Introduction
The increasing discovery of multiple
antibiotic-resistant bacteria requires urgent development of novel
and effective antibacterial reagents (1–3). Natural
defensins have attracted attention for their multiple functions
against antibiotic resistant bacteria (4,5).
Extensive studies have shown that defensins can, not only kill or
inhibit the growth of diverse pathogens directly, but also boost
specific immune responses that provide highly effective immunity
against pathogens (5,6).
Human β-defensin 2 (BD2) and BD3 are major members
of defensin family of antimicrobial peptides (AMP). In addition to
their direct bactericidal action, they have been demonstrated to
modulate the innate and adaptive immune responses (4,5,7). BD2 acts primarily by its chemotactic
ability to recruit memory T cells and immature dendritic cells,
which are key immunological molecules of the immune system during
infection or injuries (8). BD2 can
also induce mast cell migration, degranulation and histamine
release (9). BD3 has been reported
to be chemotactic for monocytes and macrophages, which plays an
important role in combating infection (10,11).
However, single use of BD2 and BD3 as new antimicrobial drugs has
to face the deficiency of low bioactivity and short half-time in
vivo due to their rapid degradation rate.
Recently, some strategies have been used to overcome
the problems of weak bioactivity and half-life period in
vivo, such as fusion of multiple AMPs (12) and the application of gene delivery
systems (11,13). The design of multivalent AMPs usually
involves conjugation of several AMP monomers together via linkers,
and have been reported to display more novel and stronger
bioactivities than the original monomeric forms (14–16). For
example, vancomycin, in the form of a component of fusion
multivalent antibiotics, has been shown to be more effective
against microbes (14) and fusion
multivalent indolicidin shows higher bioactivity against
multidrug-resistant (MDR) strains (17). These results indicate that fusion of
multiple AMPs provides a novel approach for creation of stronger
antibiotics to improve the resistance of hosts against infectious
disease.
Chitosan (CS) and liposomes (LP) have both been used
as gene delivery systems because of their non-toxic, biocompatible
properties and efficient DNA loading rates (18,19).
However, the nanoparticles containing entrapped genes in CS and LP
are rapidly cleared in the blood and are degraded by protease in
vivo. To address these problems, LP have been conjugated with
amino groups to improve cellular phagocytosis and to promote
lysosomal escape due to the positive charge (20). CS has been modified with polyethylene
glycol (PEG) and polyethylenimine (PEI) to prolong blood
circulation time and enhance escape of DNA from lysosomes (21,22).
Such advances in the application of modified CS gene carriers to
enhance DNA transfection efficiency in vivo have encouraged
further research.
Until now, little information has been available on
the synergetic antibacterial effects between BD2 and BD3 in
vitro and in vivo. Therefore, the present experiment was
carried out to compare the delivery efficiency and expression
effect of the fusion gene of BD2 and BD3 that were entrapped with
different LP, CS and its derived package molecules with the aim of
developing a safe, effective and biocompatible material to improve
the bioactivity of fusion antibacterial peptide gene in vivo
against bacterial infection in animals.
Materials and methods
General
The study was approved by the Ethics Committee of
Sichuan University (Chengdu, China).
Construction of prokaryotic expression
plasmid of fusion gene of BD2/3
To construct a fusion gene of BD2/3 (GenBank:
AF071216, AF301470), six oligodeoxynucleotide fragments were
designed and synthesized by Sangon Biotech Co., Ltd., Shanghai,
China (Table I). The fused gene of
BD2/3 was prepared by overlap extension PCR (23). The stop codon of BD3 and the start
codon of BD2 were deleted, and restriction sites BamHI,
EcoRI and BglII were added to both ends. The fused
BD2/BD3 genes were connected by linker sequence
Ser-Ser-Gly-Ser-Gly-Ser. The fusion gene BD2/3 was digested with
BamHI and EcoRI, and then cloned into the pGEX-4T-1
expression plasmid (Pharmacia Biotech; GE Healthcare, Chicago, IL,
USA), a prokaryotic expression vector containing the Tac promoter
and a glutathione S transferase (GST) tag sequence for fusion
protein expression. Sequence of the resulting plasmid was confirmed
by double restriction enzyme digestions and sequencing, and
designated as pGB2B3.
 | Table I.Primers of BD3 and BD2. |
Table I.
