Introduction
Management of postoperative pain continues to be a
challenging task. Opioid-based patient-controlled analgesia (PCA)
is widely used in postoperative analgesia, which may cause a number
of side effects, including postoperative nausea and vomiting
(PONV), respiratory depression, pruritus and urinary retention
(1). As a derivative of morphine,
butorphanol has partial agonist/antagonist activity on µ-opioid
receptors, agonist activity on κ-opioid receptors and no obvious
activity on δ-opioid receptors (2).
Butorphanol has been used for musculoskeletal pain, headaches and
perioperative analgesia safety (3).
Analgesia using butorphanol is five times greater than that using
morphine (4). A previous study
demonstrated that butorphanol exerted affective analgesia on
patients following uvulopalatopharyngoplasty (5). Butorphanol PCA rarely leads to side
effects, potential for abuse or systemic toxicity (6,7). The
majority of patients (84%) undergoing general abdominal surgery and
general anesthesia using butorphanol, a PCA, as the analgesic agent
were able to obtain excellent postoperative pain relief (8). However, similarly to other opioids,
butorphanol can lead to respiratory depression, excessive sedation,
PONV and dizziness (9). Therefore,
there has been a pursuit for combining currently available
pharmacological agents to reduce the side effects. Using a
combination of pharmacological agents that act on multiple
pharmacologic sites is the optimal method for postoperative
analgesia, which is defined as multimodal analgesia (10). This allows the use of lower doses of
opioids, which decreases the incidence of side effects. In order to
decrease the side effects resulting from opioid-based, intravenous
PCA (PCIA), the addition of various adjuncts has been broadly
studied but their efficacy has not yet been confirmed (10).
Dexmedetomidine (DEX), a novel selective
α2-adrenergic receptor agonist, has been used for sedation or
analgesia in intensive care and during surgery (11). DEX has analgesic, sedative and
sympatholytic effects but does not cause respiratory depression
(12). Due to its multiple effects,
perioperative administration of DEX is applicable as a sedative and
analgesic pharmacological agent (13). DEX also has analgesia and
opioid-sparing effects when used as an adjuvant for postoperative
analgesia (14,15). The results of one meta-analysis
indicated opioid (morphine, fentanyl or sufentanyl)-DEX combination
PCIA optimized analgesia, spared opioid consumption, deduced side
effects and increased patient satisfaction when compared with PCIA
opioid alone (16). Furthermore,
postoperative administration of DEX may serve an important role in
multimodal analgesia (16). However,
the conclusion of this review was significantly confounded by the
studies performed with differences in DEX dose, administration time
and surgery (13–15). Furthermore, DEX is only licensed for
patient sedation under intensive care and few studies have observed
the effects of DEX in patients using butorphanol-based PCIA.
Based on the aforementioned results, it was
hypothesized that DEX may improve the analgesic effect of
butorphanol-based PCIA and reduce adverse effects in patients
undergoing total hysterectomy. The aim of the present prospective,
randomized, double-blinded, controlled study was to evaluate
whether intraoperative and postoperative infusion of DEX added to
butorphanol PCIA could enhance the analgesic effect in patients 24
h post-total laparoscopic hysterectomy. Simultaneously, the adverse
effects associated with the DEX-butorphanol combination PCIA were
also investigated.
Materials and methods
Study protocol
The present trial was retrospectively registered at
the clinical trial (http://www.chictr.org.cn/index.aspx, registration
number: ChiCTR1800015675). The present study was approved by the
Institutional Human Investigations Committee of Yantai Yuhuangding
Hospital (Yantai, China) and written informed consent was obtained
from all patients prior to 88 patients undergoing total
laparoscopic hysterectomy with general anesthesia being
recruited.
Patients
The inclusion criteria were as follows: Aged between
38 and 65 years and an American Society of Anesthesiologists (ASA)
grade of I or II. The exclusion criteria were as follows: ASA grade
of ≥III, obesity [body mass index (BMI), >30], opioid addiction,
treatment with sedative-hypnotic drug(s), uncontrolled
hypertension, severe heart disease, conduction abnormality,
neuropsychiatric diseases, alcohol abuse and allergy to either
butorphanol or DEX. Prior to surgery, all patients were taught the
operation of PCA and visual analogue scale (VAS) pain score (pain
intensity on a 10-point VAS; 0, no pain and 10, the worst pain
imaginable). Patients were instructed to push the PCA button when
they experienced pain.
Randomization and blinding
A computer-generated randomization table was used to
divide the patients randomly into control (CON) or DEX groups by an
independent anesthetist prior to surgery. Another anesthetist, who
was not involved in the present study, prepared the drugs according
to the group. Anesthetists, surgeons, patients and nurses were
blinded to the proposal during the study.
