Introduction
Osteoarthritis is one of the most common joint
diseases and is primarily caused by inflammation and synovial cell
dysfunction (1). A number of
factors, including patient age, body mass index, level of physical
function and level of physical activity are associated with hip or
knee osteoarthritis (2). The
pathological causes of joint osteoarthritis development are complex
(3).
The incidence of osteoarthritis (~8% of the
population) has increased since 2012 and this disease poses a
serious threat to human health and quality of life due to the pain
and disability caused (4–6). Inflammation serves a crucial role in
osteoarthritis and is associated with joint and cartilage
destruction (7). Previous studies
have demonstrated that anti-inflammatory therapies targeting the
inflammatory factors accumulating in the synovial fluid of patients
with osteoarthritis induce therapeutic effects (8–10).
The association between synovial inflammation and
structural damage during the progression of osteoarthritis has been
evaluated and it has been suggested that inflammatory cytokines may
predict the prognosis of patients with osteoarthritis (11). The tumor necrosis factor (TNF) family
is one such cytokine that may serve an important role in the
inflammatory responses that occur during osteoarthritis (12). TNF-α-associated joint inflammation
has been analyzed in patients with rheumatoid arthritis and
osteoarthritis and TNF-α was identified as a potential target for
rheumatoid arthritis therapy (13).
Currently, anti-TNF-α-targeted therapy is being applied to treat
patients with osteoarthritis and is achieving satisfactory outcomes
by decreasing inflammation (14,15). A
number of different agents that target TNF-α, including etanercept,
trastuzumab, adalimumab and infliximab, have been developed to
treat patients with osteoarthritis (16–18).
However, to the best of our knowledge, the mechanisms mediated by
TNF-α in the synovial fibroblasts of patients with osteoarthritis
remain unknown.
The present study investigated the potential
mechanism(s) of TNF-α in synovial fibroblasts taken from a
monosodium iodoacetate-induced rat model of osteoarthritis in
vitro. Levels of inflammatory cytokines in the synovial
fibroblasts were also analyzed in vivo. The results of the
present study indicate that TNF-α is able to regulate inflammation
in a rat model of osteoarthritis by downregulating the
phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling
pathway in synovial fibroblasts.
Materials and methods
Ethics statement
The present study was performed in strict accordance
with the Guide for the Ethics Committee of The Fourth Hospital
affiliated to Harbin Medical University. All surgeries and
euthanasia were performed under intravenous injection of sodium
pentobarbital anesthesia (35 mg/kg, Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). All mice were sacrificed using isoflurane (0.5
ml) euthanasia as described previously (19).
Animal model
A total of 36 female Sprague-Dawley rats (eight
weeks old; body weight, 280–300 g) were purchased from Shanghai
SLAC laboratory Animal Co., Ltd. (Shanghai, China). All rats were
housed under controlled temperatures (23°C, 50% humidity) under a
12 h light/dark cycle with access to food and water ad
libitum. All rats were identified by ear punching. A rat model
of osteoarthritis was produced using intra-articular injections of
monosodium iodoacetate (0.2 mg per rat; Sigma-Aldrich; Merck KGaA;
n=24) administered at a volume of 30 µl once per day, for 10 days,
as previously described (20);
however, healthy control rats did not undergo this procedure
(n=12). On day 10, rats were randomly assigned to one of three
groups: A control group (n=12), a vehicle group (n = 12) and a
TNF-α inhibitor group (n = 12). All rats received subcutaneous
injections of vehicle (30 mg/kg/day; control group) or TNF-α
inhibitor (30 mg/kg/day; cat. no. 1049741-03-8, Merck KGaA).
Treatments were continued seven times and rats received the vehicle
or TNF-α inhibitor once every 2 days for a total of 14 days. All
rats were euthanized on day 14 prior to histological analysis. Rats
were weighed following 14 days of treatment.
Cells and reagents
Synovial fibroblasts were isolated from the same
rats as those described in the preceding paragraph and cultured in
Dulbecco's Modified Eagle's medium supplemented with 10% fetal
bovine serum (Sigma-Aldrich; Merck KGaA) at 37°C in a 5%
CO2 humidified atmosphere. Synovial fibroblasts were
treated with the TNF-α inhibitor (2 mg/ml) (21), PI3K inhibitor (2 mg/ml; cat. no.
526559-5MGCN, Merck KGaA) or an equal volume of PBS at 37°C for 24
h.
