Introduction
Liver serves a crucial role in the metabolism of
xenobiotics which may cause numerous hepatic diseases (1). A previous study has indicated that
oxidative stress is a key element to the pathophysiology of various
types of liver damage, including alcoholic and nonalcoholic liver
disease (2), Previous studies have
also confirmed that the reactive oxygen species (ROS) is a
crucially important factor for the initiation of oxidative stress
and that advanced glycation end products (AGEs) are associated with
enhanced oxidative stress (3,4).
D-galactose is a common oxidative stress model inducer and the
tissues of animals with chronic D-gal administration induce
oxidative damage and AGE formation (5). Oxidative stress causes the increase of
liver cell membrane permeability, releasing ALT and AST into the
blood, making these parameters suitable biochemical markers of
liver damage (6). Furthermore, other
biomarkers including superoxide dismutase (SOD), 8-hydroxy-2
deoxyguanosine (8-OH-dG), malondialdehyde (MDA), glutathione (GSH),
glutathione peroxidase (GSH-Px), may be used to assess oxidative
stress in liver disease (7).
Traditional treatment methods usually include
administration of nucleoside analogues, interferon and antiviral
drugs (8). Synthetic drugs for the
treatment of liver diseases can lead to severe side-effects
(9), and, therefore, herbal
medication may be an alternative treatment method. There is a long
history of using traditional Chinese medicines in treatment of
liver diseases (10). Ginsenoside
Rg1 (Rg 1) is one of the most active ingredients of ginseng which
induces antiaging and antioxidative effects, as well as improves
immunity and memory (11,12). Previous studies have investigated the
effect of Rg1 on liver damage and demonstrated that this compound
reduces apoptosis, and inflammatory response to protect the liver
from ischemia reperfusion injury and type 2 diabetes (13,14). Rg1
also enhanced the antioxidative defense system to ameliorate carbon
tetrachloride-induced liver injury in rats (15). The present study used a mouse model
of D-gal-induced liver injury to elucidate the function and the
underlying mechanism of Rg1.
Materials and methods
Animals
SPF male C57BL/6J mice (weight, 16±2 g; n=30; age,
6–8 weeks) were purchased from the Medical and Laboratory Animal
Center of Chongqing University (Chongqing, China) and housed at a
temperature of 22–24°C, a humidity of 40–70% and a 12 h light/dark
cycle, with free access to water and food. All animal experiments
were performed in accordance with the institutional and national
guidelines and regulations, and approved by the Chongqing Medical
University Animal Care and Use Committee.
Reagents
Rg1 (purity >95%) was purchased from Hongjiu
Biotech Co., Ltd., (Tonghua, China). D-galactose (D-gal; purity
>99%) was obtained from Sangon Biotech Co., Ltd. (Shanghai,
China). The senescence-associated β-galactosidase (SA-β-gal)
Staining kit was purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA), whereas the SOD, GSH-Px, GSH and MDA detection
kits were purchased from Nanjing Jiancheng Bioengineering Institute
(Nanjing, China). The AGEs (cat. no. abx512406) and 8-OH-dG (cat.
no. SKT-120-480) kits were purchased from Shanghai Yuanye
Biological Technology Co., Ltd. (Shanghai, China).
Mouse D-gal aging model and treatment
groups
A total of 30 mice were randomly divided into three
groups: Control group, D-gal-administration group (D-gal group) and
D-gal-administration plus Rg1 treatment group (D-gal + Rg1 group).
In the D-gal group, D-gal (120 mg/kg body weigh/day) was injected
intraperitoneally into mice for 42 days. In the D-gal + Rg1 group,
120 mg/kg body weigh/day D-gal was also administered to mice
intraperitoneally and Rg1 (20 mg/kg body weight/day) was
concomitantly injected for 35 days from the 8th day of the D-gal
injection. All control animals were given an equal volume of saline
intraperitoneally. On the 43rd day of the experiment, the animals
were weighted (27.20±1.37 g) and anesthetized by intraperitoneal
injection of sodium pentobarbital at a dose of 40 mg/kg. The blood
was collected from the retro-orbital plexus (0.3 ml per mice) and
liver samples were dissected and weighted immediately. The
anesthetized mice were sacrificed by blood loss from heart puncture
(0.6±0.1 ml per mice) until the mice succumbed to mortality
confirmed by muscle relaxation and no respiratory circulation. The
blood and animal carcass were collected and returned to Medical and
Laboratory Animal Center of Chongqing for safe disposal.