Anesthesia and PCIA
Following arrival at the operating room,
electrocardiography, blood pressure (BP), pulse oxygen saturation
(SPO2), end-tidal CO2 (pETCO2) and the bi-spectral index (BIS) were
monitored by an automated patient monitor (Philips IntelliVue MP60;
Philips Medical Systems, Inc., Bothell, WA, USA). All patients were
administered intravenously (i.v.) with midazolam (0.05 mg/kg),
fentanyl (2–3 µg/kg; Yichang Humanwell Pharmaceutical Co., Ltd.,
Yichang, China), propofol (1.5–2 mg/kg; Fresenius Kabi
Asia-Pacific, Ltd., Wanchai, Hong Kong) and cisatracurium (0.2
mg/kg; Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China) for
induction. Following intubation with a laryngeal mask, patients
were ventilated with a PetCO2 at 35–40 mmHg. Fentanyl (2–3 µg/kg)
was administered prior to skin incision. Anesthesia was maintained
with sevoflurane (end-tidal concentration of 1.5–2.5%) and a
continuous infusion of remifentanil (0.1–0.2 µg/kg/min; Yichang
Humanwell Pharmaceutical Co., Ltd.). Cisatracurium (0.05 mg/kg) was
administered during surgery until 1 h prior to the end of surgery.
Titration of anesthetics was adjusted to maintain a BIS value of
40–60. Patients in the two groups received 4–6 ml/kg/h of Ringer's
solution on the basis of fluid deficit, maintenance dose and
intraoperative losses. Either hydroxyethyl starch (130/0.4) or
ephedrine (6 mg i.v.) was administered to treat hypotension [mean
BP (MBP), <60 mmHg]. Atropine (0.2 mg i.v.) was administered to
treat bradycardia (HR, <50 bpm). A total of 0.5 µg/kg DEX
(Jiangsu Hengrui Medicine Co., Ltd.) or the equivalent volume of
0.9% normal saline was infused i.v. for ≥10 min in the DEX and CON
group following induction, respectively. A total of 30 min prior to
the end of surgery, the two groups received 1 mg butorphanol
(Jiangsu Hengrui Medicine Co., Ltd.) and 0.25 mg palonosetron.
Following surgery, 1 mg neostigmine and 0.5 mg atropine were
administered. The patients following extubation were delivered to
the post-anesthesia care unit (PACU), where they were intensively
cared for and administered with O2.
PCIA was commenced immediately following surgery. In
the CON group, the PCA regimen consisted of 10 mg butorphanol. In
the DEX group, the PCA regimen consisted of 10 mg butorphanol and
300 µg DEX. The PCA volume was made up to 100 ml with 0.9% normal
saline. The PCA was infused with a 0.5 ml bolus on-demand, with a
15 min lockout interval and a 2 ml/h background rate. Therefore, a
background infusion of DEX in the DEX group was 0.1 µg/kg/h.
Outcome measures
HR, MAP and SpO2 were recorded as follows: Arrival
at the operating room (baseline, T0); induction (T1); intubation
(T2); 30 min following intubation (T3); 60 min following intubation
(T4); extubation (T5); and 1, 2, 6, 12 and 24 h post-surgery
(T6-T10). The pump-press number and consumption of butorphanol were
recorded at T10. Pain scores at rest and movement were recorded at
T6-T10. At the same time points, the sedation level was scored
using a 5-point scale (0, fully awake; 1, drowsy, closed eyes; 2,
asleep, easily aroused with light tactile stimulation or a simple
verbal command; 3, asleep, arousable only by strong physical
stimulation; and 4, unarousable). The level of satisfaction (0,
very satisfied; 1, satisfied; 2, less satisfied; 3, not satisfied)
was assessed at T10. Bradycardia (HR, <50 bpm), hypotension
(SBP, <90 mmHg), somnolence (sedation score, ≥3), and
respiratory depression (respiration rate, <8 bpm over 5 min)
were regarded as severe adverse events and were treated
immediately. Other adverse events (PONV, itching and dizziness)
were also recorded.
Statistical analysis
A difference of 20% in butorphanol PCIA consumption
was expected. For a study power of 80% (α=0.05, β=0.2), the
required sample size in each group was 38. To allow for a possible
15% drop-out rate, 44 patients were included in each group.
Statistical analysis was performed using SPSS for Windows Version
16.0 (SPSS, Inc., Chicago, IL, USA). Normally distributed data are
expressed as the mean ± standard deviation. Patient
characteristics, including age, weight, BMI, surgery time,
anesthesia time, PACU stay time, pump-press number and butorphanol
consumption were compared between the 2 groups using unpaired
Student's t-tests. HR and MBP at different time points were
compared between the two groups using two-way analysis of variance,
followed by Bonferroni's post hoc test. The incidence of adverse
events and the degree of satisfaction were analyzed using the χ2
test. Pain and sedation scores were analyzed using the Mann-Whitney
U-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Demographic data and
surgery/anesthesia-associated information
As indicated in Fig.
1, the CONSORT flow diagram of patient recruitment was
demonstrated. A total of 88 patients were enrolled in the present
study; 4 patients were excluded due to not meeting the inclusion
criteria or declining to participate; 1 patient in the CON group
withdrew due to their surgery being canceled and 2 patients (1 from
the CON group and 1 from the DEX group) were excluded following
surgery as PCA was discontinued. Finally, 81 patients completed the
study (40 in the CON group and 41 in the DEX group). Basic
demographic data and surgery/anesthesia-associated information in
the 2 groups were compared (Table
I). There were no significant differences in age, body weight,
BMI, anesthesia time, surgery time and recovery time at PACU.
 | Table I.Basic demographic data and
surgery/anesthesia-associated information. |
Table I.