ELISA
Blood was extracted from experimental rats on day 15
and sera was obtained via centrifugation (6,000 × g for 15 min) at
4°C. TNF-α, interleukin (IL)-1β, IL-17a and IL-8 concentrations in
the serum of experimental rats were analyzed using ELISA kits (cat.
nos. MTA00B, MLB00C, DY421 and P8000, respectively; all Bio-Rad
Laboratories, Inc., Hercules, CA, USA), following the
manufacturer's protocol. The results were analyzed using the
1775×Mark™ ELISA reader system at 450 nm (Bio-Rad Laboratories,
Inc.).
Western blotting
Western blotting was performed to measure protein
expression following a previously described method (22). Briefly, synovial fibroblasts
(1×107) were lysed in a lysis buffer containing 1%
phenylmethane sulfonyl fluoride (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for three cycles of freezing-thawing and
subsequently centrifuged at 8,000 × g for 10 min at 4°C. Protein
concentrations were measured using a BCA assay kit (cat. no. 23225;
Thermo Fisher Scientific, Inc.). Proteins (20 µg) were separated by
10% SDS-PAGE and transferred onto a nitrocellulose membrane, which
was blocked with 5% (w/v) nonfat dry milk dissolved in
Tris-buffered saline plus Tween-20 (TBST) solution for 2 h at 37°C.
Membranes were subsequently incubated with primary rabbit anti-rat
antibodies against TNF-α (1:1,000, cat. no. ab6671), IL-1β
(1:1,000, cat. no. ab200478), IL-17a (1:1,000, cat. no. ab180904),
IL-8 (1:1,000, cat. no. ab34100), PI3K (1:2,000, cat. no. ab1678),
phosphorylated (p)-PI3K (1:1,000, cat. no. ab182651), AKT (1:1,000,
cat. no. ab8805), pAKT (1:1,000, cat. no. ab64148), matrix
metalloproteinase (MMP)-3 (1:1,000, cat. no. ab53015), MMP-9
(1:1,000, cat. no. ab38898), vascular endothelial growth factor
(VEGF; 1:1,000, cat. no. ab39256), ADAMTS4 (1:1,000, cat. no.
ab185722) and β-actin (1:1,000, cat. no. ab8226; all Abcam,
Cambridge, UK) for 12 h at 4°C. Horseradish peroxidase-conjugated
goat anti-rabbit immunoglobulin G monoclonal secondary antibodies
(cat. no. PV-6001; OriGene Technologies, Inc., Beijing, China) were
added for 24 h at 4°C. A Ventana Benchmark automated staining
system was used to analyze protein expression (BX51; Olympus
Corporation, Tokyo, Japan). Protein expression signals were
analyzed using scanning densitometry with a Microtek ScanMaker 8700
(Beijing Zhongjing Electronic Technology Co., Ltd., Beijing, China)
using ScanWizard 5 software (Shanghai Microtek Technology Co.,
Ltd., Shanghai, China). Band densities were analyzed using Quantity
One v.4.62 (Bio-Rad Laboratories, Inc.).
Histopathological analysis
Rats with osteoarthritis were euthanized under
pentobarbital anesthesia on day 14. Joints and articular cartilage
were separated and fixed in 10% formalin for 10 min at room
temperature. Paraffin-embedded joints and articular cartilages were
sliced into 4-µm sections. Tissue sections were stained with
hematoxylin and eosin for 30 min at room temperature for
histological evaluation. Safranin O-fast green and Toluidine blue
staining for 20 min at room temperature was used to evaluate
proteoglycans in the cartilage matrix using a light microscope at a
magnification, ×40.
Tissue preparation and
histopathological evaluation
Tissue sections were fixed in 10% formalin at 37°C
for 24 h, decalcified using Gooding and Stewart's fluid (Wako Pure
Chemical Industries, Ltd., Osaka, Japan) and subsequently embedded
in paraffin. Sections (4-µm thick) were stained with 0.05%
Toluidine blue (pH 4.1) for 20 min at room temperature and the
degree of osteonecrosis was evaluated using the modified Mankin
scoring system (23). The Mankin
scoring system was scored as follows: 0, normal; 1, irregular
surface; 2, pannus; 3, absence of superficial cartilage layers; 4,
slight disorganization; 5, fissure into the calcified cartilage
layer; and 6, disorganization. Histopathological evaluation was
performed by two independent blinded observers.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Each experiment was performed in triplicate. All data
were analyzed using SPSS software ver. 19.0 (SPSS, Inc., Chicago,
IL, USA). Statistical analyses were performed using one-way
analysis of variance followed by Tukey's multiple comparison post
hoc tests and P<0.05 was considered to indicate a statistically
significant difference.