Detection of hepatic index
The hepatic indices of each group were calculated
using the following equation: Hepatic index=liver weight (mg)/body
weight (g).
Biochemical determination
The collected blood was allowed to clot and serum
was separated by centrifugation at a speed of 1,200 × g for 15 min
at 4°C. Serum biochemical parameters of liver function including
alanine aminotransferase (ALT), aminotransferase (AST), albumin
(Alb) and total bilirubin (TBiL) were analyzed by the Department of
Laboratory Medicine of the First Affiliated Hospital Group of
Chongqing Medical University (Chongqing, China).
Histological examination
For histological studies, the liver tissues were
fixed with 4% paraformaldehyde at room temperature for 24 h,
dehydrated in graded (50–100%) alcohol series and embedded in
paraffin. Thin sections (5 µm) were cut and placed into a glass
dish with hematoxylin. Following agitation for 30 sec at room
temperature, slides were washed using water for 1 min. Slides were
then stained with 1% eosin for 30 sec at room temperature. Sections
were then dehydrated using alcohol and washed with xylene. One or
two drops of mounting medium was then placed onto samples and
covered with a coverslip. Samples were observed using bright field
microscopy (magnification, ×200) to observe sections. The initial
examination was qualitative, with the purpose of determining
histopathological lesions of the liver tissue. For transmission
electron microscopy (TEM) liver tissues were fixed with 2.5%
buffered glutaraldehyde for 4 h at 4°C. Tissues were subsequently
washed three times for 15 min with PBS. Tissues were then fixed
with 1% osmium for 1 h at 4°C and dehydrated with different
concentrations of alcohol (50, 70, 80, 90 and 95%) for 15 min each.
then infiltration and embedding tissues at 70°C overnight. Tissues
were then sliced into 100 nm sections and stained with 0.5%
toluidine blue for 30 min at 60°C. Samples were observed using
TEM.
SA-β-gal cytochemical staining
The SA-β-gal staining was carried out according to
the manufacturer's protocol of the SA-β-gal Staining kit. The
preparation of liver tissue paraffin sections (5 µm) was performed
as aforementioned. Briefly, slides were washed twice for >5 min
using PBS, fixed with the Fixative Solution for 15 min at room
temperature and stained by X-Gal Staining Solution (100 mM
sodiumphosphate, 2 mM MgCl2, 150 mM sodium chloride,
0.01% sodium deoxycholate, 0.02% NP-40, 5 mM potassium
ferricyanide, 5 mM potassium ferrocyanide and 1 mg/ml X-gal; pH 6)
for 24 h at 37°C without CO2. After the incubation,
sections were washed in PBS, stained with eosin for 30 sec at room
temperature and viewed using bright field microscopy
(magnification, ×200). Quantitative image analysis included at
least 3 random fields of view per slide and was performed in a
blinded manner. The intensity of SA-β-gal-positive staining was
evaluated using the relative optical density (ROD) value. ROD of
SA-β-gal-positive cells in the liver was obtained following
transformation of mean gray values into ROD using the following
formula: ROD=log (256/mean gray value) (16). Images were captured from five
randomly selected fields of view using Image Pro Plus software 6.0
(Media Cybernetics, Inc., Rockville, MD, USA).
Detection of oxidation-associated
biomarkers
The liver was collected and lysed in a −4°C ice bath
for 30 min. The supernatant was collected after centrifugation at
12,000 r/min and 4°C for 15 min. GSH-Px and SOD activity, and GSH
and MDA contents were detected by colorimetric analysis according
to the manufacturer's protocol.
Detection of AGEs and 8-OH-dG in the
liver homogenate by ELISA
The supernatant was collected as described above,
and the levels of AGEs and 8-OH-dG in the liver in each group were
measured by ELISA kits according to the manufacturer's
protocol.
Statistical analysis
Data were analyzed using one-way analysis of
variance followed by a post-hoc Tukey's test for multiple
comparisons. SPSS software (version 17.0; SPSS, Inc., Chicago, IL,
USA) was used to analyze data, which are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of Rg1 on the hepatic index in
a mouse model of hepatic injury
Hepatic indices in the D-gal group were
significantly higher compared with the control and the D-gal + Rg1
groups (P<0.05; Table I).
 | Table I.Effect of Rg1 on hepatic indices. |
Table I.
