Introduction
Acute lung injury (ALI) is defined as a clinical
syndrome of severe pulmonary inflammation, which is caused by
indirect or direct lung injury with alveolar-capillary barrier
disruption as well as gas exchange dysfunction (1). ALI is characterized by neutrophilic
infiltration, uncontrolled inflammatory process and oxidative
stress (2,3). Although various new therapies have
improved the survival of patients, the morbidity and mortality
rates are still very high, (4,5) which
has encouraged researchers to further study the pathophysiological
mechanisms of ALI to develop more effective therapeutic
regimens.
Until now, although many studies have tried to
demonstrate the underlying pathophysiological process, the
molecular mechanism of ALI has not been well understood (6). However, it is known that alveolar
macrophages (AMs), which serve as the first line of defense in the
lung and play a major role in the process of ALI, are activated via
a mechanism involving Toll-like receptors (TLRs) to reduce damage
to the lungs (7). In addition,
previous studies have indicated that inflammation and oxidative
stress are closely associated with the pathogenesis of ALI
(8). Nuclear factor-κB (NF-κB), a
central mediator in inflammation, strictly controls the production
of various important pro-inflammatory cytokines secreted by
activated AMs in the early stage of ALI, including tumor necrosis
factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6 (9). Therefore, the suppression of the
activation of AMs by targeting NF-κB could alleviate
inflammation-induced ALI.
MicroRNAs (miRNAs), endogenous non-coding small RNAs
selectively expressed in immunocytes, have promising potential in
the regulation of inflammation. Previous studies have revealed that
miRNAs, such as miR-155, miR-21 and miR-146a, could modulate the
biological behaviors of immunocytes in terms of activation,
proliferation and differentiation during inflammatory responses
(5,10,11). Of
these behaviors, modulations targeting the NF-κB pathway have drawn
increasing attention. It has been reported that several miRNAs
participate in the ALI process (7).
Among them, miR-21 is known to have vital functions in
immunological reactions. A study of miRNA expression during ALI
induced by aerosolized LPS in an animal model demonstrated that
miR-21 performs a pivotal function in the inflammatory response
(8). Another study showed that
miR-21 can negatively regulate LPS-induced inflammatory responses
(12). These studies indicated that
miR-21 may play a vital role in the pathological process. However,
the clear mechanism connecting miR-21 and LPS-induced ALI is still
not well-understood. Therefore, it is necessary to further
investigate the underlying mechanism by which miR-21 regulates ALI.
miR-21 has also been shown to be an inhibitor of the production of
pro-inflammatory cytokines in a manner that is associated with the
NF-κB signaling pathway (12–15).
Thus, we hypothesized that miR-21 might also participate in immune
modulation in ALI through the NF-κB pathway.
To test our hypothesis, we used an in vitro
AM model and an in vivo lipopolysaccharide (LPS)-induced ALI
model to evaluate the role of miR-21 (referred to as miR-21-5p
hereafter) in the regulation of inflammation in ALI. A miR-21 mimic
and an inhibitor were also used to investigate the potential
mechanism of miR-21-5p in ALI.
Materials and methods
Animal model
Sprague Dawley rats (approximately 8 weeks old)
purchased from the Traditional Chinese Medical Hospital of Zhuji
(Zhuji, China) were used through the study for the in vivo
assays. All animal experiments were approved by the Animal
Committee of the Traditional Chinese Medical Hospital of Zhuji. The
ALI model was established in the rats according to the methods in
previous reports (6). Briefly, rats
were intraperitoneally anesthetized with pentobarbital sodium (50
mg/kg). Then, 7.5 mg/kg LPS was intratracheally instilled. Animals
in the control group were subjected to the same procedure, but only
PBS was instilled. Rats were sacrificed 8 h later. Unilateral lungs
were processed by bronchoalveolar lavage to measure the levels of
the cytokines TNF-α, IL-6, IL-1β and IL-10. The other lungs were
used for histopathological observations and gene expression assays.