Results
Inflammatory factors are upregulated
in the iodoacetate-induced osteoarthritis rat model
The expression of inflammatory factors was measured
in the osteoarthritis rat model and compared with that of healthy
rats. The results demonstrated that serum TNF-α, IL-1β, IL-17a and
IL-8 expression was significantly higher in rats with
osteoarthritis compared with healthy rats (P<0.01; Fig. 1A). Furthermore, TNF-α, IL-1β, IL-17a
and IL-8 expression was significantly upregulated in synovial
fibroblasts isolated from rats with osteoarthritis compared with
healthy rats (P<0.01; Fig. 1B).
By contrast, treatment with the TNF-α inhibitor significantly
decreased TNF-α, IL-1β, IL-17a and IL-8 expression in the serum of
rats with osteoarthritis (P<0.01; Fig. 1C). The TNF-α inhibitor also
significantly decreased TNF-α, IL-1β, IL-17a and IL-8 expression in
synovial fibroblasts isolated from rats with osteoarthritis
(P<0.01; Fig. 1D). These results
suggest that inflammatory factors are upregulated in the serum and
synovial fibroblasts of rats with osteoarthritis.
 | Figure 1.Inflammatory factor levels in a rat
model of osteoarthritis. (A) Serum levels of TNF-α, IL-1β, IL-17a
and IL-8 in rats with osteoarthritis compared with healthy rats, as
determined by an enzyme-linked immunosorbent assay. (B) Levels of
TNF-α, IL-1β, IL-17a and IL-8 expression in synovial fibroblasts
isolated from rats with osteoarthritis or healthy rats, as measured
by western blot analysis. (C) Serum levels of TNF-α, IL-1β, IL-17a
and IL-8 in rats with osteoarthritis rat following treatment with
TNF-α inhibitor or vehicle. (D) Levels of TNF-α, IL-1β, IL-17a and
IL-8 expression in synovial fibroblasts from rats with
osteoarthritis following treatment with TNF-α inhibitor or vehicle.
Data are expressed as the mean ± standard deviation. **P<0.01.
TNF-α, tumor necrosis factor α; IL, interleukin. |