Effect of Rg1 on hepatic indices.
| Groups | Body weight
(g) | Liver weight
(g) | Hepatic indices
(mg/g) |
|---|
| Normal control | 20.00±1.22 | 1.21±0.45 | 62.43±8.49 |
| D-gal model | 23.85±2.35 | 2.01±0.36 |
83.32±6.32a |
| Rg1 + D-gal
model | 26.54±2.71 | 1.82±0.23 |
71.99±5.40b |
Effect of Rg1 on the liver damage
indexes
Administration of D-gal significantly altered the
biochemical parameters compared with the control group (all
P<0.05; Table II). Treatment
with Rg1 significantly decreased the serum levels of AST, ALT and
TBiL (all P<0.05) and elevated the serum level of Alb
(P<0.05) compared with the D-gal group.
 | Table II.Effect of Rg1 on liver damage
indexes. |
Table II.
Effect of Rg1 on liver damage
indexes.
| Groups | Alb (g/l) | ALT (U/l) | AST (U/l) | TBiL (µmol/l) |
|---|
| Normal control | 31.01±3.67 | 17.9±2.80 | 75.61±2.97 | 0.57±0.12 |
| D-gal model |
24.27±4.70a |
27.73±2.15a |
97.63±3.56a |
1.19±0.17a |
| Rg1 + D-gal
model |
28.94±2.05b |
21.65±1.91b |
76.09±5.32b |
1.04±0.17b |
Effect of Rg1 on liver
histopathology
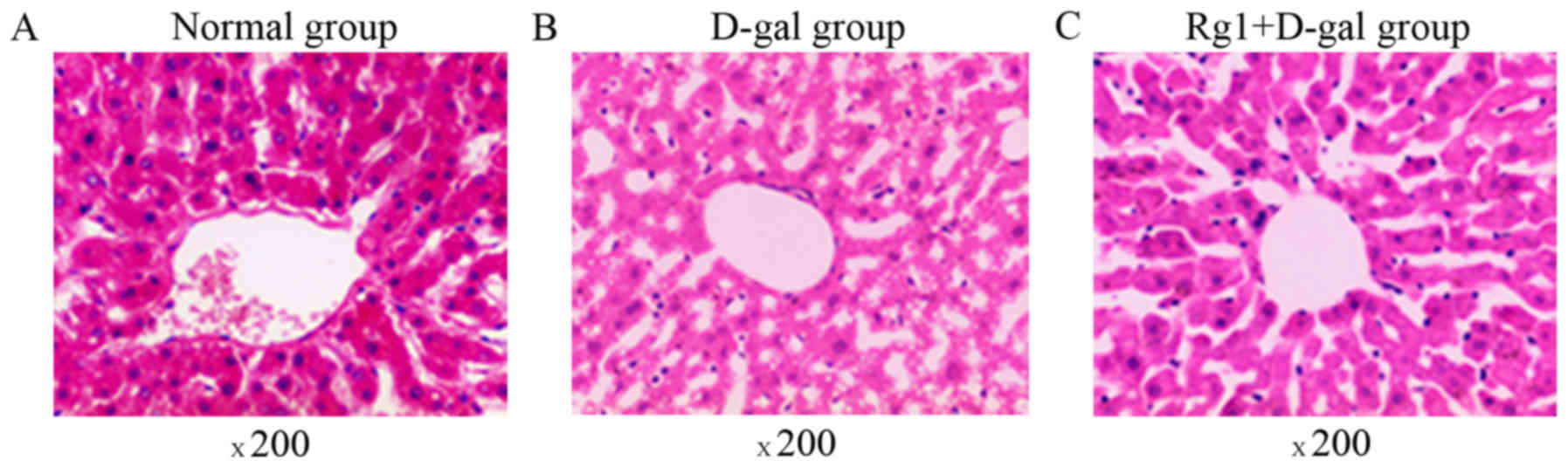
The liver tissue structure in the three groups was
assessed by optical microscopy (Fig.
1). The mice in the control group exhibited normal and intact
lobular architecture and structure of hepatocytes. Histological
examination of livers exposed to D-gal indicated severe liver
damage, including dilated liver sinusoids, disordered arrangement
of hepatocytes, multiple and extensive areas of portal inflammation
and hepatocellular necrosis, as well as an increasing number of
adipose cell. However, these pathological alterations were
attenuated in animals treated with Rg1. TEM was also used to
compare the three groups (Fig. 2).
Numerous free ribosomes were identified in the cytoplasm of the
control group hepatocytes and the cytoplasmic organelles included
mitochondria, rough endoplasmic reticulum (RER) and normal and
activated lysosomes. Following administration with D-gal for 42
days, dense deposits in the cytoplasm of hepatocytes were observed.