Primers of BD3 and BD2.
| Primer | Sequence
(5′-3′) |
|---|
| P1 |
GCCATGGGAATCATAAACACATTACAGAAATATTATTGCAGAGTCAGAGGCGGC |
| P2 |
AGAGTCAGAGGCGGCCGGTGTGCTGTGCTGAGCTGCCTTCCAAAGGAGGAACAGATCGGCAAG |
| P3 |
GAACAGATCGGCAAGTGCTCGACGCGTGGCCGAAAATGCTGCCGAAGAAAGAAAAGCAGCGGT |
| P4 |
GAAAGAAAAGCAGCGGTAGCGGAAGTGGTATAGGCGATCCTGTTACCTGCCTAAAGAGTGGAGCC |
| P5 |
TGCCTAAAGAGTGGAGCCATATGTCATCCAGTCTTTTGCCCTCGTCGGTATAAACAAATTGGAACCTG |
| P6 |
AAACAAATTGGAACCTGCGGTCTCCCTGGAACAAAATGCTGCAAAAAGCCATAAAGATCTGAATTCCG |
Construction of eukaryotic expression
plasmids for fusion BD2/3
The recombinant plasmid pGB2B3 was digested with
BamHI and BglII, and then the target fragments BD2/3
were ligated to VR1020 expression plasmid (Vical, San Diego, CA,
USA). It is a eukaryotic expression vector containing human CMV
promoter and a tissue plasminogen activator (TPA) signal sequence
for secretion. The correct recombinant plasmid was screened by
double restriction enzyme digestions, plasmid PCR and sequencing
(data not shown) and then named VRB2B3.
Bioactivity assay in vitro of fusion
BD2/3 protein expressed by E. coli
E. coli (DH5α) cells transformed with pGB2B3
and pGEX-4T-1 were induced with
isopropyl-β-D-thiogalactopyra-noside (IPTG) to express the fusion
BD2/3 protein which was purified on a GST affinity column (Amersham
Biosciences; GE Healthcare). The bioactivity of the fusion protein
was measured by inhibition of 4 standard pathogen strains (E.
coli ATCC25922, S. aureus ATCC 26112, S.
pneumoniae ATCC49619 and P. aeruginosa ATCC10211).
Minimum inhibition concentration
(MIC), minimal bactericide concentration (MBC) of fusion BD2/3
protein expressed by E. coli
Broth dilution methods were carried out to determine
the MIC of fusion BD2/3 protein against bacterial cultures of
5×105 CFU/ml (24). MBCs
were determined by transferring 100 µl samples from clear wells
onto agar plates without antibiotics. The MBC was the lowest
concentration at which there was no visible microbial growth.
Large-scale preparation of recombinant
VRB2B3
A single colony of E. coli containing the
recombinant VRB2B3 plasmid was inoculated in Luria Bertani (LB)
broth with kanamycin (100 mg/ml), with shaking at 37°C overnight.
Plasmid DNA was extracted following large-scale alkaline lysis and
precipitation by the spermine method (19), then suspended in sterile saline water
and stored at 20°C until use.
Preparation of LP
Lecithin, cholesterol, octadecylamine,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and
dimethyldistearylammonium bromide (DDAB) were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
A mixture of 3 mg lecithin, 1.7 mg cholesterol and
0.5 mg octadecylamine (30:17:5 by weight) in 10 ml chloroform was
added to a 250 ml round bottom flask and evaporated under vacuum in
a rotary evaporator at 37°C, forming a thin film on the inner
surface. The 20 ml of ddH2O was added at 37°C and the
flask was shaken with intermittent sonication in a bath
sonicator.
Preparation of nanoparticles using LP
modified CS
Five different delivery systems (CS, PEG-O-CS-PEI,
LP, PCL and PCL-protamine) with and without entrapped VRB2B3
(VRB2B3-CS, VRB2B3-PEG-O-CS-PEI, VRB2B3-LP, VRB2B3-PCL, VR
B2B3-PCL-protamine) were prepared by the ionotropic gelation method
(26). Briefly, biomaterials (CS,
PEG-O-CS-PEI, LP, PCL and PCL-protamine) were diluted,
respectively, by buffer CH3COOH/CH3COONa (pH
5.5) containing triphosphate and heated for 10 min at 65°C with
mild magnetic stirring. Then, the solution of plasmid was added
slowly to the solution of biomaterial drop by drop, and the mixed
solution was remixed and left for 5 min. The average diameter and
zeta potential of the polymeric micelles were detected by Zetasizer
3000 HS/IHPL (Malvern Instruments Ltd., Malvern, UK).
Polyamine cationic liposomes (PCL) were prepared
using 30 mg DOPE, 10 mg cholesterol and 10 mg DDAB per round bottom
flask and were used to produce PCL as described above.
PCL/protamine formulations were prepared by adding protamine to the
PCL (Vprotamine:VPCL = 1.5:1). CS (95%
deacylated, MW = 150 kDa) was supplied by Chengdu Organic Chemistry
Institute of China Academy of Science;
polyethyleneglycol-O-chitosan-polyethyleneimine (PEG-O-CS-PEI) was
provided by the College of Chemistry of Sichuan University
(25).