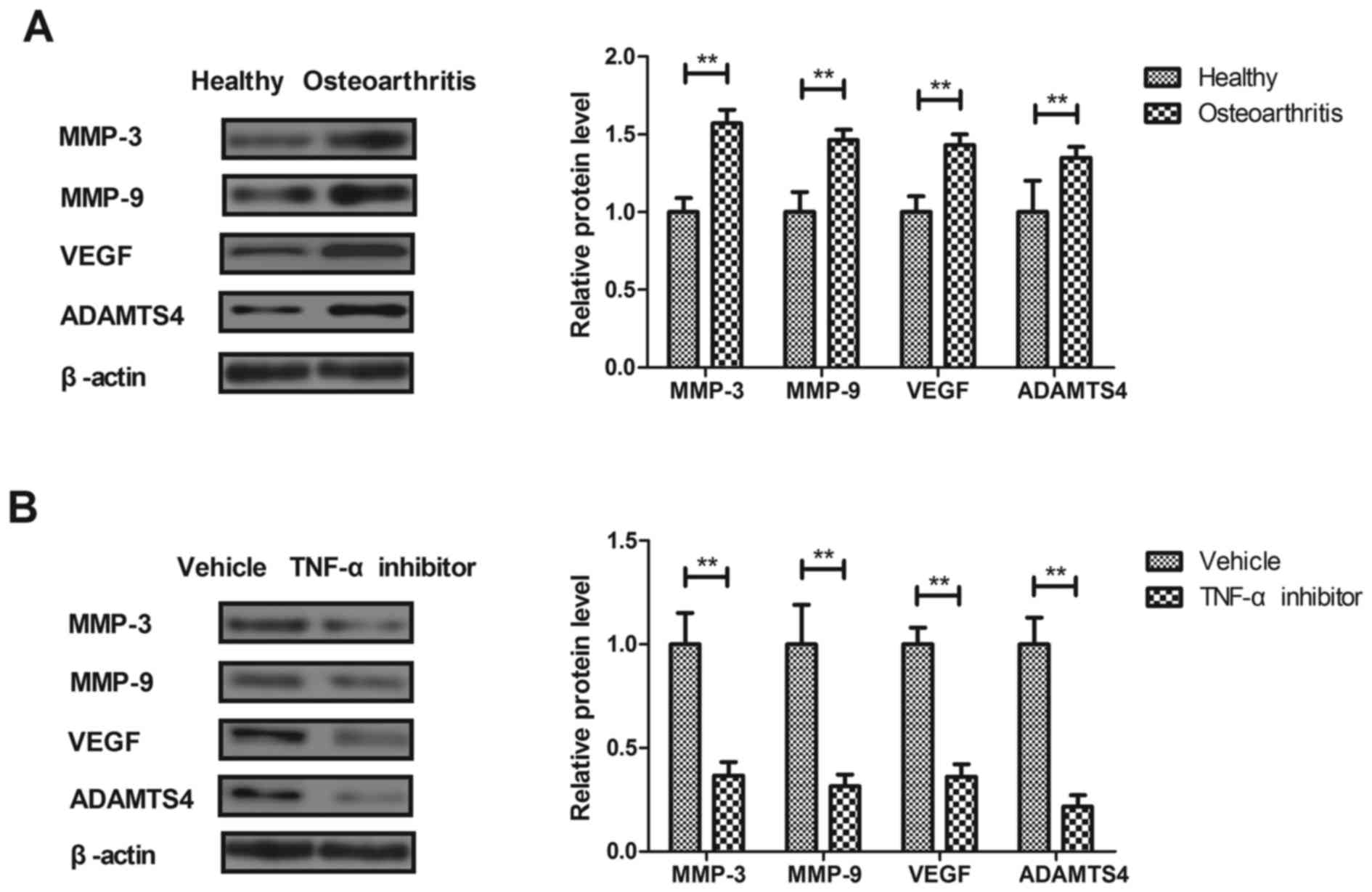
Effects of TNF-α on the expression of
pro-inflammatory factors in synovial fibroblasts
The effects of TNF-α on macrophage-regulated MMP
levels were analyzed in the synovial fibroblasts of rats with
osteoarthritis. MMP-3, MMP-9, VEGF and ADAMTS4 expression was
significantly increased in the synovial fibroblasts of rats with
osteoarthritis compared with healthy rats (P<0.01; Fig. 2A). Treatment with the TNF-α inhibitor
significantly decreased MMP-3, MMP-9, VEGF and ADAMTS4 expression
in synovial fibroblasts from rats with osteoarthritis compared with
those from healthy rats (P<0.01; Fig.
2B). These results suggest that TNF-α inhibition may be used to
treat osteoarthritis.
TNF-α regulates the expression of
inflammatory factors via the PI3K/AKT signaling pathway
It has been reported that the PI3K/AKT signaling
pathway is involved in inflammation during the progression of
osteoarthritis (24). Therefore, the
current study investigated the effects of TNF-α on the PI3K/AKT
signaling pathway in synovial fibroblasts. PI3K and AKT expression
and phosphorylation were significantly upregulated in synovial
fibroblasts from rats with osteoarthritis compared with those from
healthy rats (P<0.05; Fig. 3A).
However, PI3K and AKT expression and phosphorylation were
significantly decreased in the synovial fibroblasts of rats treated
with the TNF-α inhibitor compared with those from rats treated with
vehicle (P<0.01; Fig. 3B). The
results of an in vitro assay revealed that the PI3K
inhibitor significantly reversed the TNF-α-induced increase in
IL-1β, IL-17a and IL-8 expression in synovial fibroblasts from rats
with osteoarthritis (P<0.01; Fig.
3C). These results suggest that TNF-α regulates the expression
of inflammatory factors in synovial fibroblasts via the PI3K/AKT
signaling pathway.
