months and weighing 250–300 g, were provided by the Laboratory
Animal Center of Qingdao University (Qingdao, China). Rats were
housed at a temperature of 20–25°C and a humidity of 30–50%, with a
12 h light/dark circulation and free access to food and water. The
study protocol was approved by the Ethics Committee for Animal
Experiments of the Affiliated Hospital of Qingdao University
(Qingdao, China). Rats were randomly divided into a sham-surgery
control group (n=40) and an ICH group (n=40). Following the
establishment of the ICH model, 5 rats from each group were
sacrificed at each of the following time points: 0, 3, 6 and 12 h
and 1, 2, 3 and 5 days following surgery, and their brain tissues
were isolated. Rats were sacrificed via the following method: The
rats were intraperitoneally anesthetized with 10% chloral hydrate
(350 mg/kg; China National Pharmaceutical Group Corporation,
Beijing, China; batch no. 20080325), then the chest was opened and
the right atrium was cut, inducing mortality via exsanguination.
Successful sacrifice was confirmed when heartbeat and breathing
stopped, and the volume of blood extracted by exsanguination was
10–13 ml. In the present study, no rats exhibited signs of
peritonitis following the administration of 10% chloral hydrate.
The weight of the animals at the time of sacrifice was 250–300
g.
Analysis of NF-κB expression
For immunohistochemistry, rat brains were obtained
and immediately fixed in 40 g/l formaldehyde (pH 7.0) for 24 h at
room temperature, and later embedded in paraffin wax. Samples were
sliced into 5 µm-thick sections using a paraffin slicing machine
(RM2235; Leica Microsystems GmbH, Wetzlar, Germany). Following
deparaffinization and rehydration, paraffin-embedded tissue
sections were treated with heat-induced antigen retrieval buffer
(pH 6.0 citrate buffer) and blocked using 3% hydrogen peroxide at
room temperature. Samples were then incubated with mouse anti-rat
NF-κB p65 monoclonal antibodies (1:50; cat. no. sc-8008; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. Tissue was
then incubated with secondary mouse HRP (cat. no. sc-516102; Santa
Cruz Biotechnology, Inc.) for 30 min at room temperature and
developed using a DAB kit (cat. no. ZL1-9017; OriGene Technologies,
Inc., Rockville, MD, USA) at room temperature for 1 min. Samples
and then counterstained with hematoxylin for 30 sec at room
temperature.
Teriminal dexynucleotidyl
tranferase-mediated dUTP nick end labeling (TUNEL) assay
Following deparaffinization and rehydration,
paraffin-embedded tissue sections were treated with PBS at room
temperature. To observe DNA strand breaks in nuclei, sections were
treated with Proteinase K (cat. no. CW 2584M; CWBio, Beijing,
China) for 15 min and 3% H2O2 for 5 min. The
TUNEL assay was performed using the TUNEL Apoptosis Detection kit
(cat. no. 40307; Yeasen Biotechnology Co., Ltd., Shanghai, China)
according to the manufacturer's protocol. DAPI (cat. no. C0060;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) was used for the coloration of apoptotic cells at room
temperature for 15 min. Cell apoptosis was analyzed using a FACS
caliber flow cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Images were obtained at a magnification of ×400. Five
non-overlapping visual fields were selected and the number of
positive cells was counted. An average value of each field was then
calculated.
ICH model establishment
The ICH model was established as described by
Deinsberger et al (13). The
rats were intraperitoneally anesthetized using 10% chloral hydrate
(350 mg/kg), and placed onto a stereotaxic instrument in the prone
position. A 10-mm incision was made along the center of the scalp,
and the anterior fontanelle was exposed when a 0.5-mm hole was made
0.2 mm in front of the anterior fontanelle, 3 mm to the right of
the midline. An injection of 50 µl blood from the tail tip was
administered slowly within 8–10 min. The injection was administered
5.5 mm deep, into the caudate putamen. The injection needle
remained in place for 10 min following injection, then slowly
withdrawn. The hole was sealed with sterilized medical bone wax,
and the skin was sutured. All steps were performed under sterilized
conditions. The rat was returned to normal housing post-surgery,
with free food and water access. The rats in the control group
underwent equivalent procedures, but no blood injection was
administered.