Agarose gel electrophoresis assay of
nanoparticles
The DNA binding ability of biomaterials (CS,
PEG-O-CS-PEI, LP, PCL and PCL-protamine) were evaluated by agarose
gel electrophoresis. The nanoparticle solutions of plasmid DNA with
biomaterials (CS, PEG-O-CS-PEI, LP, PCL and PCL-protamine)
copolymer were loaded into individual wells of 0.7% agarose gel,
electrophoresed at 100 V for 45 min and stained with 0.01%
gold-view. The plasmid migration pattern was revealed under UV
irradiation.
Transfection and efficiency analysis
of fusion BD2/3 gene in eukaryotic cells in vitro
293 cells (human embryonic kidney cells; ATCC no.
CRL-1573TM) were purchased from the Chinese Academy of Science Cell
bank (Shanghai, China). 293 cells were cultured in 6 well plates
(1.5×106 cells/well) for 24 h and grown in 2 ml
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 4.0 mM L-glutamine,
10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin (Thermo
Fisher Scientific, Inc.), and maintained at 37°C in a 5%
CO2 humidified incubator (Sanyo Electric Co., Ltd.,
Tokyo, Japan) until the cell confluency of 293 achieved 80%. The
complexes of nanoparticles containing CS, PEG-O-CS-PEI, LP, PCL and
PCL-protamine each containing 5 µg VRB2B3 plasmid were added into
each well to transfect cells, respectively. Then, the transfected
cells were incubated for 48 h and the supernatants were collected
for antibacterial bioactivity detection (E. coli and S.
Pneumoniae) of fusion BD2/3.
Animal inoculation and challenge with
nanoparticles
Sixty female 3-week-old healthy Kunming mice (Animal
Center of Sichuan University) were randomly divided into 6 groups
(5 treatment groups and an untreated control). Mice in the
treatment groups were injected intramuscularly (quadriceps) with
150 µg plasmids, respectively, in VRB2B3-LP (A group), VRB2B3-CS (B
group), VRB2B3-PEG-O-CS-PEI (C group), VRB2B3-PCL (D group) or
VRB2B3-PCL-protamine (E group). Controls received 150 µg blank
plasmid VR1020-CS. Blood samples were collected by tail vein 0, 7,
14 and 21 days post-injection (p.i.). Two weeks after injection,
all the experimental mice were challenged intraperitoneally (i.p.)
with 0.2 ml 3×109 CFU/ml virulent EPEC E. coli
strain O139:K88 (Center of Animal Disease Control of Sichuan
Province). Mice were euthanized at 28 days post-challenge.
The care and use of experimental mice fully complied
with Chinese animal welfare laws, guidelines and regulations.
Immunological assays of immunized
mice
Assay of IgG, IgG1and IgG2a by
sandwich ELISA
Total serum IgG, IgG1 and IgG2a were measured by
mouse Ig ELISA quantitation kits (Bethyl Laboratories, Montgomery,
TX, USA) according to manufacturer's instructions. Capture
antibody-coated 96-well plates were incubated with 100 µl serially
diluted sera samples and standards for 1 h at ambient temperature.
HRP-conjugated goat anti-mouse secondary antibodies IgG, IgG1 and
IgG2a were added to the wells in triplicate and incubated for 1 h
at 37°C. Absorbance was measured in a multi-functional microplate
reader 680 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 450
nm.
Assay of IL-2, IL- 6 and IFN-γ by
sandwich ELISA
Serum IL-2, IL-6 and IFN-γ were assayed by mouse
IL-2, IL-6 and IFN-γ ELISA kits (eBioscience, San Diego, CA, USA),
according to the manufacturer's instructions. The OD450
values of the samples were measured in triplicate by a microplate
reader 680 (Bio-Rad Laboratories, Inc.).
Assay of immune cell quantity in the
peripheral blood
The immune cells of mice were counted using an
automatic Excell™ 22 blood cell counter (Denam Co., New York, NY,
USA).
Assay of CD4 and CD8 T cells by
FCM
Mouse anti-mouse CD4 and CD8 mAbs, labeled with
fluorescein isothiocyanate (FITC) and R-phycoerythrin (R-PE),
respectively, were purchased from SouthernBiotech (Birmingham, AL,
USA). A total of 2 µl of FITC-conjugated anti-CD4 and 2 µl R-PE
labeled anti-CD8 were added to 1.0 ml EDTA-stabilized blood and
incubated for 30 min. Erythrocytes were lysed and the remaining
cells were washed. Two-color-stained samples were analyzed using a
FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ). Cells
labeled with a single conjugated mAb served as controls. For each
sample, 2×104 cells were analyzed by Cell-Quest™
software. The absolute numbers of each T subpopulation in
peripheral blood were calculated as follows: absolute number
(106/ml) = (% positive cells of all cells analyzed ×
WBC)/100.