Basic demographic data and
surgery/anesthesia-associated information.
| Variables | CON group, n=40 | DEX group, n=41 |
|---|
| Age (years) | 46.5±9.2 | 47.2±10.3 |
| Weight (kg) | 63.2±7.4 | 64.3±10.2 |
| BMI (kg/m2) | 22.3±1.8 | 21.8±2.0 |
| Operation time
(min) | 79.2±11.3 | 81.2±12.8 |
| Anesthesia time
(min) | 95.6±12.1 | 96.1±10.6 |
| PACU stay time
(min) | 35.8±7.9 | 36.6±8.2 |
Hemodynamic changes from the baseline to 24 h
post-surgery were presented (Fig.
2). With respect to the baseline MBP and HR, there was a
decrease induced by anesthesia induction and an increase evoked by
intubation. There was a trend of a lower HR and MBP in the DEX
group following infusion with the loading dose of DEX but none of
the patients required their doses to be corrected. Furthermore,
there was no significant difference between the two groups during
surgery and 24 h after surgery with regards to MAP and HR.
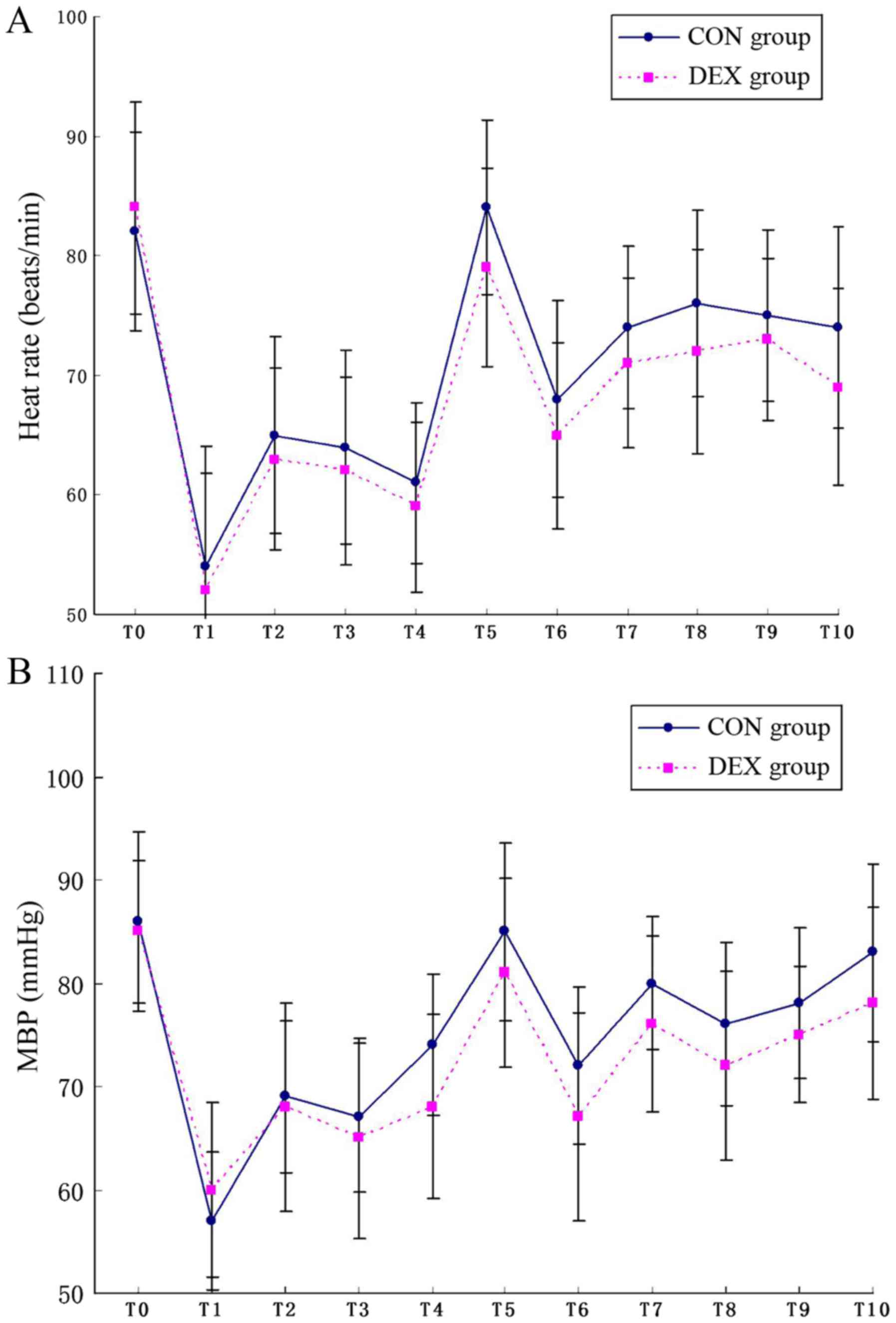 | Figure 2.HR and MBP. (A) HRs at different time
points. (B) MBP at different time points. T0, baseline; T1,
induction; T2, intubation; T3, 30 min after intubation; T4, 60 min
after intubation; T5, extubation; and T6-T10, 1, 2, 6, 12 and 24 h
post-surgery. MBP, mean blood pressure; HR, heart rates; CON,
control; DEX, dexmedetomidine. |
PCIA evaluation
PCIA was commenced immediately following surgery.