For the bronchoalveolar lavage, the trachea was dissected after the
rat was sacrificed by cervical dislocation. Then, the trachea at
the telecentric end and the other bronchus were ligated, and an
intravenous catheter was inserted into the remaining bronchus. The
syringe was used to lavage the lung with 0.8 ml PBS. The lung
tissue was gently massaged to collect the lavage fluid, and the
lavage process was repeated 3 times. Then, the lavage fluid was
centrifuged. The supernatant was collected for further measurement
of cytokines. The precipitates were further treated with red blood
cell lysis buffer. Subsequently, the AMs were obtained though the
attachment culture method. Finally, the miR-21 and TNF-α mRNA
expression levels of the AMs were measured.
Histological analysis of lung
Lung samples from the rats were fixed in 4%
paraformaldehyde for subsequent staining with hematoxylin and
eosin. The degree of lung damage was observed and recorded under
light microscopy.
Cell culture
NR8383 AMs (derived from Sprague Dawley rats) were
obtained from Cellcook Biotechnology Co., Ltd. (Guangzhou, China)
and were used for the in vitro assays. The cells were seeded
in a 6-well plate at concentration of 2×106 cell/ml, and
then LPS (1 mg/l) was used to stimulate the cells after 90 min. The
supernatant was collected at 0, 6, 12 and 24 h after stimulation,
and the cells were harvested. The production of TNF-α, IL-6, IL-1β
and IL-10 in the supernatant was measured by ELISA (MultiSciences
Co. Ltd., Hangzhou, China), and the expression levels of miR-21and
TNF-α mRNA were detected by real-time PCR.
Transfection
To selectively induce the upregulation of miR-21 in
NR8383 cells, mimics of miRIDIAN™ miR-21 were introduced
into the cells. The function of miR-21 was effectively silenced by
a miRIDIAN™ hairpin inhibitor. For transfection, miRNA
mimics (0.4 nmol) or anti-miRNA inhibitors (0.4 nmol) were mixed
with 15 µl of GenePOTER 2 Transfection Reagent (Genlantis, San
Diego, CA, USA). Then, the mixtures were transfected into
1×106 cells for 8 h. After a 48-h incubation, the cells
were used for other assays. NR8383 cells transfected with the
miR-21 mimic or anti-miR-21 were stimulated with LPS (1 mg/l).
After incubating for 6 h, the supernatant and the cells were
collected. Quantitative PCR was used to detect the expression level
of miR-21. The changes in the secretion of inflammatory cytokines
were measured by ELISA. The expression changes of TLR-4 and
intra-nuclear NF-κB p65 protein were detected by western blot.
Cytokines detection
The concentrations of TNF-α, IL-6, IL-1β and IL-10
in the cell culture supernatants and the bronchoalveolar lavage
fluid (BALF) were detected by ELISA kits according to the
instruction manuals (MultiSciences Co., Ltd). The absorbance at 450
nm was measured to determine the concentrations.
RNA expression levels
Quantitative real-time PCR was performed to measure
the TNF-α mRNA and miR-21 expression levels in cells and in the
lungs of rats with ALI in different groups. Total RNA was extracted
from lungs and NR8383 cells with TRIzol (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) according to the instructions. RNA expression
levels were evaluated by the ratio of 260/280. Total RNA was
reverse-transcribed to complementary DNA with the oligo (dT)
primer. The data were normalized to glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) using the 2−∆∆Cq method (16). The primer sequences for miR-21 were
5′-TGCGCTAGCTTATCAGACTGAT-3′ (sense) and
5′-CCAGTGCAGGGTCCGAGGTATT-3′ (antisense). The primer sequences for
TNF-α were 5′-TCTCAAAACTCGAGTGACAAG-3′ (sense) and
5′-AGTTGGTTGTCTTTGAGATCC-3′ (antisense). The primer sequences for
GAPDH were 5′-TGCGCTAGCTTATCAGACTGAT-3′ (sense) and
5′-GCGTGGAATACATTGGAACATGT-3′ (antisense). U6 snRNA was used as an
internal control to quantify and normalize the expression of
miR-21. The primer sequences for U6 snRNA were
5′-GCTTCGGCAGCACATATACTAAAAT-3′, and
5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Dual-luciferase reporter assay
We created the TLR-4 3′-UTR luciferase reporter
construct using the psiCHECK luciferase reporter vector and named
it TLR-4-WT (5′…CGAAACCUCAAAUAAGCUC…3′). We created the
TLR-4 3′-UTR luciferase reporter construct with miR-21
(5′-AGUUGUAGUCAGACUAUUCGAU-3′) target site mutation using
the miR-21-binding site mutation primers and named it TLR-4-MUT
(5′…CGAAACCUCAAAGAUCAUC…3′). The target sites were showed in
bold. Then, we co-transfected cells with TLR-4-WT or TLR-4-MUT and
miR-21. The relative luciferase activity was measured using the
dual-luciferase reporter assay system twenty-four hours later.