Treatment with TNF-α inhibitor helps
to treat rats with iodoacetate-induced osteoarthritis
Finally, the effect of the TNF-α inhibitor on the
iodoacetate-induced osteoarthritis rat model was assessed in
vivo. The results indicated that mice treated with the TNF-α
inhibitor experienced a significantly decreased total Mankin score
compared with rats treated with vehicle (P<0.01; Fig. 4A), indicating that the TNF-α
inhibitor ameliorates bone osteoarthritis. Furthermore, treatment
with the TNF-α inhibitor significantly increased the body weight of
rats compared with the vehicle group (P<0.01; Fig. 4B). Histological analyses indicated
that treatment with the TNF-α inhibitor inhibited inflammatory cell
infiltration (Fig. 4C) and decreased
bone destruction in the joints and cartilage of rats with
osteoarthritis (Fig. 4D). These
results suggest that TNF-α inhibition may improve inflammation,
inflammatory cell infiltration and bone destruction in a rat model
of osteoarthritis.
Discussion
Synovial inflammation leads to structural damage and
bone destruction in osteoarthritis and may decrease synovial
function in osteoarthritis (25);
therefore, alleviating synovial inflammation may be an effective
method of treating patients with osteoarthritis (25). It has been demonstrated that the
TNF-α inhibitor etanercept may alleviate pain in patients with
moderate to severe osteoarthritis, suggesting that TNF-α may be an
important pathological factor during the progression of
osteoarthritis (26). The aim of the
present study was to analyze the association between TNF-α and the
PI3K/AKT signaling pathway in synovial fibroblasts from rats with
osteoarthritis. The results indicated that treatment with TNF-α
inhibitor decreased the expression of IL-1β, IL-17a and IL-8 by
downregulating the PI3K/AKT signaling pathway in synovial
fibroblasts taken from rats with osteoarthritis.
Previous studies have indicated that inflammatory
cytokines are associated with synovial injury during the
development of osteoarthritis (24,27,28). A
previous study has also demonstrated that IL-1β and/or TNF-α
induces the upregulation of MMP-1 and MMP-3 expression in
chondrocyte subpopulations, which may be a pathogenic cause of
osteoarthritis (29). The present
study demonstrated that IL-1β and TNF-α are upregulated in synovial
fibroblasts taken from rats with osteoarthritis. This is in
accordance with the results of a previous study, which demonstrated
that levels of IL-6 and IL-8 cytokines are upregulated in human
osteoarthritis (30). Additionally,
IL-17a expression is increased in inflammatory osteoarthritis,
which may explain the non-response to anti-IL-17 therapy in subsets
of patients with osteoarthritis (31). The present study indicated that
IL-17a and IL-8 expression was upregulated in synovial fibroblasts
taken from rats with osteoarthritis. Notably, TNF-α inhibition
decreased levels of IL-1β, IL-17a and IL-8 in synovial fibroblasts
isolated from rats with osteoarthritis (32–34).
Chen et al (35) indicated that treatment with the TNF-α
inhibitor confers many benefits including the decrease of
inflammation and pain for patients with osteoarthritis of the hand,
who are refractory to analgesia. It has also been demonstrated that
elevated VEGF levels may increase bone destruction in an in
vivo model of osteoarthritis (33). Furthermore, it has been suggested
that ADAMTS4 may be upregulated in osteoarthritis, which, in turn,
increases the expression of the proinflammatory cytokine NF-κB
(34). In the present study, VEGF
and ADAMTS4 expression were upregulated in the synovial fibroblasts
of rats with osteoarthritis, but were downregulated following
treatment with TNF-α inhibitor. Taken together, these results
suggest that treatment with TNF-α inhibitor may be beneficial in
the treatment of osteoarthritis.
Inhibiting the PI3K/AKT signaling pathway may be
developed as a promising method of treating patients with
osteoarthritis (36,37). It has been reported that PI3K/AKT
mediates the expression of TNF-α mRNA and NF-κB activation in
calyculin A-treated primary osteoblasts (38). Notably, another study indicated that
regulation of the PI3K/AKT signaling pathway may inhibit
inflammation and the apoptosis of chondrocytes in a rat model of
osteoarthritis (39). The present
study demonstrated that treatment with TNF-α inhibitor
downregulated levels of the inflammatory factors IL-1β, IL-17a and
IL-8 via the PI3K/AKT signaling pathway. Treatment with the TNF-α
inhibitor also decreased IL-1β, IL-17a and IL-8 levels by
decreasing PI3K and AKT expression in synovial fibroblasts. It has
been demonstrated that inhibiting TNF-α may decrease inflammation
by downregulating the PI3K/AKT signaling pathway (39). These reports suggest that the
PI3K/AKT signaling pathway may be a potential target for the
treatment of osteoarthritis. The present study identified that
treatment with a TNF-α inhibitor downregulated the PI3K/AKT
signaling pathway in synovial fibroblasts isolated from rats with
osteoarthritis. Previous studies have demonstrated that the
activation of the NF-κB-mediated inflammation may be induced by
TNF-α (40–42). However, the present study did not
analyze the effects of TNF-α inhibitor on NF-κB, IL-6 and TGF-β
levels. Further studies are required to assess the other mechanisms
mediated by the TNF-α inhibitor.