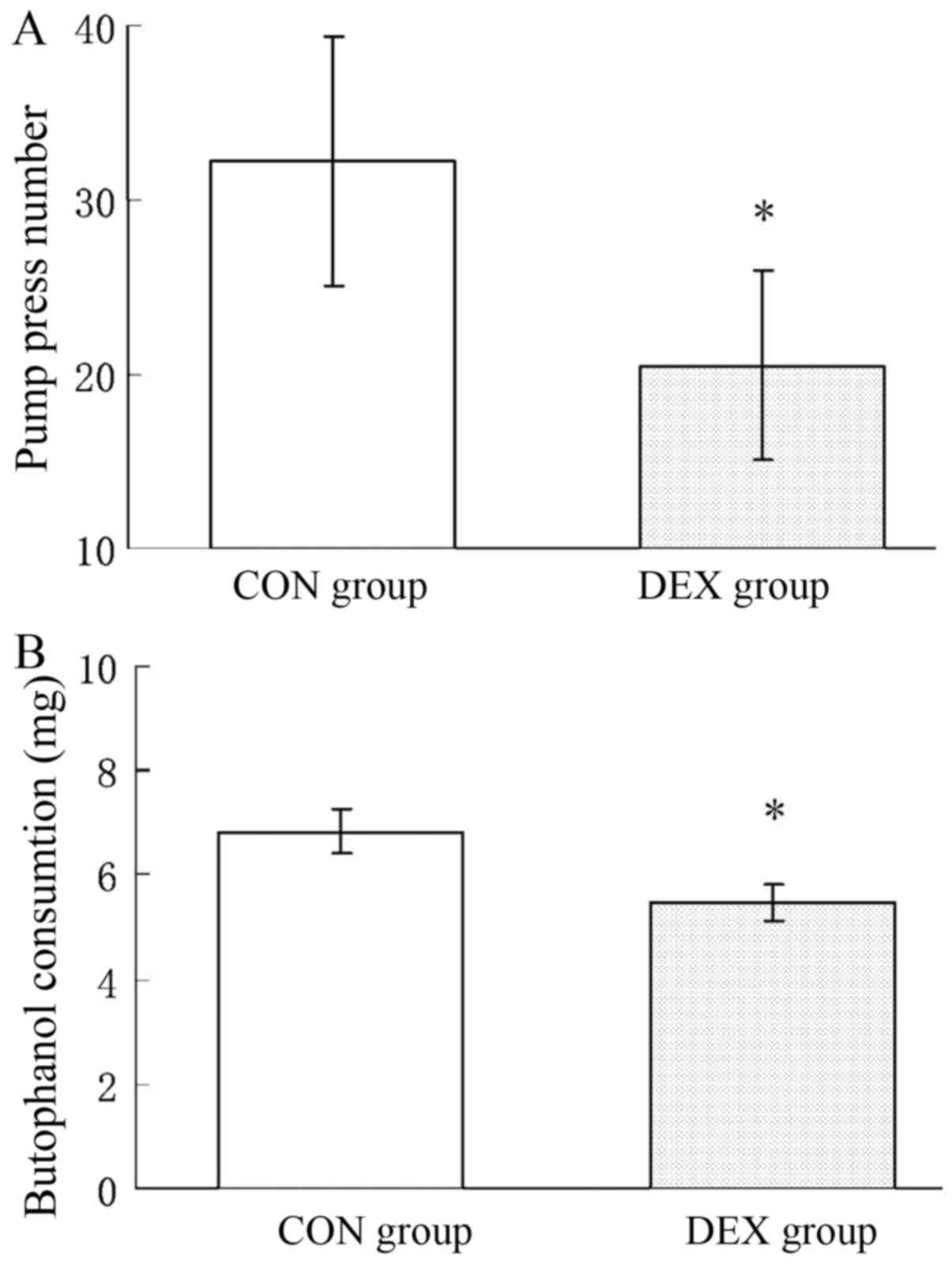
Patients in the CON group exhibited a significantly higher
pump-press number and significantly increased butorphanol
consumption compared with those in the DEX group (P<0.05).