Data processing and statistical
analysis
Cell counting was performed for each single specimen
under microscopy at ×400 magnification. Positively stained cells
were counted under an automatic morphology measuring instrument
(HPIAS21000) in 5 perihematomal fields of view. All data are
presented as the mean ± standard deviation. Comparisons between
more than two groups were made using analysis of variance, and
comparisons between 2 groups were made using Student's t-test. The
association between two parameters was analyzed via simple linear
regression. P<0.05 was considered to indicate a statistically
significant difference. SPSS software (version 13.0; SPSS, Inc.,
Chicago, IL, USA) was used for all statistical analysis.
Results
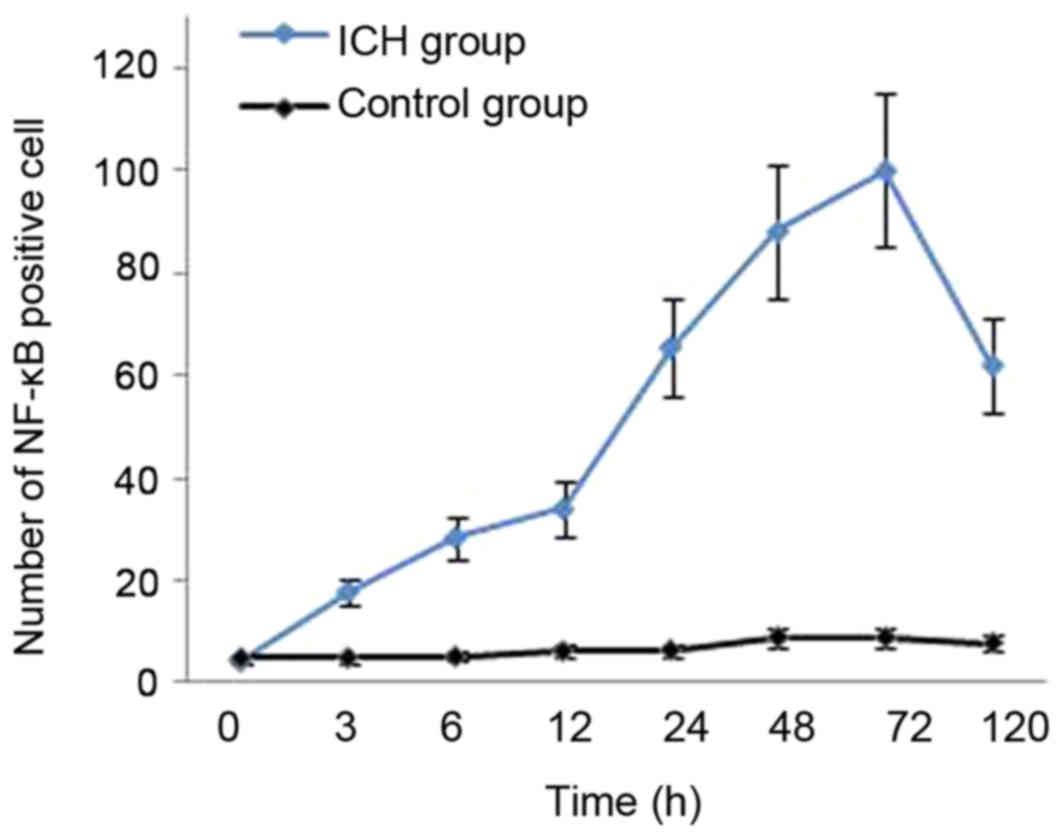
Dynamic expression of NF-κB in the
cerebral tissue around ICH
A small number of NF-κB-positive cells were observed
in the tissue around the needle path in rats in the control group.