Western blot analysis
Western blotting was performed to detect the changes
in expression of TLR-4 and the intra-nuclear NF-κB p65 protein in
different groups of NR8383 cells. In short, 50 µg of total protein
was separated by sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE; 12%) from the cellular protein extracts
and transferred to nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA). After blocking with Tris-buffered saline
containing Tween-20 (TBS-T; 0.1%) and blocking buffer (nonfat dried
milk, 5%) at room temperature for 2 h, the membranes were incubated
with primary antibodies (diluted in blocking buffer, 1:200) against
TLR-4 and intra-nuclear NF-κB p65 at 4°C overnight. Subsequently,
the membranes were further incubated with secondary antibodies
(diluted in blocking buffer, 1:2,000) at room temperature for 1 h
and then washed with TBS-T six times. Finally, the membranes were
exposed to an enhanced chemiluminescence kit, and the results were
recorded on X-ray film. GAPDH was used as a control. All
experiments were repeated in triplicate.
Statistical analysis
All quantitative data are presented as the means ±
SEM. Statistical P-values were calculated by Student's
t-tests or one-way ANOVA followed by the Tukey's test as indicated
with GraphPad Prism 7 software (GraphPad Software, Inc., La Jolla,
CA, USA).
Results
LPS caused pro-inflammation and
upregulation of miR-21 in the model of ALI
LPS was used to induce ALI in rats to study the
inflammatory response in ALI. Eight hours after challenge with or
without LPS, the lung tissues of the rats were collected for
histological analysis. As shown in Fig.
1A, LPS administration caused obvious obstruction of the
tubular lumen, infiltration of inflammatory cells and impairment.
TNF-α is one of the most commonly used inflammation markers of ALI.
The TNF-α level was approximately 9-fold higher in ALI rats than in
the control group. The mRNA expression of TNF-α in the AMs of ALI
rats was also significantly increased (about 4-fold) compared with
that of the control group, as shown in Fig. 1B. In addition, IL-6 and IL-1β, two
other inflammatory cytokines that play vital roles in lung injury,
had markedly increased expression levels after LPS stimulation
(Fig. 1C and D). In contrast, the
expression level of IL-10 (an anti-inflammatory cytokine) was
significantly decreased in rats after LPS treatment (Fig. 1E). According to information from
previous studies on the upregulation of miR-21 in macrophages after
LPS administration, miR-21 expression was measured by qRT-PCR 8 h
after LPS treatment. As shown in Fig.
1F, the miR-21 expression level increased approximately
3.6-fold in AMs from lung tissues in ALI rats compared to the
control group. Therefore, LPS-induced ALI displayed histological
impairments of the lungs, pro-inflammatory responses and
upregulation of miR-21.