An increased number of vacuoles, mitochondrial swelling and
shortened, disrupted RER were observed in the cytoplasm. Clumping
of nuclear chromatin was observed. However, the damage of
hepatocytes was attenuated following treatment with Rg1.
Antioxidative effect of Rg1 in the
mouse model of hepatic injury
Oxidative stress caused by ROS is one of the main
causes of cell injury (17). SOD and
GSH-Px are enzymes that participate in the removal of ROS from the
cellular environment (18,19). MDA is an end-product of ROS-induced
peroxidation and it is widely used as an oxidative stress biomarker
(20); while GSH is the substrate of
GSH-Px. Compared with the control group, SOD and GSH-Px activities,
and GSH content decreased significantly in the D-group liver while
the MDA content increased (all P<0.05; Table III). By contrast, Rg1 rescued the
SOD and GSH-Px activities, lowered the GSH consumption
significantly, and partially reversed the increase in MDA compared
with the D-gal group (Table
III).
 | Table III.Effect of Rg1 on expression levels of
SOD, MDA, GSH-Px and GSH. |
Table III.
Effect of Rg1 on expression levels of
SOD, MDA, GSH-Px and GSH.
| Groups | SOD (U/mg
prot) | MDA (nmol/mg
prot) | GSH-Px (U/ml) | GSH (µmol/mg) |
|---|
| Normal control | 178.47±13.32 | 4.24±1.29 | 49.68±7.91 | 7.80±0.62 |
| D-gal model |
136.32±21.59a |
8.72±2.04a |
33.22±5.44a |
6.63±0.81a |
| Rg1 + D-gal
model |
155.43±13.80b |
6.02±1.15b |
43.22±5.41b |
7.52±0.75b |
Rg1 reduced the SA-β-gal staining in
the liver
Asymmetric ROS are molecules containing unpaired,
highly reactive electrons, leading to cell injury and aging
(21). SA-β-gal is one of commonly
used biomarkers of cell senescence (22), and, therefore, in the present study
the cytoplasm of ageing cells stained blue (Fig. 3; arrows). Few SA-β-gal-positive cells
were observed in the control group (Fig.
3A). The intensity of SA-β-gal staining was evaluated using the
ROD value. D-gal administration induced a significant increase in
the ROD value of the SA-β-gal staining, compared with the control
group (Figs. 3 and 4). However, in the D-gal + Rg1 group, the
ROD value significantly decreased compared with the D-gal group
(Figs. 3 and 4).
Effect of Rg1 on the DNA damage of
hepatocytes
The oxidation of guanine to form 8-OH-dG is a marker
of oxidative DNA damage (23). Based
on the levels of 8-OH-dG, oxidative DNA damage was elevated in the
D-gal group compared with the control group. The level of 8-OH-dG
in mice treated with Rg1 remained stable at a level of 44.49±4.20
ng/ml, and was significantly reduced compared with the D-gal group
(Table IV).
 | Table IV.Effect of Rg1 on 8-OH-dG and AGEs in
the liver of injured model mice. |
Table IV.
Effect of Rg1 on 8-OH-dG and AGEs in
the liver of injured model mice.
| Groups | 8-OH-dG
(ng/ml) | AGEs (ng/ml) |
|---|
| Normal control | 35.52±4.15 | 92.82±19.70 |
| D-gal model |
56.44±3.36a |
140.99±15.95a |
| Rg1 + D-gal
model |
44.49±4.20b | 117.15±12.3 |
Treatment with Rg1 decreased the
levels of AGEs in hepatocytes from the mouse model of liver
injury
AGEs are a group of modified molecular species
formed by non-enzymatic reactions of reducing sugars and proteins,
lipids or nucleic acids (24). The
accumulation of AGEs is associated with hepatocyte damage and the
process of aging (25). The
administration of D-gal increased the liver accumulation of AGEs.
However, treatment with Rg1 decreased the levels of AGEs compared
with the D-gal model (Table
IV).
Discussion
D-gal has been previously used to establish an
experimental model of aging (26). A
previous study has demonstrated that the primary advantage of
long-term D-gal administration to mice is that it mimics the
natural aging effect of the liver (27). The underlying mechanism of
D-gal-induced injury is a decrease in the cellular UTP
concentration leading to the inhibition of RNA and protein
synthesis (28,29). Furthermore, oxidative damage and
inflammation have also been hypothesized to serve roles in
age-associated alterations in the liver (30). Hepatic aging and necrosis induced by
the excessive levels of D-gal are associated with increased
expression levels of oxygen-derived free radicals in mouse
hepatocytes (31). Based on the
results of previous studies, the present study established a
D-gal-induced model to further investigate hepatic injury. In the
present study, liver injury was induced by administration of D-gal
and confirmed by alterations in liver damage indices, oxidative
stress, histopathology, DNA damage, and levels of AGEs. All
D-gal-induced alteration in the liver were significantly attenuated
by administration of Rg1.