NF-κB was mainly located in the cytoplasm, and there was no
statistically significant difference between any time points
(P>0.05). NF-κB-positive cells were observed in the
perihematomal edema at all time points in rats of the ICH group.
There was significantly more NF-κB staining in the ICH group
compared with the control group (P<0.01). NF-κB was mainly
located in nerve cells and gliacytes in the ICH group. NF-κB
expression was increased at 3 h following hemorrhage, mainly in the
cytoplasm. Following 6 h, NF-κB was also observed in the nucleus.
The expression peaked at 72 h following hemorrhage, and then
decreased gradually. However, NF-κB expression could still be
detected following 5 days in the ICH group. (Figs. 1–3;
Table I).
 | Table I.Nuclear factor-κB-positive cells in
the cerebral tissue in both groups. |
Table I.
Nuclear factor-κB-positive cells in
the cerebral tissue in both groups.
| Time post surgery
(h) | Rats (n) | Control group, cells
(n) | ICH group, cells
(n) |
|---|
| 0 | 5 | 4.89±0.46 | 4.35±0.52 |
| 3 | 5 | 5.01±0.54 |
17.86±2.74a,b |
| 6 | 5 | 5.24±0.61 |
28.42±3.24a,b |
| 12 | 5 | 6.36±0.64 |
34.04±4.69a,b |
| 24 | 5 | 6.51±0.52 |
65.53±7.50a,b |
| 48 | 5 | 9.06±0.74 |
88.14±9.34a,b |
| 72 | 5 | 9.05±0.82 |
100.13±12.88a,b |
| 120 | 5 | 7.89±0.96 |
62.30±6.48a,b |
Apoptosis in the cerebral tissue
around ICH
The mean number of apoptotic cells in the
sham-surgery control group at each subsequent time point following
surgery was 2.85±0.26, 2.96±0.36, 2.76±0.39, 2.87±0.29, 3.21±0.41,
3.24±0.36, 3.05±0.37 and 3.11±0.32, whereas that of the ICH group
was 2.80±0.32, 3.79±0.46, 7.83±1.01, 22.01±3.94, 42.28±5.55,
43.26±4.74, 52.88±5.97 and 69.03±5.93 (Fig. 4; Table
II). In the ICH group, a small number of apoptotic nerve cells
were identified in the cerebral tissue around the hematoma at 3 h
following ICH. Following 6 h, the apoptotic cell number increased,
and it had increased significantly by 6 h. The number of apoptotic
cells continued to increase at 72 and 120 h following ICH. The
difference in apoptotic cell number at each time point 6 h post
surgery was statistically significant compared with those in the
control group (P<0.01). Changes observed in an apoptotic cell
include nucleus condensation, cell shrinkage, nuclear envelope
shrinkage, chromatin condensation to nuclear envelope, irregular
condensation and light brown granules within the nucleus, which
became dark brown upon staining. Brain tissue around the hematoma
exhibiting distinct color alteration following the TUNEL assay
indicated dense areas of apoptotic cells. Only small amounts of
apoptotic nerve cells were observed in normal tissue following
staining, as was observed in the sham-surgery control group.
(Figs. 5 and 6).
 | Table II.Apoptotic cells in the cerebral
tissue in both groups. |
Table II.
Apoptotic cells in the cerebral
tissue in both groups.
| Time post surgery
(h) | Rats (n) | Control group,
cells (n) | ICH group, cells
(n) |
|---|
| 0 | 5 | 2.85±0.26 | 2.80±0.32 |
| 3 | 5 | 2.95±0.34 | 3.79±0.46 |
| 6 | 5 | 2.76±0.39 |
7.83±1.01a,b |
| 12 | 5 | 2.87±0.29 |
22.01±3.94a,b |
| 24 | 5 | 3.21±0.41 |
42.28±5.55a,b |
| 48 | 5 | 3.24±0.36 |
43.26±4.74a,b |
| 72 | 5 | 3.05±0.37 |
52.88±5.97a,b |
| 120 | 5 | 3.11±0.32 |
69.03±5.93a,b |
Correlation between NF-κB expression
and apoptosis in the cerebral tissue around ICH
The number of apoptotic cells was significantly and
positively correlated with the number of NF-κB-positive cells
(r=0.753; P<0.01). The correlation between the number of
NF-κB-positive cells and the number of apoptotic cells in the ICH
group is presented in Fig. 7.