Statistical analysis
Data from all the groups were presented as mean ±
SD. Statistical analysis between groups was performed using
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Construction of fusion gene of BD2 and
BD3
As shown in Fig. 1, a
band of ~300 bp was detected by agarose gel electrophoresis
following double digestion and plasmid PCR (Fig. 1A and B), indicating that fusion BD2/3
had been successfully inserted into pGEX-4T-1 vector. Sequencing of
pGB2B3 confirmed this result (data not shown). SDS-PAGE analysis
(Fig. 1C) revealed that GST-tagged
fusion proteins were successfully induced to express mainly in the
supernatant of lysed cultures.
 | Figure 1.Electrophoretic and SDS-PAGE analysis
of recombinant pGB2B3. (A) Electrophoretic identification of
digested pGB2B3 recombinant (1.0% agarose gel). Lane M, DL2000
marker; lane 1, pGB2B3/BamHI+EcoRI; lane 2, pGEX-4T-1 plasmid. (B)
Electrophoresis of the PCR product from pGB2B3 (1.0% agarose gel).
Lane M, 50 bp marker; lane 1, PCR product of pGB2B3 plasmid; lane
2, PCR product of pGEX-4T-1 plasmid. (C) SDS-PAGE analysis of
GST-tagged BD2/3 fusion protein expression. Lane M, protein MW
marker; lane 1, expression products of pGEX-4T-1 induced by IPTG;
lane 2, expression products of pGB2B3 induced by IPTG; lane 3,
supernatant of lysed culture of pGB2B3 induced by IPTG; lane 4,
pellet of lysed culture of pGB2B3 induced by IPTG; lane 5,
expression products of pGB2B3 without IPTG induction. BD,
β-defensin; IPTG, isopropyl-β-D-thiogalactopyranoside; GST,
glutathione S transferase. |
Antimicrobial activity of fusion BD2/3
protein in vitro
Fig. 2 and the values
of MIC and MBC (Table II)
demonstrated that the fusion BD2/3 protein induced by IPDG
significantly inhibited the proliferation of Gram-positive bacteria
(S. aureus, S. pneumoniae) and Gram-negative bacteria (P.
aeruginosa, E. coli) in comparison with controls and the GST
group (P<0.05). Compared with the ampicillin control group, the
fusion BD2/3 protein had higher antimicrobial activity against
Gram-negative bacteria. That means the fusion BD2/3 protein can be
used as an anti-bacterial infection drug.
 | Table II.The MIC and MBC of fusion BD2/3
antibacterial peptide. |
Table II.
The MIC and MBC of fusion BD2/3
antibacterial peptide.
| Bacterial
strain | MIC (µg/ml) | MBC (µg/ml) |
|---|
| S. aureus
ATCC26112 | 2 | 4 |
| S.
pneumoniae ATCC49619 | 2 | 8 |
| P.
aeruginosa ATCC27853 | 2 | 8 |
| E. coli
ATCC25922 | 2 | 8 |
Characterization of nanoparticles
Observation by Zetasizer 3000 HS/IHPL (Malvern
Instruments Ltd., Malvern, UK) revealed that most of the
liposomes/polymer VRB2/3 were spherical nanoparticles ranging from
178–381 nm with zeta potentials +11.1–22.1 mV (Table III), confirming that the DNA was
fully entrapped in the nanoparticles and indicating an almost 100%
package rate. Through agarose gel electrophoresis assay of
nanoparticles, the condensation capability of CS, PEG-O-CS-PEI, LP,
PCL and PCL-protamine with DNA were evaluated by measuring the
emitted fluorescence when adding the goldview into the
nanoparticles. It was shown that the complexes were positively
charged when the mass ratios were 20:1, while the migration of DNA
plasmid was suppressed completely when the mass ratio of CS,
PEG-O-CS-PEI, LP, PCL and PCL-protamine to DNA was 20:1. It showed
that DNA plasmids were entrapped into CS, PEG-O-CS-PEI, LP, PCL and
PCL-protamine successfully.
 | Table III.Size and zeta potential of the
nanoparticles. |
Table III.