Liver damage induced by D-gal disrupts liver cell
metabolism which leads to characteristic alterations in serum
enzyme activity (32). Alterations
in the membrane permeability of liver cells indicate the severity
of hepatocellular damage induced by D-gal (6). In the present study, serum ALT, AST and
TBiL activities increased following treatment with D-gal and
significantly decreased following administration of Rg1.
Administration of Rg1 increased the level of Alb in serum which
decreased following treatment with D-gal. Furthermore, hepatic
indices in the D-gal + Rg1 group were reduced compared with the
D-gal group. The above results indicate that Rg1 may attenuate
liver injury induced by D-gal in mice and therefore serve a
protective role in liver damage.
Histological examination is necessary for
determination of drug-induced liver protection. Tissues and cells
are subjected to oxidative injury when large quantities of free
radicals are generated or the activity of the antioxidant system
deteriorates. The normal lobular architecture and structure of
hepatic cells in the control group was altered in the D-gal group
as indicated by hepatocellular necrosis and cytoplasm vacuolar
degeneration. An improved architecture of the liver tissue was
observed following administration of Rg1. TEM results revealed
large numbers of free ribosomes in hepatocytes of the control
group; cytoplasmic organelles were present and included
mitochondria, RER and normal lysosomes. Following the
administration of D-gal, there were numerous electron-dense
deposits throughout hepatocyte cytoplasm and cytoplasmic organelles
exhibited degenerative signs, including mitochondrial edema,
shortened and disrupted RER, or vacuolation. Clumping of nuclear
chromatin was observed. The D-gal-induced hepatic injury was
attenuated by the administration of Rg1. Therefore, in the present
study, administration of Rg1 served a protective role against the
development of hepatic necrosis.
D-gal contributes to increased oxidative stress and
formation of reactive oxygen species, which may result in
hepatocyte damage or death (33).
Free radicals may damage a number of cellular components including
DNA, proteins and lipids (34,35).
Antioxidation serves a protective role against D-gal-induced liver
injury. SOD, which reduces the levels of superoxide anions and aids
in the synthesis of hydrogen peroxide, is widely distributed in
cells and protects from oxidative damage (36). GSH-Px catalyzes the reduction of
hydrogen peroxide and lipid peroxide to non-toxic products and
scavenges highly reactive lipid peroxides in the aqueous phase of
the cell membrane (37). SOD and
GSH-Px are important enzymes that participate in the removal of ROS
from the cellular environment. GSH, an endogenous antioxidant,
exhibits a particularly high concentration in the liver and is
known for its key function as an electron donor during ROS
metabolism and scavenge (38).
Furthermore, GSH, GSH-Px and SOD decrease the levels of ROS to
prevent the oxidation of proteins, lipids and DNA (39). MDA is an end-product of ROS-induced
peroxidation, and it is commonly used as an oxidative stress
biomarker (40). 8-OH-dG, a product
of DNA oxidation, is a marker of oxidative DNA damage (41). The activity of SOD and GSH-PX, and
the contents of GSH, MDA and 8-OH-dG may be used to measure the
level of oxidative stress in cells (42), and to evaluate the protective effect
of Rg1. In the present study, Rg1 increased the activity of SOD and
GSH-Px and the contents of GSH compared with the D-gal group
(43). Furthermore, treatment with
Rg1 reduced the increase in the expression levels of MDA and
8-OH-dG caused by injury (44).
These results indicated that Rg1 may act by enhancing the activity
of endogenous antioxidative enzymes in cells and reducing the
levels of peroxidation products.
Cell senescence is due to cellular damage caused by
ROS (45). It has been reported that
D-gal causes hepatocyte damage, induces cellular senescence and
results in hepatic injury (46,47).
Lysosomal dysfunction results in accumulation of SA-β-gal in aging
cells, and, therefore, SA-β-gal is one of the most widely used
biomarkers of aging (22).
Furthermore, determination of cellular accumulation of AGEs may
also be used to detect cellular aging (48). Both SA-β-gal and AGEs are common
biomarkers used to evaluate senescence (49,50). The
present study indicated that Rg1 inhibited cellular aging, which
supports the results of a previous study where Rg1 alleviated
senescence of hematopoietic stem/progenitor cells (51). Treatment with D-gal markedly
increased the ROD value of SA-β-gal-positive cells and increased
the accumulation of AGEs compared with the control group.