Discussion
NF-κB is an important multidirectional transcription
factor. Without stimulation, NF-κB exists in cytoplasm bound to its
inhibitory factor, IκB (14).
Certain environmental stimuli, including cytokines, free radicals,
ultraviolet irradiation, ischemia, anoxia and bacterial or viral
antigens, stimulate NF-κB, leading to the promotion of target gene
transcription (15–17). The target is then recruited for
physiological or pathological processes, including the inflammatory
reaction, immunoreaction, cell apoptosis and free radical injury
(18–21).
The majority of current studies of the role of NF-κB
in cerebrovascular disease focus on brain ischemia. Animal
experiments and clinical autopsies have demonstrated that,
following ischemic cerebral injury, existing NF-κB was activated
from the rest state, and its mRNA and protein expression levels
were enhanced (22,23). Due to varying experimental
conditions, the results of previous studies are inconsistent. Among
the extensive research of the role of NF-κB in ischemic cerebral
injury (24–26), one study indicated that
administration of the NF-κB inhibitor, N-acetylcysteine, resulted
in a significant decrease in cerebral infarct volume in middle
cerebral artery occlusion (MACO) ischemia/reperfusion rats
(27). It has been demonstrated in
numerous animal experiments and clinical trials that mild
hypothermia can reduce infarct volume and accelerate the recovery
of nerve function (28,29). Han et al (30) used MACO ischemia/reperfusion rats to
observe the effect of mild hypothermia on the expression of NF-κB,
and it was demonstrated that rats with mild hypothermia exhibited
significantly reduced activity of NF-κB. Thus, it is speculated
that the inhibition of NF-κB activity may be a mechanisms of brain
protection.
The pathogenesis of brain injury following ICH is
similar to that of cerebral infarction, suggesting that NF-κB may
also be associated with brain cell injury following ICH (31). Therefore the present study evaluated
the dynamic expression of NF-κB and its association with apoptosis
in the cerebral tissue surrounding hematoma in rats following ICH.
It was demonstrated that, in the sham-surgery group, minimal NF-κB-
positive cells were observed around the needle path with no
significance regarding time or nuclear translocation. In the ICH
group, NF-κB was activated 3 h following ICH, and a large number of
positive cells were observed in the cerebral tissue around the
hematoma. The NF-κB-positive cells were mainly located in the
cytoplasm, indicating that NF-κB had not exerted biological
activity in nucleus. At 6 h following ICH, an NF-κB nuclear shift
was observed, and NF-κB-positive staining was evident in the
cytoplasm and nucleus, or in the nucleus alone. NF-κB activation
also occurred in the surrounding tissues, including the cortex,
hippocampus and hypothalamus. NF-κB-activation peaked at 72 h
following ICH, then decayed gradually. The number of positive cells
at 5 days was twice that at 3 h. This demonstrated that NF-κB
remained activated following ICH, and suggests that it may be
associated with pathogenesis of cerebral injury following ICH.
Previous studies have demonstrated that penumbra also exists in the
cerebral tissue around hematoma following ICH, with a similar
pathological mechanism to that of cerebral infarction (32). Therefore, it was speculated that the
continuous activation of NF-κB following ICH may aggravate cerebral
injury.