In conclusion, the present study investigated the
potential mechanisms mediated by TNF-α in a rat model of
osteoarthritis induced by monosodium iodoacetate. The results
indicate that the PI3K/AKT signaling pathway is an inflammatory
pathway that may be mediated by TNF-α in osteoarthritis. The
expression of inflammatory cytokines in synovial fibroblasts
significantly decreased following treatment with TNF-α inhibitor.
These results suggest that inhibiting the PI3K/AKT signaling
pathway may contribute to the inhibition of inflammation in
osteoarthritis and may therefore be developed as novel method of
treating osteoarthritis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HoL performed the experiments. SX, YQ, HuL, and RZ
prepared and analyzed experimental data. YL designed the
experiment.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Fourth Hospital affiliated to Harbin Medical
University (Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Onishi K, Utturkar A, Chang E, Panush R,
Hata J and Perret-Karimi D: Osteoarthritis: A critical review. Crit
Rev Phys Rehabil Med. 24:251–264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Veenhof C, Huisman PA, Barten JA, Takken T
and Pisters MF: Factors associated with physical activity in
patients with osteoarthritis of the hip or knee: A systematic
review. Osteoarthritis Cartilage. 20:6–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis AM: Osteoarthritis year in review:
Rehabilitation and outcomes. Osteoarthritis Cartilage. 20:201–206.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuijt MT, Inklaar H, Gouttebarge V and
Frings-Dresen MH: Knee and ankle osteoarthritis in former elite
soccer players: A systematic review of the recent literature. J Sci
Med Sport. 15:480–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taylor R Jr, Raffa RB and Pergolizzi JV
Jr: Controlled release formulation of oxycodone in patients with
moderate to severe chronic osteoarthritis: A critical review of the
literature. J Pain Res. 5:77–87. 2012.PubMed/NCBI
|
|
6
|
Jotanovic Z, Mihelic R, Sestan B and
Dembic Z: Role of interleukin-1 inhibitors in osteoarthritis: An
evidence-based review. Drugs Aging. 29:343–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lieberthal J, Sambamurthy N and Scanzello
CR: Inflammation in joint injury and post-traumatic osteoarthritis.
Osteoarthritis Cartilage. 23:1825–1834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hermann J, Lipp RW, Dunzinger A, Spreizer
C, Schaffler G, Kvaternik H, Ofner P and Graninger W: Anti-TNF
scintigraphy to assess TNF-α-associated joint inflammation in
rheumatoid arthritis and osteoarthritis. Clin Exp Rheumatol.
32:6142014.PubMed/NCBI
|
|
9
|
Gross JB, Guillaume C, Gégout-Pottie P,
Mainard D and Presle N: Synovial fluid levels of adipokines in
osteoarthritis: Association with local factors of inflammation and
cartilage maintenance. Biomed Mater Eng. 24 Suppl 1:S17–S25.
2014.
|
|
10
|
Ballegaard C, Riis RG, Bliddal H,
Christensen R, Henriksen M, Bartels EM, Lohmander LS, Hunter DJ,
Bouert R and Boesen M: Knee pain and inflammation in the
infrapatellar fat pad estimated by conventional and dynamic
contrast-enhanced magnetic resonance imaging in obese patients with
osteoarthritis: A cross-sectional study. Osteoarthritis Cartilage.
22:933–940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mancarella L, Addimanda O, Cavallari C and
Meliconi R: Synovial inflammation drives structural damage in hand
osteoarthritis: A narrative literature review. Curr Rheumatol Rev.