D-gal-induced alterations were attenuated following treatment with
Rg1. These results of the present suggest that Rg1 may alleviate
the effects of D-gal on liver aging, and the underlying mechanism
may be associated with the reduction of oxidative stress injury in
cells.
In conclusion, the present study indicated that Rg1
improved the resistance of hepatic cells to D-gal-induced subacute
liver injury in an in vivo mouse model. The results
indicated that Rg1 exerted protective effects through its
antioxidative properties, alleviation of DNA damage induced by
chronic oxidative stress and enhanced activity of endogenous
antioxidative defense enzymes. The present study may provide a
theoretical and experimental basis for the application of Rg1 in
the treatment of liver injury.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81673748).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MHX, JYX and YPW conceived and designed the
experiments of the current study. Performed the experiments: JYX,
MHX, ZWL, WXH, YLF, DYJ, JL, PWJ and LW performed the experiments.
JYX, MHX, JL and LW analyzed the data. WL, WXH and YLF provided
reagents, materials and analysis tools. MHX and JX wrote the
manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the institutional and national guidelines and regulations, and
approved by the Chongqing Medical University Animal Care and Use
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kashaw V, Kumar AN and Agarwal A:
Hepatoprotective prospective of herbal drugs and their vesicular
carriers-a review. Int J Res Pharmaceutical Biomedical Sci.
2:360–374. 2011.
|
|
2
|
Lieber CS: Role of oxidative stress and
antioxidant therapy in alcoholic and nonalcoholic liver diseases.
Adv Pharmacol. 38:601–628. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Plumel MI, Benhaim-Delarbre M, Rompais M,
Thiersé D, Sorci G, van Dorsselaer A, Criscuolo F and Bertile F:
Differential proteomics reveals age-dependent liver oxidative costs
of innate immune activation in mice. J Proteomics. 135:181–190.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan HD, Li XZ, Xie JM and Li M: Effects of
advanced glycation end products on renal fibrosis and oxidative
stress in cultured NRK-49F ceils. Chin Med J (Engl). 120:787–793.
2007.PubMed/NCBI
|
|
5
|
Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J,
Packer L and Liu J: Chronic systemic D-galactose exposure induces
memory loss, neurodegeneration, and oxidative damage in mice:
Protective effects of R-alpha-lipoic acid. J Neurosci Res.
83:1584–1590. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ktari N, Nasri R, Mnafgui K, Hamden K,
Belguith O, Boudaouara T, El Feki A and Nasri M: Antioxidative and
ACE inhibitory activities of protein hydrolysates from zebra blenny
(Salaria basilisca) in alloxan-induced diabetic rats. Process
Biochemistry. 49:890–897. 2014. View Article : Google Scholar
|
|
7
|
Arauz J, Ramos-Tovar E and Muriel P: Redox
state and methods to evaluate oxidative stress in liver damage:
From bench to bedside. Ann Hepatol. 15:160–173. 2016.PubMed/NCBI
|
|
8
|
Cordoba J: New assessment of hepatic
encephalopathy. J Hepatol. 54:1030–1040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Triantafyllou K, Vlachogiannakos J and
Ladas SD: Gastrointestinal and liver side effects of drugs in
elderly patients. Best Pract Res Clin Gastroenterol. 24:203–215.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue XL, Wu XY, Xing JM, Li L, Zhao Y, Wang
JJ, Zhang YJ, Wang QB, Tang Y, Li GR, et al: Xiaopiyishen herbal
extract granule improves the quality of life among people with
fatigue-predominant Subhealth and liver-qi stagnation and spleen-qi
deficiency syndrome. Evid Based Complement Alternat Med.
2012:5097052012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng Y, Shen LH and Zhang JT:
Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and
its mechanism of action. Acta Pharmacol Sin. 26:143–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Zhang J, Fang Y, Zhao C and Zhu Y:
Ginsenoside Rg1 delays tert-butyl hydroperoxide-induced premature
senescence in human WI-38 diploid fibroblast cells. J Gerontol A
Biol Sci Med Sci. 63:253–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tao T, Chen F, Bo L, Xie Q, Yi W, Zou Y,
Hu B, Li J and Deng X: Ginsenoside Rg1 protects mouse liver against
ischemia-reperfusion injury through anti-inflammatory and
anti-apoptosis properties. J Surg Res. 191:231–238. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian W, Chen L, Zhang L, Wang B, Li XB,
Fan KR, Ai CH, Xia X, Li SD and Li Y: Effects of ginsenoside Rg1 on
glucose metabolism and liver injury in streptozotocin-induced type
2 diabetic rats. Genet Mol Res. 16:2017. View Article : Google Scholar
|
|
15
|
Qi B, Zhang S, Guo D, Guo S, Jiang X and
Zhu X: Protective effect and mechanism of ginsenoside Rg1 on carbon
tetrachloride-induced acute liver injury. Mol Med Rep.