Previous studies have demonstrated apoptosis was
associated with secondary cerebral injury following ICH (30,33–34). In
the present study, TUNEL assays indicated that an increased number
of apoptotic nerve cells were observed in the cerebral tissue
surrounding hematomas in the ICH surgery group. In these rats,
chromatin was condensed into sharply delineated masses with
irregular shapes, including crescent, annulus, rectangular, or
fragments, which were marginated against the nuclear membranes. In
contrast, in the sham-surgery rats, very few apoptotic cells were
observed in the brain tissue. The increase in apoptosis up to 120 h
following ICH, was a contrasting finding to that of Matsushita
et al (35), which may be due
to differences in the size of the hematoma or model-establishment
methodology. The present study demonstrated that apoptotic cells
were mostly observed in the cerebral tissue surrounding the
hematoma, whereas necrotic cells were located near the edge of the
hematoma. No apoptotic cells were observed in tissue far from the
hematoma, indicating that the apoptotic mechanism is associated
with nerve cell injury following ICH. Furthermore, apoptotic cells
were also observed in tissue without pathological change,
suggesting that the range of nerve cell injury around the hematoma
is more extensive than the pathological changes observed under a
light microscope. A total of 6 h following ICH, the level of NF-κB
activation increased significantly and the protein translocated
into the nucleus, followed by the emergence of a large number of
apoptotic cells at 12 h. This illustrates the association between
apoptosis and NF-κB activation following ICH. Furthermore,
correlation analysis revealed a significant positive correlation
between NF-κB protein expression and apoptosis, indicating that
NF-κB activation may enhance cerebral apoptosis in perihematomal
edema of rats following ICH. However, the mechanism of how NF-κB
regulates apoptosis remains unclear. Future studies will include
investigation of the regulatory effect of NF-κB on the expression
of apoptosis- associated genes.
In conclusion, NF-κB protein expression and
apoptotic-cell number were demonstrated to be increased in the
cerebral tissue following ICH, and there was a significant positive
correlation between NF-κB expression and apoptosis. Therefore, the
present study indicates that NF-κB activation may promote cerebral
apoptosis in perihematomal edema of rats following ICH. However,
further research is required to elucidate how NF-κB promotes
apoptosis.
Acknowledgements
The authors would like to thank the Central
Laboratory of Affiliated Hospital of Qingdao University (Qingdao,
China) for technical support, and to the Department of Neurology of
Tianjin People's Hospital (Tianjin, China) for their valuable
cooperation.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL designed the current study and
analyzed/interpreted the data. LM acquired the data, performed the
experiments and analyzed/interpreted the data. JS acquired the
data, performed data analysis/interpretation and supervised the
current study. JG performed the experiments and statistical
analysis.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee for Animal Experiments of the Affiliated Hospital of
Qingdao University (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Referenc
|
1
|
Satopää J, Meretoja A, Koivunen RJ,
Mustanoja S, Putaala J, Kaste M, Strbian D, Tatlisumak T and
Niemelä MR: Treatment of intracerebellar haemorrhage: Poor outcome
and high long-term mortality. Surg Neurol Int. 8:2722017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang JJ, Khorchid Y, Dillard K, Kerro A,
Burgess LG, Cherkassky G, Goyal N, Chapple K, Alexandrov AW,
Buechner D, et al: Elevated pulse pressure levels are associated
with increased in-hospital mortality in acute spontaneous
intracerebral hemorrhage. Am J Hypertens. 30:719–727. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhai W, Chen D, Shen H, Chen Z, Li H, Yu Z
and Chen G: A1 adenosine receptor attenuates intracerebral
hemorrhage-induced secondary brain injury in rats by activating the
P38-MAPKAP2-Hsp27 pathway. Mol Brain. 9:662016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cai P, Luo H, Xu H, Zhu X, Xu W, Dai Y,
Xiao J, Cao Y, Zhao Y, Zhao BQ and Fan W: Recombinant ADAMTS 13
attenuates brain injury after Intracerebral hemorrhage. Stroke.