Size and zeta potential of the
nanoparticles.
| Sample | Zeta potential
(mV) | Size (nm) |
|---|
| VRB2B3-LP | +17.8±0.62 | 335±16.2 |
| VRB2B3-CS | +15.8±0.66 | 230±12.3 |
|
VRB2B3-PEG-O-CS-PEI | +21.9±0.92 | 178±10.5 |
| VRB2B3-PCL | +22.1±0.69 | 350±14.6 |
|
VRB2B3-PCL-protamine | +11.1±0.33 | 381±17.9 |
Expression efficiency of nanoparticles
in vitro
Compared with those in the blank plasmid-CS control
group, the antibacterial activity against E. coli and S.
pneumoniae of BD2/3 significantly increased in VRB2B3-LP,
VRB2B3-CS, VRB2B3-PEG-O-CS-PEI, VRB2B3-PLC and VRB2B3-PLC-protamine
groups (P<0.05). Furthermore, the antibacterial ability against
E. coli of BD2/3 in VRB2B3-PEG-O-CS-PEI group was
significantly higher than those in the other groups. The
antimicrobial activity against S. pneumoniae was also
significantly raised in VRB2B3-CS and VRB2B3-PEG-O-CS-PEI groups
(P<0.05), though not significantly raised between them
(P>0.05) (Fig. 3). These results
demonstrated that VRB2B3 was successfully transfected into 293
cells by the five gene delivery systems, LP, CS, PEG-O-CS-PEI, PC
and PCL-protamine. Of these, the expression efficiency of
PEG-O-CS-PEI was the highest.
Quantitation of IgG, IgG1 and
IgG2a
The level of IgG significantly improved in sera of
treated mice compared with that of the blank control from 21 days
post-injection (p.i.), and IgG1 and IgG2a significantly increased
from 14 days p.i. with the dominant increase in IgG2a (P<0.05),
while there was no significant difference between treatment groups
A to E, the amount of IgG, IgG1 and IgG2a in group C was higher
than those in the other groups (Fig.
4). Fig. 4D shows that there was
no difference in IgG1/IgG2a ratios after intramuscular
administration of plasmid nanoparticles.
 | Figure 4.Levels of IgG (A), IgG1 (B) and IgG2a
(C) in the sera of experimental mice. Group A, B, C, D and E were
intramuscularly injected with VRB2B3-LP, VRB2B3-CS,
VRB2B3-PEG-O-CS-PEI, VRB2B3-PCL and VRB2B3-PCL-protamine,
respectively; group F was the blank control group. (D) IgG1/IgG2a.
No obvious change was found compared with that in blank control
group, which indicated Th1/Th2 homeostasis during the whole
experiment. The sign ($) indicates the challenge day with virulent
EPEC E. coli. LP, liposomes; CS, chitosan; PEG-O-CS-PEI,
polyethylene glycol-O-chitosan-polyethylenimine; PCL, polyamine
cationic liposomes. |
Cytokine levels in treated mice
Compared with those of the controls, the levels of
IL-2, IL-6 and IFN-γ were significantly increased in the sera of
treated mice from 7 days p.i. (Fig.
5; P<0.05). The levels of IL-2 and IFN-γ of group C were
higher than those in group A, B, D and E, while the differences in
groups A, B, D and E were not significant (P>0.05). After
challenged with virulent bacteria, cytokine levels in treated
groups were still significantly higher than those in controls
except for IL-2 at 14 days post-challenge.
Effect on immune cell numbers
Fig. 6A shows that
lymphocyte numbers were significantly increased in treated mice
from 7 days p.i. onwards (P<0.05), and remained high
post-challenge. As shown in Fig. 6B and
C, neutrophil and monocyte counts were increased to different
degrees following treatment except for neutrophils at 7 days
post-challenge, but not significantly (P>0.05).
Response to challenge
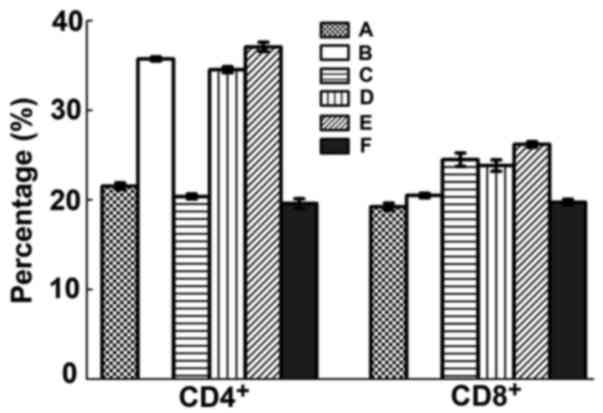
CD4 and CD8 positive T cells
After challenge, levels of CD4+ Th cells
of B, D and E groups were significantly increased (P<0.05) but
it was not significant between groups (P>0.05). CD8+
Tc cell counts were also significantly raised in group B, C, D
(P<0.05) but again not significant between groups (P>0.05)
(Fig. 7).