LPS caused inflammation and the
upregulation of miR-21 in AMs
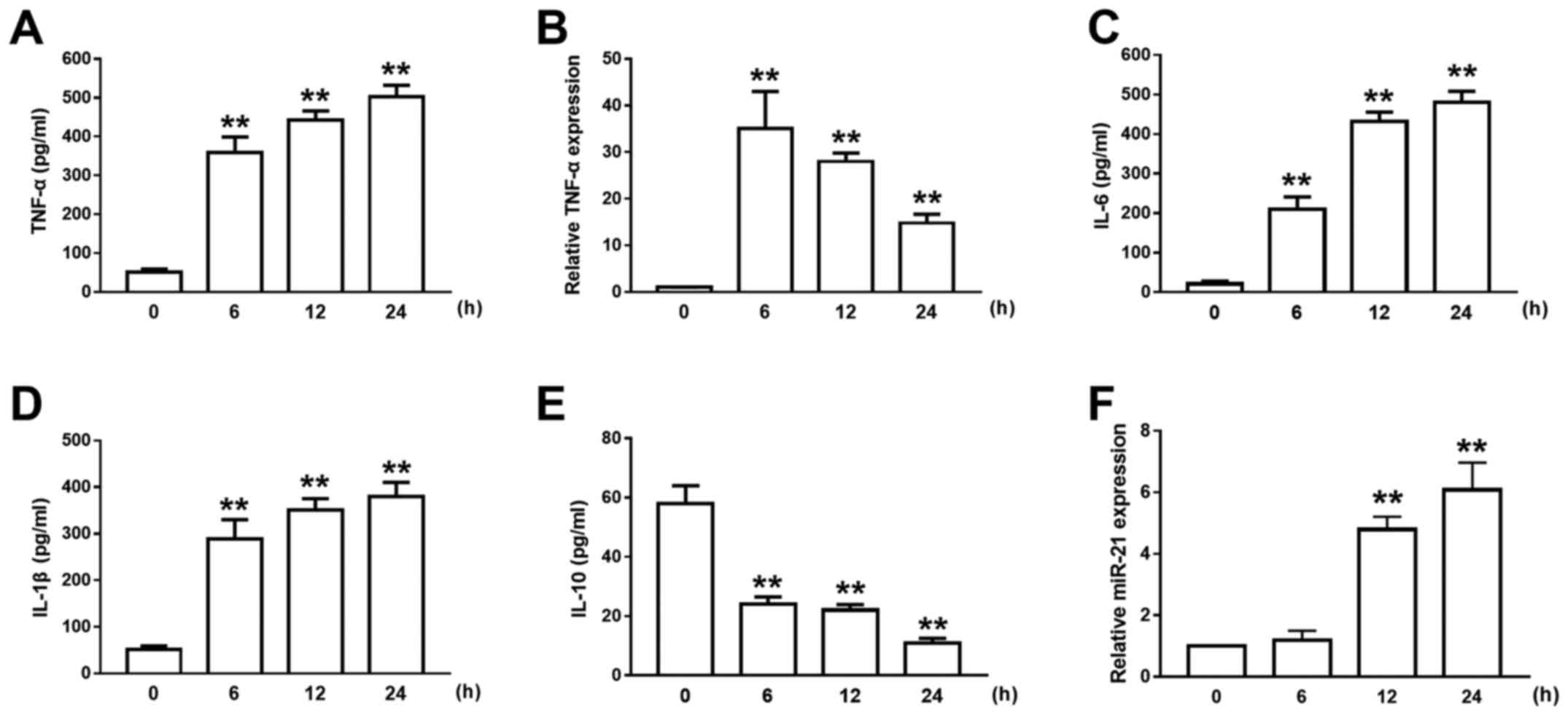
In the physiopathology of ALI, AMs play an important
role in the regulation of inflammation. After LPS treatment of
NR8383 cells (AM cell line), the expression level of TNF-α
increased in a time-dependent manner (Fig. 2A), while the expression of TNF-α mRNA
peaked 6 h after the LPS treatment (Fig.
2B). In the meantime, the concentrations of the proinflammatory
cytokines IL-6 and IL-1β were gradually increased after LPS
administration and lasted for at least 12 h (Fig. 2C and D). However, the expression
level of IL-10 was significantly decreased after LPS treatment
(Fig. 2E). An increase in miR-21
expression after LPS treatment was also observed, and a significant
4.2-fold increase in miR-21 expression was detected 12 h after LPS
administration (Fig. 2F). Due to the
mismatch between the early peak in TNF-α expression and the delayed
increase in miR-21 expression after LPS challenge in AMs, we
propose the hypothesis that miR-21 may participate in the negative
regulation of ALI inflammatory responses.
Expression of miR-21 regulates
inflammatory responses in ALI
To confirm the underlying role of miR-21 in the
regulation of inflammation in ALI, NR8383 cells transfected with
the miR-21 mimic or the anti-miR-21 inhibitor were investigated.