13:43–50. 2016. View Article : Google Scholar
|
|
12
|
Han L, Song JH, Yoon JH, Park YG, Lee SW,
Choi YJ, Nam SW, Lee JY and Park WS: TNF-α and TNF-β polymorphisms
are associated with susceptibility to osteoarthritis in a Korean
population. Korean J Pathol. 46:30–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaneko K, Sugitani M, Goto M and Murashima
A: Tocilizumab and pregnancy: Four cases of pregnancy in young
women with rheumatoid arthritis refractory to anti-TNF biologics
with exposure to tocilizumab. Modern rheumatology. 26:672–675.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan PW, Liu DY, Chu XD, Hao YQ, Zhu C and
Qu Q: Effects of preventive administration of juanbi capsules on
TNF-alpha, IL-1 and IL-6 contents of joint fluid in the rabbit with
knee osteoarthritis. J Tradit Chin Med. 30:254–258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stannus O, Jones G, Cicuttini F,
Parameswaran V, Quinn S, Burgess J and Ding C: Circulating levels
of IL-6 and TNF-α are associated with knee radiographic
osteoarthritis and knee cartilage loss in older adults.
Osteoarthritis Cartilage. 18:1441–1447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olson SA, Furman BD, Kraus VB, Huebner JL
and Guilak F: Reply to ‘Does progranulin account for the opposite
effects of etanercept and infliximab/adalimumab in osteoarthritis?’
by Wei et al. J Orthop Res. 34:15–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martín G, Cañueto J, Santos-Briz A, Alonso
G, Unamuno PD and Cruz JJ: Interstitial granulomatous dermatitis
with arthritis associated with trastuzumab. J Eur Acad Dermatol
Venereol. 24:493–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Güler-Yüksel M, Allaart CF, Watt I,
Goekoop-Ruiterman YP, de Vries-Bouwstra JK, van Schaardenburg D,
van Krugten MV, Dijkmans BA, Huizinga TW, Lems WF and Kloppenburg
M: Treatment with TNF-α inhibitor infliximab might reduce hand
osteoarthritis in patients with rheumatoid arthritis.
Osteoarthritis Cartilage. 18:1256–1262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Golledge HD: Response to Roustan et al.
Evaluating methods of mouse euthanasia on the oocyte quality:
Cervical dislocation versus isoflurane inhalation: Animal welfare
concerns regarding the aversiveness of isoflurane and its inability
to cause rapid death. Lab Anim. 46:358–359; author reply 360. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barve RA, Minnerly JC, Weiss DJ, Meyer DM,
Aguiar DJ, Sullivan PM, Weinrich SL and Head RD: Transcriptional
profiling and pathway analysis of monosodium iodoacetate-induced
experimental osteoarthritis in rats: Relevance to human disease.
Osteoarthritis Cartilage. 15:1190–1198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ali MS, Starke RM, Jabbour PM, Tjoumakaris
SI, Gonzalez LF, Rosenwasser RH, Owens GK, Koch WJ, Greig NH and
Dumont AS: TNF-α induces phenotypic modulation in cerebral vascular
smooth muscle cells: Implications for cerebral aneurysm pathology.
J Cereb Blood Flow Metab. 33:1564–1573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kurien BT and Scofield RH: Western
blotting. Methods. 38:283–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bar-Yehuda S, Rath-Wolfson L, Del Valle L,
Ochaion A, Cohen S, Patoka R, Zozulya G, Barer F, Atar E,
Piña-Oviedo S, et al: Induction of an antiinflammatory effect and
prevention of cartilage damage in rat knee osteoarthritis by CF101
treatment. Arthritis Rheum. 60:3061–3071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang C, Zeng L, Zhang T, Liu J and Wang W:
Tenuigenin prevents IL-1β-induced inflammation in human
osteoarthritis chondrocytes by suppressing PI3K/AKT/NF-κB signaling
pathway. Inflammation. 39:807–812. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen J, Abu-Amer Y, O'Keefe RJ and
McAlinden A: Inflammation and epigenetic regulation in
osteoarthritis. Connect Tissue Res. 58:49–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohtori S, Orita S, Yamauchi K, Eguchi Y,
Ochiai N, Kishida S, Kuniyoshi K, Aoki Y, Nakamura J, Ishikawa T,
et al: Efficacy of direct injection of etanercept into knee joints
for pain in moderate and severe knee osteoarthritis. Yonsei Med J.