Patients in the DEX group consumed 19% less butorphanol during 0–24
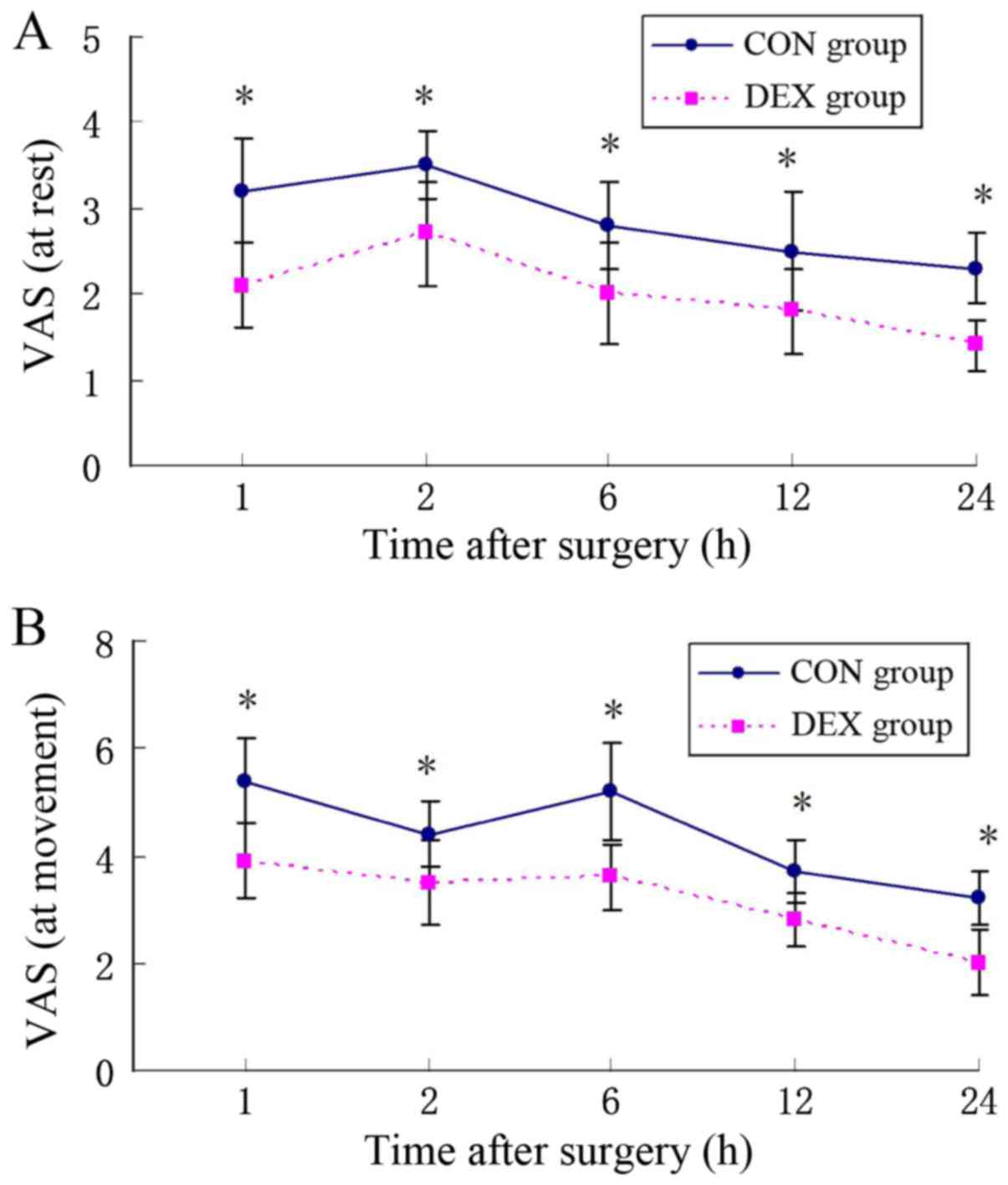
h post-surgery compared with the CON group (Fig. 3). At the same time, patients in the
DEX group exhibited a significantly lower VAS score at rest and
movement states compared with the CON group (P<0.05; Fig. 4). There were no significant
differences in the sedation score between the groups. None of the
patients had a sedation score ≥3 (Fig.
5). Furthermore, results indicated that the satisfaction scores
were significantly greater in the DEX group compared with those in
the CON group (P<0.05; Table
II).
 | Table II.Comparison of patient satisfaction in
two groups. |
Table II.
Comparison of patient satisfaction in
two groups.
| Satisfaction
rating | CON group (%),
n=40 | DEX group (%),
n=41 |
|---|
| Very satisfied | 7 (17.5) | 19
(46.3)a |
| Satisfied | 22 (55.0) | 16
(39.0)a |
| Moderately
satisfied | 9 (22.5) | 5 (12.2)a |
| Not satisfied | 2 (5.0) | 1 (2.4) |
Post-operative adverse effects
Compared with the CON group, the DEX group exhibited
a significantly lower incidence of nausea and vomiting (P<0.05).
However, the incidence of itching and dizziness was similar in the
two groups. Changes in the respiratory rate were not significantly
different between the groups. Notably, there was no instance of
serious adverse events (respiratory depression, hypotension,
bradycardia or somnolence; Table
III).
 | Table III.Postoperative side effects from
patients in two groups. |
Table III.
Postoperative side effects from
patients in two groups.
| Side effect | CON group (%),
n=40 | DEX group (%),
n=41 |
|---|
| Nausea | 12 (30.0) | 7 (17.1)a |
| Vomiting | 7 (17.1) | 2 (4.9)a |
| Itching | 2 (5.0) | 2 (4.9) |
| Respiratory
depression | 0 (0) | 0 (0) |
| Dizziness | 8 (20.0) | 6 (14.6) |
| Bradycardia | 0 (0) | 0 (0) |
Discussion
The results of the present trial indicated that the
combination treatment of DEX and butorphanol strengthened the
analgesic effect of butorphanol, and reduced butorphanol
consumption and the butorphanol-induced PONV, without severe
adverse events.