Detection of the post challenged
mice
Following i.p. injection with virulent EPEC E.
coli for 2 weeks, most mice injected with VRB2B3 packaged in
all the tested forms survived without symptoms while the controls
became lethargic and developed severe diarrhea (Table IV). Moreover, gross pathological
inspection showed that the organs and tissues of surviving treated
mice were normal. In contrast, control mice died of infection
presented with visible lesions, including necrosis of the liver and
spleen, bleeding of the stomach, and duodenal and jejunal catarrh,
with E. coli being isolated from the affected organs by
microbiological culture.
 | Table IV.Effect of challenge with virulent
EPEC E. coli. |
Table IV.
Effect of challenge with virulent
EPEC E. coli.
| Group | Challenge
number | Morbidity | Mortality | Survival rate
(%) |
|---|
| A | 10 | 2 | 2 | 80 |
| B | 10 | 1 | 0 | 100 |
| C | 10 | 0 | 0 | 100 |
| D | 10 | 1 | 1 | 90 |
| E | 10 | 1 | 1 | 90 |
| F | 10 | 10 | 10 | 0 |
Discussion
In this study, a novel fusion gene BD2/3 was
successfully constructed, containing sequences from human BD-2 and
BD3 genes, which individually had been found to exert significant
antimicrobial ability and immunological stimulatory activity
(4,5,7). Fusion
gene BD2/3 contained 304 bases and encoded 93 amino acids. The
molecular weight of the BD2/3 expressed fusion protein was 11.2
kDa. We proved that this novel fusion protein displayed remarkable
antibacterial bioactivity in vitro and immunological
enhancement in vivo. By antimicrobial assay, the results
have demonstrated a significant reduction of bacterial growth in
vitro. Most notably, the antimicrobial activity of BD2/3 fusion
protein against gram negative pathogen was more potent than that of
ampicillin, indicating its potential as a candidate for new
antibiotic drugs.
In BD2/3 plasmid-injected mice, the levels of IgG,
IgG1 and IgG2a increased considerably, so as levels of IL-2, IL-6
and IFN-γ. Moreover, CD4+ Th and CD8+ Tc cell
counts were also significantly elevated in all treated groups after
EPEC E. coli challenge. These observations clearly showed
that BD2/3 was able to promote the specific immune response in
mice, and to provide robust immune protection of mice against
pathogenic infection.
When treated mice were challenged i.p. with EPEC
E. coli at 14 days p.i., levels of all measured parameters
(immunoglobulins, interleukins and immune cells) were increased to
different extents, illustrating the potent enhancement of immunity
by BD2/3. Immune responses are mainly regulated by the activity of
two functional T helper cell types, Th1 and Th2, Th1 cells
primarily promote cellular immune responses and Th2 cells generally
drive humoral immunity (27), and
appropriate Th2/Th1 balance is critical for maintenance of
homeostasis. Many diseases are induced by the skewing of Th2/Th1
ratios (28). Dominant Th1 responses
may contribute to diseases such as rheumatoid arthritis (RA)
(29), multiple sclerosis (MS)
(30) and type 1 diabetes (31). Atopic allergy is an example of Th2
dominance (32). The ratio of
IgG1/IgG2a is usually interpreted as a reflection of different T
helper cell (Th2-Th1) reactivity. Over the course of our
experiments these ratios did not reveal any significant difference
following treatment, indicating that BD2/3 did not influence
Th2/Th1 balance. Therefore, we conclude that BD2/3 can enhance the
immunity of mice to a remarkable degree and display the same
biological safety as defensins in nature.
To raise the low efficiency of gene expression and
to achieve stable effective gene delivery systems in vivo,
the BD2/3 recombinant plasmid was incorporated into 5 different
gene carriers: LP, CS, PEG-O-CS-PEI, PCL and PCL-protamine.
Although there was no clear difference between them, except for
better transfection efficiency and immune response in group C
(VRB2B3-PEG-O-CS-PEI), all 5 carriers resulted in highly
significant increased antibacterial bioactivity in HEK293 cells and
enhancements of immunity both pre- and post-challenge. Based on
these findings, it can be concluded that LP, CS, PEG-O-CS-PEI, PC
and PCL-protamine are all effective gene delivery vectors in
vitro and in vivo, while PEG-O-CS-PEI was the most
effective and practicable. This may be attributed to the
modification of PEI ligation on CS. PEI is known to improve
transfection efficiency of genes in vivo by enhancing
cellular endocytosis and escape from lysosomes, and its coupling
with CS may extend the expression time of wrapped gene to prolong
blood circulation time and reduce reticuloendothelial clearance.