After transfection, significant increases and decreases in miR-21
expression were observed in miR-21 mimic- and anti-miR-21
inhibitor-transfected cells, respectively, compared to the single
LPS-treated group (Fig. 3A).
Subsequently, the effects of miR-21 overexpression or
downregulation on the expression of TNF-α, IL-6, IL-1β and IL-10
after LPS treatment in NR8383 cells were investigated. As shown in
Fig. 3B-E, NR8383 cells
overexpressing miR-21 showed significant suppression of
pro-inflammatory cytokines and increased levels of
anti-inflammatory cytokines after exposure to LPS. In contrast,
downregulation of miR-21 promoted the inflammatory responses of
NR8383 cells after LPS exposure. Taken together, it demonstrated
that miR-21 could negatively regulate LPS-induced inflammatory
responses.
miR-21 inhibited inflammation induced
by LPS by targeting the TLR4-NF-κB pathway
According to previous research, TLR-4 is a common
receptor of LPS, and its downstream signaling effector, NF-κB,
plays a crucial role in the inflammatory responses in ALI. The
potential targeting interaction between miR-21 and TLR-4 was
verified by the dual-luciferase reporter assays. The result showed
that the luciferase activity was significantly decreased after
co-transfection with TLR-4-WT and miR-21 but not after
co-transfection with TLR-4-MUT and miR-21 (Fig. 4A), indicating a targeting interaction
between miR-21 and TLR-4. To illuminate the underlying mechanism of
miR-21-regulated inflammatory events in AMs, the TLR4-NF-κB pathway
was investigated. As shown in Fig.
4B-D, transfection with miR-21 mimics markedly inhibited the
expression of TLR-4 and intra-nuclear NF-κB p65. Similarly,
downregulation of miR-21 significantly increased TLR-4 expression
and the activation of NF-κB p65 in LPS-treated AMs. Therefore,
these results demonstrated that miR-21 could impede the activation
of the TLR4-NF-κB pathway induced by LPS in AMs.
Discussion
ALI is a frequent, severe complication following
sepsis in patients with critical illness, and it is characterized
by widespread lung inflammation and high pulmonary vascular
permeability (8,9,17,18). LPS
administration is a classical method to create ALI models in
vivo (6,19–21). In
the present work, we chose intra-tracheal instillation of LPS for
ALI induction over intravenous administration to avoid potential
systemic injuries. The NR8383 cells used in the present study to
illuminate the underlying role of miR-21 in the modulation of
LPS-induced inflammation were derived from normal rat macrophages,
which have the typical features of phagocytosis and inflammatory
cytokines secretion.
LPS is transferred by the LPS-binding protein and
binds to macrophages via TLR-4 and CD14; subsequently, NF-κB
translocates into the nucleus, the production of pro-inflammatory
cytokines (such as TNF-α, IL-6, and IL-1β) is activated and the
level of anti-inflammatory cytokines (like IL-10) is decreased
(9,18,19,22). In
our ALI model, the concentrations of TNF-α, IL-6 and IL-1β in the
BALF were obviously increased 8 h after LPS administration, which
was also verified histopathologically. In contrast, the level of
IL-10 was reduced, facilitating the pro-inflammatory events. Taken
together, LPS can activate AMs and induce ALI.
Many studies have shown that miRNAs participate in
the regulation of the inflammatory response (6,17,23).
Various miRNAs such as miR-9, miR-147 and miR-132 have been
reported to have an inhibitory effect on inflammatory responses
(24–28). Studies from Sheedy et al
showed that miR-21 inhibits the pro-inflammatory mediator PDCD4,
blocking NF-κB activation and impeding the inflammatory response
(29). However, the role of miR-21
in the inflammatory responses of ALI have not been extensively
studied. In the present study, the early expression of TNF-α was
suppressed by the increased expression of miR-21 after LPS
challenge in AMs. In addition, according to previous research, the
TLR-4 (a common receptor of LPS) and NF-κB pathway plays a crucial
role in the inflammatory responses in ALI (13,29,30).