PCIA has been studied extensively for postoperative
analgesia. The use of opioids for postoperative analgesia typically
results in nausea, vomiting and other adverse events. In multimodal
analgesia, adding an adjunct to an opioid in PCIA for pain control
is popular (17). An important
aspect of multimodal analgesia is that the PCIA must have superior
analgesic effects with minimal side effects. Compared with
clonidine, DEX has a more favorable pharmacokinetic profile,
including a higher α2:α1 specificity ratio, 1,600:1 vs. 200:1; a
shorter plasmatic half-life T1/2, 2–2.5 vs. 9–12 h; and a higher
protein binding, 94 vs. 50%. The advantages of DEX for
postoperative analgesia, either as an opioid-sparing effect or
reducing pain scores, have been verified in numerous studies
(18,19). Notably, adding DEX to opioids may
improve postoperative analgesia. A number of studies have applied
this method but the DEX doses have been different and the
conclusion remains unclear (16).
When butorphanol is infused at a background rate, it
may produce potential analgesia and a certain degree of sedation
(6). Butorphanol being infused
continuously can maintain stable plasma concentrations, resulting
in extended analgesia (7). Patients
who underwent abdominal surgery expressed satisfaction with the
butorphanol PCIA (7). Reedy et
al (20) indicated that the
respiratory rate and sedation status of patients who received
butorphanol or morphine were similar. Patients received effective
analgesia and minimal sedation.
The recommended loading dose of DEX is 0.5–1 µg/kg
in adults (12). A previous study
demonstrated that a loading dose 0.5 µg/kg DEX alleviated labor
pain (21). A larger loading dose of
DEX may delay anesthetic recovery. The onset time, distribution
half-life and elimination half-life of DEX are ~15 min, 6 min and 2
h respectively (12,22). The duration of total laparoscopic
hysterectomy is relatively short (~80 min), so a loading dose of
0.5 µg/kg was administered.
In the present study, a significant reduction in the
pain score and butorphanol consumption was observed in the DEX
groups, suggesting an analgesic effect of DEX. The opioid-sparing
effects of DEX in the present study were similar to those observed
in other studies (14,15,19).
Possible mechanisms underlying DEX that were suggested included the
following: Inhibition of nociceptive neurotransmission via
activating peripheral, spinal and supraspinal α2-adreno receptors;
attenuation of the stress response and the affective-motivational
components of pain; and alleviation of hyperalgesia resulting from
opioid administration or surgical inflammation (22,23).
A specific level of sedation following surgery is
necessary for patients to reduce worry and anxiety. Moderate
sedation during the early post-operative period is regarded as a
clinical method to maintain hemodynamic stability and to provide
comfort and analgesia without interfering with the evaluation of
the conscious state (7).
Furthermore, sedation can prevent aimless movement from inadequate
analgesia and promote recovery (7).
Given the sedative properties of butorphanol, butorphanol infused
at the background dose resulted in a low level of sedation. The use
of DEX, recognized as a sedative and analgesic drug, combined with
butorphanol following surgery may arouse concerns of unnecessary or
excessive sedation. However, no excessive sedation following DEX
was observed during postoperative PCIA in the present study. Such
moderate sedation ensures that patients adequately remain
orientated and calm, cooperative, breathing and coughing. This
observation may be due to: i) The doses of DEX used in the present
study being lower than the recommended maintenance infusion for
sedation and ii) the reduced consumption of butorphanol serving a
vital role in mitigating sedation.
The present study demonstrated that adding DEX to
butorphanol PCIA decreased PONV compared with butorphanol alone.
This may be due to the antiemetic properties of DEX since higher
plasma concentrations of catecholamines is an important factor
leading to PONV (24). Additionally,
the butorphanol-sparing effect of DEX, which resulted in a
reduction in PONV, has been demonstrated in gynecological patients
(25). A meta-analysis demonstrated
that DEX can reduce the occurrence of PONV, which is likely
attributable to the reduced consumption of opioids (26). A decreased consumption of butorphanol
in patients receiving DEX may explain the reduced incidence of
PONV.
Owing to the lack of evidence for the off-label use
of DEX in non-intensive care unit settings, the risk of respiratory
depression from the combination of DEX and butorphanol requires
consideration. Therefore, the basal rate was set at 0.1 µg/kg/h
with a maximum limit of 0.2 µg/kg/h. This is far below the
manufacturer's recommended dosage (0.2–0.7 µg/kg/h). No respiratory
depression was observed in the present study, indicating that
adding DEX to butorphanol PCA does not affect respiratory
stability.