16:2814–2822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garcia G, Tagliaferro P, Ferri A, de
Duffard Evangelista AM, Duffard R and Brusco A: Study of tyrosine
hydroxylase immunoreactive neurons in neonate rats lactationally
exposed to 2,4-dichlorophenoxyacetic acid. Neurotoxicology.
25:951–957. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chandra K, Salman AS, Mohd A, Sweety R and
Ali KN: Protection against FCA induced oxidative stress induced DNA
damage as a model of arthritis and In vitro anti-arthritic
potential of Costus speciosus Rhizome extract. Int J Pharmacognosy
Phytochemical Res. 7:383–389. 2015.
|
|
18
|
Schlieve CR, Lieven CJ and Levin LA:
Biochemical activity of reactive oxygen species scavengers do not
predict retinal ganglion cell survival. Invest Ophthalmol Vis Sci.
47:3878–3886. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu J, Chen Y, Zhai L, Zhang L, Xu Y, Wang
S and Hu S: Antioxidative effect of ginseng stem-leaf saponins on
oxidative stress induced by cyclophosphamide in chickens. Poult
Sci. 94:927–933. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang YT, Chang WN, Tsai NW, Huang CC,
Kung CT, Su YJ, Lin WC, Cheng BC, Su CM, Chiang YF and Lu CH: The
roles of biomarkers of oxidative stress and antioxidant in
Alzheimer's disease: A systematic review. Biomed Res Int.
2014:1823032014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sigler K, Chaloupka J, Brozmanová J,
Stadler N and Höfer M: Oxidative stress in microorganisms-I.
Microbial vs. higher cells-damage and defenses in relation to cell
aging and death. Folia Microbiologica. 44:587–624. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O,
et al: A biomarker that identifies senescent human cells in culture
and in aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kregel KC and Zhang HJ: An integrated view
of oxidative stress in aging: Basic mechanisms, functional effects,
and pathological considerations. Am J Physiol Regulatory
Integrative Comp Physiol. 292:R18–R36. 2007. View Article : Google Scholar
|
|
24
|
Goldin A, Beckman JA, Schmidt AM and
Creager MA: Advanced glycation end products: Sparking the
development of diabetic vascular injury. Circulation. 114:597–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park S, Kim CS, Lee J, Kim Suk J and Kim
J: Effect of regular exercise on the histochemical changes of
d-galactose-induced oxidative renal injury in high-fat diet-fed
rats. Acta Histochem Cytochem. 46:111–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kaviani E, Rahmani M, Kaeidi A,
Shamsizadeh A, Allahtavakoli M, Mozafari N and Fatemi I: Protective
effect of atorvastatin on d-galactose-induced aging model in mice.
Behav Brain Res. 334:55–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ho SC, Liu JH and Wu RY: Establishment of
the mimetic aging effect in mice caused by D-galactose.
Biogerontology. 4:15–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keppler D, Lesch R, Reutter W and Decker
K: Experimental hepatitis induced by D-galactosamine. Exp Mol
Pathol. 9:279–290. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El-Mofty SK, Scrutton MC, Serroni A,
Nicolini C and Farber JL: Early, reversible plasma membrane injury
in galactosamine-induced liver cell death. Am J Pathol. 79:579–596.