Previous studies from our laboratory have also shown that the
expression efficiency of plasmids can be improved by entrapment
with PEG-O-CS-PEI nanoparticles in vitro (20,25).
Furthermore, none of the 5 treated mouse groups
displayed any systemic or local symptom and lesion, such as local
injuries in injected sites, fever or loss of weight gain following
intraperitoneal challenge with EPEC E. coli. Whereas,
control mice exhibited severe gross lesions. This indicates that
the BD2/3-PEG-O-CS-PEI and other nanoparticles have the potential
to be applied as safe and effective gene delivery systems.
In conclusion, our results suggest that the novel
fusion BD2/3 is a safe and effective molecule not only to inhibit
bacteria pathogens directly but also to enhance the immunity of
mice against infection. In particular, the recombinant gene packed
within PEG-O-CS-PEI nanoparticles has shown promise for development
as a novel effective and biocompatible delivery system for control
of infection caused by antibiotic-resistant pathogens.
Acknowledgements
We sincerely thank Dr Gang Wang (National
Engineering Research Center for Biomaterials) for technical
support.
Funding
This study was supported by the National Natural
Science Foundation of China (no. 30871855), National International
Cooperation Program (no. 2011DFA10101103) and key project from
Sichuan Province of China (2016NYZ0042).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW, JC, CC, ZW and RG conceived and designed the
study. XW, JC, CC, HZ, SZ, JL and XL were responsible for the
collection and analysis of the patient data. XW, JC, ZW and RG
interpreted the data and drafted the manuscript. CC and RG revised
the manuscript critically for important intellectual content. All
authors read and approved the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Sichuan University (Chengdu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Norrby SR, Nord CE and Finch R; European
Society of Clinical Microbiology and Infectious Diseases, : Lack of
development of new antimicrobial drugs: A potential serious threat
to public health. Lancet Infect Dis. 5:115–119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hegstad K, Langsrud S, Lunestad BT, Scheie
AA, Sunde M and Yazdankhah SP: Does the wide use of quaternary
ammonium compounds enhance the selection and spread of
antimicrobial resistance and thus threaten our health? Microb Drug
Resist. 16:91–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spellberg B, Blaser M, Guidos RJ, Boucher
HW, Bradley JS, Eisenstein BI, Gerding D, Lynfield R, Reller LB,
Rex J, et al: Infectious Diseases Society of America (IDSA):
Combating antimicrobial resistance: Policy recommendations to save
lives. Clin Infect Dis. 52 Suppl 5:S397–S428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ganz T: Defensins: Antimicrobial peptides
of innate immunity. Nat Rev Immunol. 3:710–720. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Selsted ME and Ouellette AJ: Mammalian
defensins in the antimicrobial immune response. Nat Immunol.
6:551–557. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Midorikawa K, Ouhara K, Komatsuzawa H,
Kawai T, Yamada S, Fujiwara T, Yamazaki K, Sayama K, Taubman MA,
Kurihara H, et al: Staphylococcus aureus susceptibility to innate
antimicrobial peptides, β-defensins and CAP18, expressed by human
keratinocytes. Infect Immun. 71:3730–3739. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang D, Biragyn A, Kwak LW and Oppenheim
JJ: Mammalian defensins in immunity: More than just microbicidal.
Trends Immunol. 23:291–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang D, Chertov O, Bykovskaia SN, Chen Q,
Buffo MJ, Shogan J, Anderson M, Schröder JM, Wang JM, Howard OM, et
al: β-defensins: Linking innate and adaptive immunity through
dendritic and T cell CCR6. Science. 286:525–528. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa
H and Nagaoka I: Epithelial cell-derived human β-defensin-2 acts as
a chemotaxin for mast cells through a pertussis toxin-sensitive and
phospholipase C-dependent pathway. Int Immunol. 14:421–426. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
García JR, Jaumann F, Schulz S, Krause A,
Rodríguez-Jiménez J, Forssmann U, Adermann K, Klüver E, Vogelmeier
C, Becker D, et al: Identification of a novel, multifunctional
β-defensin (human β-defensin 3) with specific antimicrobial
activity. Its interaction with plasma membranes of Xenopus oocytes
and the induction of macrophage chemoattraction. Cell Tissue Res.
306:257–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng R, Feng F, Meng F, Deng C, Feijen J
and Zhong Z: Glutathione-responsive nano-vehicles as a promising
platform for targeted intracellular drug and gene delivery. J
Control Release. 152:2–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu SP, Zhou L, Lakshminarayanan R and
Beuerman RW: Multivalent antimicrobial peptides as therapeutics:
Design principles and structural diversities. Int J Pept Res Ther.