When DEX was administered in a large bolus dose,
hemodynamic effects, including hypotension and bradycardia, were
the most frequent adverse events (27). A previous study reported that
postoperative infusion of DEX with sufentanil without a bolus dose
could avoid hemodynamic effects; however, anti-PONV effects of DEX
were lessened in the first 4 h post-surgery. Additionally, a study
involving healthy volunteers demonstrated that a 0.5 µg/kg loading
dose can provide sufficient analgesia without clinically
significant hypotension or bradycardia (28). Therefore, in order to minimize the
adverse effects (e.g., hypotension, hypertension and bradycardia),
a small bolus-loading dose of DEX (0.5 µg/kg) was selected instead
of the manufacturer's recommended dose (1 µg/kg). Significant
hypotension or bradycardia was not observed in the present study,
which may be due to the low loading dose of intraoperative DEX and
a relatively lower maintenance dose. However, hemodynamic
deterioration associated with DEX has been reported (29). In the present study, there were not
statistically significant differences in HR and MAP between the two
groups; however, HR and MAP were lower in the DEX group. The
hemodynamic effects associated with DEX being added to butorphanol
PCA should be appreciated.
The present study has certain limitations. To begin
with, different groups with different doses of DEX to examine
whether lower or higher doses of DEX were more effective were not
designed. This requires verification in future studies. Secondly,
SBP <90 mmHg was defined as hypotension and HR <50 bpm was
defined as bradycardia. However, others have defined MBP <60
mmHg or a 20% drop from the baseline as hypotension and HR <50
bpm or a 20% drop from the baseline as bradycardia (14,24).
Finally, the present study was performed at one hospital. Further
studies including more patients from different centers and
undergoing different types of surgery may provide more definitive
results.
In conclusion, compared with butorphanol PCIA alone,
a small loading dose of DEX infusion (0.5 µg/kg) followed by a
continuous infusion (0.1 µg/kg/h) as an adjunct to butorphanol PCIA
can reduce butorphanol consumption, improve the analgesic effect
and increase the patient satisfaction level. The present study
suggested that DEX combined with butorphanol PCIA is a potential
analgesia for patients following total laparoscopic hysterectomy.
Post-operative DEX administration may serve a role in multimodal
pain therapy. Further studies are required to establish the
effect-dose balance between optimal postoperative analgesia and
minimal side effects in DEX-butorphanol PCIA.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Nature and
Science Fund of Shandong Province, China (grant no. ZR2014HL109)
and the Science and Technology Program Foundation of Yantai, China
(grant no. 2014WS009).
Authors' contributions
JD and JL registered the clinical trial, recruited
patients, collected data and wrote the manuscript. JJ and CS
analyzed the data. JM designed the study and edited the manuscript.
All authors approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Human Investigations Committee of Yantai Yuhuangding Hospital of
Qingdao University and written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walder B, Schafer M, Henzi I and Tramèr
MR: Efficacy and safety of patient controlled opioid analgesia for
acute postoperative pain. A quantitative systematic review. Acta
Anesthesiol Scand. 45:795–804. 2001. View Article : Google Scholar
|
|
2
|
Sellon DC, Monroe VL, Roberts MC and
Papich MG: Pharmacokinetics and adverse effects of butorphanol
administered by single intravenous injection or continuous
intravenous infusion in horses. Am J Vet Res. 62:183–189. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ganesh A and Maxwell LG: Pathophysiology
and management of opioid-induced pruritus. Drugs. 67:2323–2333.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du BX, Song ZM, Wang K, Zhang H, Xu FY,
Zou Z and Shi XY: Butorphanol prevents morphine-induced pruritus
without increasing pain and other side effects: A systematic review
of randomized controlled trials. Can J Anaesth. 60:907–917. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Q, Li Z, Wang ZP and Cui C:
Preemptive analgesic effect of parecoxib sodium in patients
undergoing laparoscopic colorectal surgery. Nan Fang Yi Ke Da Xue
Xue Bao. 30:2556–2557. 2010.(In Chinese). PubMed/NCBI
|
|
6
|
Nelson KE and Eisenach JC: Intravenous
butorphanol, meperidine, and their combination relieve pain and
distress in women in labor. Anesthesiology. 102:1008–1013. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang F, Shen X, Liu Y, Xu S and Guo X:
Continuous infusion of butorphanol combined with intravenous
morphine patient-controlled analgesia after total abdominal
hysterectomy: A randomized, double-blind controlled trial. Eur J
Anaesthesiol. 26:28–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wermeling DP, Foster TS, Farrington EA,
Witt WO, Gallion HH, Donaldson E, van Nagell JR Jr, Outman WR and
McPherson DP: Patient-controlled analgesia using butorphanol for
postoperative pain relief: An open-label study. Acute Care. 12
Suppl 1:S31–S39. 1988.
|
|
9
|
Dawn AG and Yosipovitch G: Butorphanol for
treatment of intractable pruritus. J Am Acad Dermatol. 54:527–531.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elia N, Lysakowski C and Tramèr MR: Does
multimodal analgesia with acetaminophen, nonsteroidal
antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors
and patient-controlled analgesia morphine offer advantages over
morphine alone? Meta-analyses of randomized trials. Anesthesiology.