1975.PubMed/NCBI
|
|
30
|
Wei H, Li L, Song Q, Ai H, Chu J and Li W:
Behavioural study of the D-galactose induced aging model in
C57BL/6J mice. Behav Brain Res. 157:245–251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guerrieri F, Pellecchia G and Papa S:
Reactive oxygen species (ROS) and alteration of F0F1-ATP synthase
in aging and liver regeneration. Free Radicals Oxidative Stress and
Antioxidants. 296:109–1191. 1998. View Article : Google Scholar
|
|
32
|
Wu YH, Hao BJ, Cao HC, Xu W, Li YJ and Li
LJ: Anti-Hepatitis B virus effect and possible mechanism of action
of 3,4-O-Dicaffeoylquinic acid in vitro and in vivo. Evid Based
Complement Alternat Med. 2012:1–9. 2012. View Article : Google Scholar
|
|
33
|
Liu LM, Zhang JX, Luo J, Guo HX, Deng H,
Chen JY and Sun SL: A role of cell apoptosis in lipopolysaccharide
(LPS)-induced nonletheal liver inury in D-galactosamine
(D-GalN)-sensitised rats. Dig Dis Sci. 53:1316–1324. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Das SK and Vasudevan DM: Alcohol-induced
oxidative stress. Life Sci. 81:177–187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lieber CS: Pathogenesis and treatment of
alcoholic liver disease: Progress over the last 50 years. Rocz Akad
Med Bialymst. 50:7–20. 2005.PubMed/NCBI
|
|
36
|
Muller FL, Song W, Liu Y, Chaudhuri A,
Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ II, Csete
M, et al: Absence of CuZn superoxide dismutase leads to elevated
oxidative stress and acceleration of age-dependent skeletal muscle
atrophy. Free Radic Biol Med. 40:1993–2004. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Halliwell B and Gutteridge JMC: Free
radicals in biology and medicine. J Free Radicals Biol Med.
1:331–332. 2007. View Article : Google Scholar
|
|
38
|
Townsend DM, Tew KD and Tapiero H: The
importance of glutathione in human disease. Biomed Pharmacother.
57:145–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yuan L and Kaplowiyz N: Glutathione in
liver diseases and hepatotoxicity. Mol Aspects Med. 30:29–41. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dahake H, Warade J, Kansara G, Pawade Y
and Ghangle S: Study of malondialdehyde as an oxidative stress
marker in schizophrenia. Int J Res Med Sei. 4:730–4734. 2016.
|
|
41
|
Mizoue T, Tokunaga S, Kasai H, Kawai K,
Sato M and Kubo T: Body mass index and oxidative DNA damage: A
longitudinal study. Cancer Sci. 98:1254–1258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sharma JB, Sharma A, Bahadur A, Vimala N,
Satyam A and Mittal S: Oxidative stress marker and antioxidant
levels in normal pregnancy and preeclampsia. Int J Gynecol Obstet.
94:23–27. 2006. View Article : Google Scholar
|
|
43
|
Hailiqian T, Kang JS and Sun L: Effects of
aqueous extract of Hedysarum austrosibiricum on metabolism of oxyen
free radicals in subacute aging mice caused by D-galactose.
Zhongguo Zhong Yao Za Zhi. 32:729–731. 2007.(In Chinese).
PubMed/NCBI
|
|
44
|
Fan Y, Xia J, Jia D, Zhang M, Zhang Y,
Huang G and Wang Y: Mechanism of ginsenoside Rg1 renal protection
in a mouse model of d-galactose-induced subacute damage. Pharm
Biol. 54:1815–1821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Davalli P, Mitic T, Caporali A, Lauriola A
and D'Arca D: ROS, cell senescence, and novel molecular mechanisms
in aging and age-related diseases. Oxid Med Cell Longev.
2016:35651272016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Schulte BA, LaRue AC, Ogawa M and
Zhou D: Total body irradiation selectively induces murine
hematopoietic stem cell senescence. Blood. 107:358–366. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Liu L, Pazhanisamy SK, Li H, Meng
A and Zhou D: Total body irradiation causes residual bone marrow
injury by induction of persistent oxidative stress in murine
hematopoietic stem cells. Free Radic Biol Med. 48:348–356. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu XF and Zou HD: PEDF in diabetic
retinopathy: A protective effect of oxidative stress. J Biomed
Biotechnol. 2012:5806872012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gruber HE, Ingram JA, Norton HJ and Hanley
EN Jr: Senescence in cells of the aging and degenerating
intervertebral disc: Immunolocalization of senescence-associated
beta-galactosidase in human and sand rat discs. Spine (Phila Pa
1976). 32:321–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Park CH and Kim JW: Effect of advanced
glycation end products on oxidative stress and senescence of
trabecular meshwork cells. Korean J Ophthalmol. 26:123–131. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen C, Mu XY, Zhou Y, Shun K, Geng S, Liu
J, Wang JW, Chen J, Li TY and Wang YP: Ginsenoside Rg1 enhances the
resistance of hematopoietic stem/progenitor cells to
radiation-induced aging in mice. Acta Pharmacol Sin. 35:143–150.
2014. View Article : Google Scholar : PubMed/NCBI
|