46:2647–2653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Skevas P C, Knospe V, Vettorazzi E,
Richard G, Wagenfeld L, Westphal M and Regelsberger J: Terson
syndrome in subarachnoid hemorrhage, intracerebral hemorrhage, and
traumatic brain injury. Neurosurg Rev. 38:129–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wasserman JK and Schlichter LC: White
matter injury in young and aged rats after intracerebral
hemorrhage. Exp Neurol. 214:266–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehmood T, Maryam A, Tian X, Khan M and Ma
T: Santamarine inhibits NF-κB and STAT3 activation and induces
apoptosis in HepG2 liver cancer cells via oxidative stress. J
Cancer. 8:3707–3717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Neill LA and Kaltschmidt C: NF-kappa B:
A crucial transcription factor for glial and neuronal cellfunction.
Trends Neurosci. 20:252–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu S, Tang S and Su F: Dioscin inhibits
ischemic strokeinduced inflammation through inhibition of the
TLR4/MyD88/NF-κB signaling pathway in a rat model. Mol Med Rep.
17:660–666. 2018.PubMed/NCBI
|
|
11
|
Jeong J, Kim S, Lim DS, Kim SH, Doh H, Kim
SD and Song YS: TLR5 Activation through NF-κB is a neuroprotective
mechanism of postconditioning after cerebral ischemia in mice. Exp
Neurobiol. 26:213–226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simmons LJ, Surles-Zeigler MC, Li Y, Ford
GD, Newman GD and Ford BD: Regulation of inflammatory responses by
neuregulin-1 in brain ischemia and microglial cells in vitro
involves the NF-kappa B pathway. J Neuroinflammation. 13:2372016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deinsberger W, Vogel J, Kuschinsky W, Auer
LM and Böker DK: Experimental intracerebral hemorrhage: Description
of double injection model in rats. Neurol Res. 18:475–477. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niu J, Wang K, Graham S, Azfer A and
Kolattukudy PE: MCP-1-induced protein attenuates endotoxin-induced
myocardial dysfunction by suppressing cardiac NF-κB activation via
inhibition of IκB kinase activation. J Mol Cell Cardiol.
51:177–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunsch C and Rosen C: NF-kappaB
subunit-specific regulation of the interleukin-8 promoter. Mol Cell
Biol. 13:6137–6146. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zambrano S, de Toma I, Piffer A, Bianchi
ME and Agresti A: NF-κB oscillations translate into functionally
related patterns of gene expression. Elife. 5:1–38. 2016.
View Article : Google Scholar
|
|
17
|
Lee REC, Walker SR, Savery K, Frank DA and
Gaudet S: Fold change of nuclear NF-κB determines TNF-induced
transcription in single cells. Mol Cell. 53:867–879. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Rasheed NM, Al-Rasheed NM, Bassiouni
YA, Hasan IH, Al-Amin MA, Al-Ajmi HN and Mohamad RA: Vitamin D
attenuates pro-inflammatory TNF-α cytokine expression by inhibiting
NF-κB/p65 signaling in hypertrophied rat hearts. J Physiol Biochem.