56:1379–1383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang X, Guan Y, Tian S, Wang Y, Sun K and
Chen Q: Mechanical and IL-1β responsive miR-365 contributes to
osteoarthritis development by targeting histone deacetylase 4. Int
J Mol Sci. 17:4362016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma Z, Wang Y, Piao T and Liu J:
Echinocystic acid inhibits IL-1β-induced COX-2 and iNOS expression
in human osteoarthritis chondrocytes. Inflammation. 39:543–549.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kunisch E, Kinne RW, Alsalameh RJ and
Alsalameh S: Pro-inflammatory IL-1beta and/or TNF-alpha up-regulate
matrix metalloproteases-1 and −3 mRNA in chondrocyte subpopulations
potentially pathogenic in osteoarthritis: In situ hybridization
studies on a single cell level. Int J Rheum Dis. 19:557–566. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Gao SG, Zhang FJ, Luo W, Xue JX
and Lei GH: Effects of osteopontin on the expression of IL-6 and
IL-8 inflammatory factors in human knee osteoarthritis
chondrocytes. Eur Rev Med Pharmacol Sci. 18:3580–3586.
2014.PubMed/NCBI
|
|
31
|
van Baarsen LG, Lebre MC, van der Coelen
D, Aarrass S, Tang MW, Ramwadhdoebe TH, Gerlag DM and Tak PP:
Heterogeneous expression pattern of interleukin 17A (IL-17A),
IL-17F and their receptors in synovium of rheumatoid arthritis,
psoriatic arthritis and osteoarthritis: Possible explanation for
nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 16:4262014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chevalier X, Ravaud P, Maheu E, Baron G,
Rialland A, Vergnaud P, Roux C, Maugars Y, Mulleman D, Lukas C, et
al: Adalimumab in patients with hand osteoarthritis refractory to
analgesics and NSAIDs: A randomised, multicentre, double-blind,
placebo-controlled trial. Ann Rheum Dis. 74:1697–1705. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan Q, Sun L, Li JJ and An CH: Elevated
VEGF levels contribute to the pathogenesis of osteoarthritis. BMC
Musculoskelet Disord. 15:4372014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bondeson J, Wainwright S, Hughes C and
Caterson B: The regulation of the ADAMTS4 and ADAMTS5 aggrecanases
in osteoarthritis: A review. Clin Exp Rheumatol. 26:139–145.
2008.PubMed/NCBI
|
|
35
|
Chen J, Crawford R and Xiao Y: Vertical
inhibition of the PI3K/Akt/mTOR pathway for the treatment of
osteoarthritis. J Cell Biochem. 114:245–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Young SR, Gerard-O'Riley R, Harrington M
and Pavalko FM: Activation of NF-kappaB by fluid shear stress, but
not TNF-alpha, requires focal adhesion kinase in osteoblasts. Bone.
47:74–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Isozaki T, Kasama T, Takahashi R, Odai T,
Wakabayashi K, Kanemitsu H, Nohtomi K, Takeuchi HT, Matsukura S and
Tezuka M: Synergistic induction of CX3CL1 by TNF alpha and IFN
gamma in osteoblasts from rheumatoid arthritis: Involvement of
NF-kappa B and STAT-1 signaling pathways. J Inflamm Res. 1:19–28.
2008.PubMed/NCBI
|
|
38
|
Qiu L, Zhang L, Zhu L, Yang D, Li Z, Qin K
and Mi X: PI3K/Akt mediates expression of TNF-alpha mRNA and
activation of NF-kappaB in calyculin A-treated primary osteoblasts.
Oral Dis. 14:727–733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen HW, Lin AH, Chu HC, Li CC, Tsai CW,
Chao CY, Wang CJ, Lii CK and Liu KL: Inhibition of TNF-α-induced
inflammation by andrographolide via down-regulation of the PI3K/Akt
signaling pathway. J Nat Prod. 74:2408–2413. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Inam A, Shahzad M, Shabbir A, Shahid H,
Shahid K and Javeed A: Carica papaya ameliorates allergic asthma
via down regulation of IL-4, IL-5, eotaxin, TNF-α, NF-κB, and iNOS
levels. Phytomedicine. 32:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sanchavanakit N, Saengtong W,
Manokawinchoke J and Pavasant P: TNF-α stimulates MMP-3 production
via PGE2 signalling through the NF-κB and p38 MAPK pathway in a
murine cementoblast cell line. Arch Oral Biol. 60:1066–1074. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim JM, Cho HH, Lee SY, Hong CP, Yang Jw,
Kim YS, Suh KT and Jung JS: Role of IRAK1 on TNF-induced
proliferation and NF-κB activation in human bone marrow mesenchymal
stem cells. Cell Physiol Biochem. 30:49–60. 2012. View Article : Google Scholar : PubMed/NCBI
|