103:1296–1304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lirola T, Ihmsen H, Laitio R, Kentala E,
Aantaa R, Kurvinen JP, Scheinin M, Schwilden H, Schüttler J and
Olkkola KT: Population pharmacokinetics of dexmedetomidine during
long-term sedation in intensive care patients. Br J Anaesth.
108:460–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerlach AT and Dasta JF: Dexmedetomidine:
An updated review. Ann Pharmacother. 41:245–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blaudszun G, Lysakowski C, Elia N and
Tramèr MR: Effect of perioperative systemic α2 agonists on
postoperative morphine consumption and pain intensity: Systematic
review and meta-analysis of randomized controlled trials.
Anesthesiology. 116:1312–1322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin TF, Yeh YC, Lin FS, Wang YP, Lin CJ,
Sun WZ and Fan SZ: Effect of combining dexmedetomidine and morphine
for intravenous patient-controlled analgesia. Br J Anaesth.
102:117–122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SY, Chang CH, Lee JS, Kim YJ, Kim MD
and Han DW: Comparison of the efficacy of dexmedetomidine plus
fentanyl patient-controlled analgesia with fentanyl
patient-controlled analgesia for pain control in uterine artery
embolization for symptomatic fibroid tumors or adenomyosis: A
prospective, randomized study. J Vasc Interv Radiol. 24:779–786.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng K, Liu HY, Wu SR, Cheng H and Ji FH:
Effects of combining dexmedetomidine and opioids for postoperative
intravenous patient controlled analgesia: A systematic review and
meta-analysis. Clin J Pain. 31:1097–1104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Joshi GP: Multimodal analgesia techniques
and postoperative rehabilitation. Anesthesiol Clin North America.
23:185–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McQueen-Shadfar LA, Megalla SA, White WD,
Olufolabi AJ, Jones CA and Habib AS: Impact of intraoperative
dexmedetomidine on postoperative analgesia following gynecologic
surgery. Curr Med Res Opin. 27:2091–2097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheung CW, Qiu Q, Ying AC, Choi SW, Law WL
and Irwin MG: The effects of intra-operative dexmedetomidine on
postoperative pain, side-effects and recovery in colorectal
surgery. Anesthesia. 69:1214–1221. 2014. View Article : Google Scholar
|
|
20
|
Reedy ME, Morris LE, Brown DL, Snow D,
Koehl M and Stone CK: Double-blind comparison of butorphanol and
morphine in patient-controlled analgesia. Acute Care. 12 Suppl
1:S40–S46. 1988.
|
|
21
|
Palanisamy A, Klickovich RJ, Ramsay M,
Ouyang DW and Tsen LC: Intravenous dexmedetomidine as an adjunct
for labor analgesia and cesarean delivery anesthesia in a
parturient with a tethered spinal cord. Int J Obstet Anesth.
18:258–261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X and Bai X: New therapeutic uses
for an alpha2 adrenergic receptor agonist-dexmedetomidine in pain
management. Neurosci Lett. 561:7–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng Y, Cui S, Liu Y, Zhang J, Zhang W,
Zhang J, Gu X and Ma Z: Dexmedetomidine prevents
remifentanil-induced postoperative hyperalgesia and decreases
spinal tyrosine phosphorylation of N-methyl-d-aspartate receptor 2B
subunit. Brain Res Bull. 87:427–431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song Y, Shim JK, Song JW, Kim EK and Kwak
YL: Dexmedetomidine added to an opioid-based analgesic regimen for
the prevention of postoperative nausea and vomiting in highly
susceptible patients: A randomised controlled trial. Eur J
Anaesthesiol. 32:75–83. 2016. View Article : Google Scholar
|
|
25
|
Massad IM, Mohsen WA, Basha AS, Al-Zaben
KR, Al-Mustafa MM and Alghanem SM: A balanced anesthesia with
dexmedetomidine decreases postoperative nausea and vomiting after
laparoscopic surgery. Saudi Med J. 30:1537–1541. 2009.PubMed/NCBI
|
|
26
|
Liang X, Zhou M, Feng JJ, Wu L, Fang SP,
Ge XY, Sun HJ, Ren PC and Lv X: Efficacy of dexmedetomidine on
postoperative nausea and vomiting: A meta-analysis of randomized
controlled trials. Int J Clin Exp Med. 8:12113–12134.
2015.PubMed/NCBI
|
|
27
|
Schnabel A, Meyer-Frießem CH, Reichl SU,
Zahn PK and Pogatzki-Zahn EM: Is intraoperative dexmedetomidine a
new option for postoperative pain treatment? A meta-analysis of
randomized controlled trials. Pain. 154:1140–1149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cortinez LI, Hsu YW, Sum-Ping ST, Young C,
Keifer JC, Macleod D, Robertson KM, Wright DR, Moretti EW and Somma
J: Dexmedetomidine pharmacodynamics: Part II: Crossover comparison
of the analgesic effect of dexmedetomidine and remifentanil in
healthy volunteers. Anesthesiology. 101:1077–1083. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gerlach AT and Murphy CV:
Dexmedetomidine-associated bradycardia progressing to pulseless
electrical activity: Case report and review of the literature.
Pharmacotherapy. 29:14922009. View Article : Google Scholar : PubMed/NCBI
|