Therefore, we proposed the hypothesis that miR-21 may play a vital
role in the negative regulation of ALI inflammation responses. The
mechanism by which miR-21 affects the TLR-4-NF-κB signaling pathway
needs to be investigated. The expression level of miR-21 was
manipulated in LPS-stimulated NR8383 cells, and the secretion of
pro/anti-inflammatory cytokines, the expression of TLR-4 and the
intra-nuclear NF-κB p65 level were measured. The results suggested
that miR-21 could relieve LPS-induced inflammation by inhibiting
the TLR-4-NF-κB pathway.
However, ALI is a complex pathological process
involving many miRNAs and target genes. The main limitation of this
study is the sole focus on the TLR-4-NF-κB pathway. The underlying
relationship between this pathway and other related pathways needs
further investigations.
In summary, our research investigated the potential
role of miR-21 in the regulation of inflammation in LPS-induced
ALI. In the present study, LPS exposure induced a high expression
level of miR-21 both in vivo and in NR8383 cells. Further
analysis revealed that miR-21 participated in the negative
regulation of inflammatory responses in LPS-stimulated NR8383 cells
through the TLR4-NF-κB signaling pathway, indicating the vital role
of miR-21 in the progression of ALI. Therefore, this study has
provided further insight into the molecular mechanism of ALI
progression.
Acknowledgements
The present study was supported by the Basic Medical
Center of the Traditional Chinese Medical Hospital of Zhuji.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
W-DZ designed the study and revised the manuscript.
JX drafted the manuscript. The experiments were performed by JX, MZ
and T-MZ. Y-HZ and KS analyzed and processed the data.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Committee of the Traditional Chinese Medical Hospital of Zhuji.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guo Z, Gu Y, Wang C, Zhang J, Shan S, Gu
X, Wang K, Han Y and Ren T: Enforced expression of miR-125b
attenuates LPS-induced acute lung injury. Immunol Lett. 162:18–26.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Z, Zhang C, Cheng L, Hu M, Tao H and
Song L: The microRNA miR-17 regulates lung FoxA1 expression during
lipopolysaccharide-induced acute lung injury. Biochem Biophys Res
Commun. 445:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao J, Li X, Zou M, He J, Han Y, Wu D,
Yang H and Wu J: miR-135a inhibition protects A549 cells from
LPS-induced apoptosis by targeting Bcl-2. Biochem Biophys Res
Commun. 452:951–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen XY, Dou YX, Luo DD, Zhang ZB, Li CL,
Zeng HF, Su ZR, Xie JH, Lai XP and Li YC: β-Patchoulene from
patchouli oil protects against LPS-induced acute lung injury via
suppressing NF-κB and activating Nrf2 pathways. Int
Immunopharmacol. 50:270–278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song L, Zhou F, Cheng L, Hu M, He Y, Zhang
B, Liao D and Xu Z: MicroRNA-34a suppresses autophagy in alveolar
type II epithelial cells in acute lung injury by inhibiting FoxO3
expression. Inflammation. 40:927–936. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Qiu X, Jiang H, Han Y, Wei D and Liu
J: Downregulation of miR-181a protects mice from LPS-induced acute
lung injury by targeting Bcl-2. Biomed Pharmacother. 84:1375–1382.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng Z, Gong H, Li Y, Jie K, Ding C, Shao
Q, Liu F, Zhan Y, Nie C, Zhu W and Qian K: Upregulation of miR-146a
contributes to the suppression of inflammatory responses in
LPS-induced acute lung injury. Exp Lung Res. 39:275–282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li W, Ma K, Zhang S, Zhang H, Liu J, Wang
X and Li S: Pulmonary microRNA expression profiling in an immature
piglet model of cardiopulmonary bypass-induced acute lung injury.
Artif Organs. 39:327–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SM, Choi H, Yang G, Park KC, Jeong S
and Hong S: microRNAs mediate oleic acid-induced acute lung injury
in rats using an alternative injury mechanism. Mol Med Rep.