71:289–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lian S, Xia Y, Khoi PN, Ung TT, Yoon HJ,
Kim NH, Kim KK and Jung YD: Cadmium induces matrix
metalloproteinase-9 expression via ROS-dependent EGFR, NF-κB, and
AP-1 pathways in human endothelial cells. Toxicology. 338:104–116.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khaksar S and Bigdeli MR: Intra-cerebral
cannabidiol infusion-induced neuroprotection is partly associated
with the TNF-α/TNFR1/NF-κB pathway in transient focal cerebral
ischaemia. Brain Inj. 31:1932–1943. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Candel S, Tyrkalska SD, García-Moreno D,
Meseguer J and Mulero V: Identification of Evolutionarily Conserved
Md1 Splice Variants That Regulate Innate Immunity through
Differential Induction of NF-κB. J Immunol. 197:1379–1388. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang M, Cui Z, Cui H, Wang Y and Zhong C:
Astaxanthin protects astrocytes against trauma-induced apoptosis
through inhibition of NKCC1 expression via the NF-κB signaling
pathway. BMC Neurosci. 18:422017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qi W, Zhou F, Li S, Zong Y, Zhang M, Lin
Y, Zhang X, Yang H, Zou Y, Qi C, Wang T and Hu X: Remote ischemic
postconditioning protects ischemic brain from injury in rats with
focal cerebralischemia/reperfusion associated with suppression of
TLR4 and NF-κB expression. Neuroreport. 27:469–475. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen W, Zhang C and Zhang G: Nuclear
factor kappaB activation is mediated by NMDA and non-NMDA receptor
and L-type voltage-gated Ca(2+) channel following severe global
ischemia in rat hippocampus. Brain Res. 933:23–30. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nurmi A, Lindsberg PJ, Koistinaho M, Zhang
W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N,
Schwaninger M and Koistinaho J: Nuclear factor-kappaB contributes
to infarction after permanent focal ischemia. Stroke. 35:987–991.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clemens JA, Stephenson DT, Smalstig EP,
Smalstig EB, Mincy RE, Rash KS and Little SP: Global ischemia
activates nuclear factor-kappa B in forebrain neurons of rats.
Stroke. 28:1073–1080. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carroll JE, Howard EF, Hess DC, Wakade CG,
Chen Q and Cheng C: Nuclear factor-kappa B activation during
cerebral reperfusion: Effect of attenuation with N-acetylcysteine
treatment. Brain Res Mol Brain Res. 56:186–191. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma LL, Song L, Yu XD, Yu TX, Liang H and
Qiu JX: The clinical study on the treatment for acute cerebral
infarction by intra-arterial thrombolysis combined with mild
hypothermia. Eur Rev Med Pharmacol Sci. 21:1999–2006.
2017.PubMed/NCBI
|
|
29
|
Geurts M, Scheijmans FE, van Seeters T,
Biessels GJ, Kappelle LJ, Velthuis BK and van der Worp HB: DUST
investigators: Temporal profile of body temperature in acute
ischemic stroke: Relation to infarct size and outcome. BMC Neurol.
1:2332016. View Article : Google Scholar
|
|
30
|
Han HS, Karabiyikoglu M, Kelly S, Sobel RA
and Yenari MA: Mild hypothermia inhibits nuclear factor-kappaB
translocation in experimental stroke. J Cereb Blood Flow Metab.
23:589–598. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bihl JC, Zhang C, Zhao Y, Xiao X, Ma X and
Chen Y, Chen S, Zhao B and Chen Y: Angiotensin-(1–7) counteracts
the effects of Ang II on vascular smooth muscle cells, vascular
remodeling and hemorrhagic stroke: Role of the NFκB inflammatory
pathway. Vascul Pharmacol. 73:115–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Menon BK, Hill MD, Eesa M, Modi J, Bhatia
R, Wong J, Hudon ME, Morrish W, Demchuk AM and Goyal M: Initial
experience with the Penumbra Stroke System for recanalization of
large vessel occlusions in acute ischemic stroke. Neuroradiology.
53:261–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xue M and Del Bigio MR: Intracerebral
injection of autologous whole blood in rats: Time course of
inflammation and cell death. Neurosci Lett. 283:230–232. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei N, Wei Y, Li B and Pang L: Baicalein
Promotes Neuronal and Behavioral Recovery after intracerebral
hemorrhage via suppressing apoptosis, oxidative stress and
neuroinflammation. Neurochem Res. 42:1345–1353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsushita K, Meng W, Wang X, Asahi M,
Asahi K, Moskowitz MA and Lo EH: Evidence for apoptosis after
intercerebral hemorrhage in rat striatum. J Cereb Blood Flow Metab.
20:396–404. 2000. View Article : Google Scholar : PubMed/NCBI
|