16:199–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gan Q, Wang T, Cochrane C and McCarron P:
Modulation of surface charge, particle size and morphological
properties of chitosan-TPP nanoparticles intended for gene
delivery. Colloids Surf B Biointerfaces. 44:65–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L and Xu B: Multivalent vancomycins and
related antibiotics against infectious diseases. Curr Pharm Des.
11:3111–3124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Z, Deshazer H, Rice AJ, Chen K, Zhou C
and Kallenbach NR: Multivalent antimicrobial peptides from a
reactive polymer scaffold. J Med Chem. 49:3436–3439. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arnusch CJ, Branderhorst H, de Kruijff B,
Liskamp RM, Breukink E and Pieters RJ: Enhanced membrane pore
formation by multimeric/oligomeric antimicrobial peptides.
Biochemistry. 46:13437–13442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Young AW, Liu Z, Zhou C, Totsingan F,
Jiwrajka N, Shi Z and Kallenbach NR: Structure and antimicrobial
properties of multivalent short peptides. MedChemComm. 2:308–314.
2011. View Article : Google Scholar
|
|
18
|
Roy K, Mao HQ, Huang SK and Leong KW: Oral
gene delivery with chitosan - DNA nanoparticles generate
immunologic protection in a murine model of peanut allergy. Nat
Med. 5:387–391. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Templeton NS, Lasic DD, Frederik PM, Strey
HH, Roberts DD and Pavlakis GN: Improved DNA: Liposome complexes
for increased systemic delivery and gene expression. Nat
Biotechnol. 15:647–652. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Almofti MR, Harashima H, Shinohara Y,
Almofti A, Baba Y and Kiwada H: Cationic liposome-mediated gene
delivery: Biophysical study and mechanism of internalization. Arch
Biochem Biophys. 410:246–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Z, Wan X, Zhang W, Wang Z, Peng R, Tao
F, Cai L, Li Y, Jiang Q and Gao R: Synthesis of biodegradable
polycationic methoxy poly (ethylene
glycol)-polyethylenimine-chitosan and its potential as gene
carrier. Carb Polym. 78:46–53. 2009. View Article : Google Scholar
|
|
22
|
Li ZT, Guo J, Zhang JS, Zhao YP, Lv L,
Ding C and Zhang XZ: Chitosan-graft-polyethylenimine with improved
properties as a potential gene vector. Carb Polym. 80:254–259.
2010. View Article : Google Scholar
|
|
23
|
Horton RM, Hunt HD, Ho SN, Pullen JK and
Pease LR: Engineering hybrid genes without the use of restriction
enzymes: Gene splicing by overlap extension. Gene. 77:61–68. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wiegand I, Hilpert K and Hancock RE: Agar
and broth dilution methods to determine the minimal inhibitory
concentration (MIC) of antimicrobial substances. Nat Protoc.
3:163–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu YY, Wang Z, Cai L, Wang G, Yang X, Li Y
and Gao R: Synthesis and characterization of methoxy poly(ethylene
glycol)-O-chitosan-polyethylenimine for gene delivery. Carb Polym.
81:269–274. 2010. View Article : Google Scholar
|
|
26
|
Bodmeier R, Chen HG and Paeratakul O: A
novel approach to the oral delivery of micro- or nanoparticles.
Pharm Res. 6:413–417. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mosmann TR and Sad S: The expanding
universe of T-cell subsets: Th1, Th2 and more. Immunol Today.
17:138–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kidd P: Th1/Th2 balance: The hypothesis,
its limitations, and implications for health and disease. Altern
Med Rev. 8:223–246. 2003.PubMed/NCBI
|
|
29
|
Yamada H, Nakashima Y, Okazaki K, Mawatari
T, Fukushi JI, Kaibara N, Hori A, Iwamoto Y and Yoshikai Y: Th1 but
not Th17 cells predominate in the joints of patients with
rheumatoid arthritis. Ann Rheum Dis. 67:1299–1304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lassmann H and Ransohoff RM: The CD4-Th1
model for multiple sclerosis: A crucial re-appraisal. Trends
Immunol. 25:132–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kis J, Engelmann P, Farkas K, Richman G,
Eck S, Lolley J, Jalahej H, Borowiec M, Kent SC, Treszl A, et al:
Reduced CD4+ subset and Th1 bias of the human iNKT cells
in Type 1 diabetes mellitus. J Leukoc Biol. 81:654–662. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seki Y, Inoue H, Nagata N, Hayashi K,
Fukuyama S, Matsumoto K, Komine O, Hamano S, Himeno K,
Inagaki-Ohara K, et al: SOCS-3 regulates onset and maintenance of
T(H)2-mediated allergic responses. Nat Med. 9:1047–1054. 2003.
View Article : Google Scholar : PubMed/NCBI
|