10:292–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tao Z, Yuan Y and Liao Q: Alleviation of
lipopolysaccharides-induced acute lung injury by miR-454. Cell
Physiol Biochem. 38:65–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang W, Liu Z, Su J, Chen WS, Wang XW, Bai
SX, Zhang JZ and Yu SQ: Macrophage micro-RNA-155 promotes
lipopolysaccharide-induced acute lung injury in mice and rats. Am J
Physiol Lung Cell Mol Physiol. 311:L494–L506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng J, Li A, Deng J, Yang Y, Dang L, Ye
Y, Li Y and Zhang W: miR-21 attenuates lipopolysaccharide-induced
lipid accumulation and inflammatory response: Potential role in
cerebrovascular disease. Lipids Health Dis. 13:272014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z,
Qi H, Guo H and Yin H: Dendritic cell-derived exosomes elicit tumor
regression in autochthonous hepatocellular carcinoma mouse models.
J Hepatol. 67:739–748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Jia Z, Li A, Jenkins G, Yang X, Hu J
and Guo W: Resveratrol repressed viability of U251 cells by miR-21
inhibiting of NF-κB pathway. Mol Cell Biochem. 382:137–143. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong X, Chung AC, Chen HY, Dong Y, Meng
XM, Li R, Yang W, Hou FF and Lan HY: miR-21 is a key therapeutic
target for renal injury in a mouse model of type 2 diabetes.
Diabetologia. 56:663–674. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei C, Li L, Kim IK, Sun P and Gupta S:
NF-κB mediated miR-21 regulation in cardiomyocytes apoptosis under
oxidative stress. Free Radic Res. 48:282–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Xian PF, Yang L and Wang SX:
MicroRNA-21 promotes proliferation of fibroblast-like synoviocytes
through mediation of NF-κB nuclear translocation in a rat model of
collagen-induced rheumatoid arthritis. Biomed Res Int.
2016:92790782016.PubMed/NCBI
|
|
19
|
Tang R, Pei L, Bai T and Wang J:
Down-regulation of microRNA-126-5p contributes to overexpression of
VEGFA in lipopolysaccharide-induced acute lung injury. Biotechnol
Lett. 38:1277–1284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao J, Chen C, Guo M, Tao Y, Cui P, Zhou
Y, Qin N, Zheng J, Zhang J and Xu L: MicroRNA-7 deficiency
ameliorates the pathologies of acute lung injury through elevating
KLF4. Front Immunol. 7:3892016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen C, Luo F, Liu X, Lu L, Xu H, Yang Q,
Xue J, Shi L, Li J, Zhang A and Liu Q: NF-κB-regulated exosomal
miR-155 promotes the inflammation associated with arsenite
carcinogenesis. Cancer Lett. 388:21–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Mao G, Lv Y, Huang Q and Wang G:
MicroRNA-181b stimulates inflammation via the nuclear factor-κB
signaling pathway in vitro. Exp Ther Med. 10:1584–1590. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Bai X, Song Q, Fan F, Hu Z, Cheng
G and Zhang Y: miR-223 inhibits lipid deposition and inflammation
by suppressing toll-like receptor 4 signaling in macrophages. Int J
Mol Sci. 16:24965–24982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Z and You Z: Mesenchymal stem cells
alleviate LPS-induced acute lung injury in mice by
miR-142a-5p-controlled pulmonary endothelial cell autophagy. Cell
Physiol Biochem. 38:258–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee HM, Kim TS and Jo EK: miR-146 and
miR-125 in the regulation of innate immunity and inflammation. BMB
Rep. 49:311–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bao MH, Li JM, Luo HQ, Tang L, Lv QL, Li
GY and Zhou HH: NF-κB-regulated miR-99a modulates endothelial cell
inflammation. Mediators Inflamm. 2016:53081702016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang D, Cao X, Li J and Zhao G: miR-210
inhibits NF-κB signaling pathway by targeting DR6 in
osteoarthritis. Sci Rep. 5:127752015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao J, Tang J, Chen Q, Tang D, Liu M, Luo
M, Wang Y, Wang J, Zhao Z, Tang C, et al: miR-429 regulates
alveolar macrophage inflammatory cytokine production and is
involved in LPS-induced acute lung injury. Biochem J. 471:281–291.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sheedy FJ, Palsson-McDermott E, Hennessy
EJ, Martin C, O'Leary JJ, Ruan Q, Johnson DS, Chen Y and O'Neill
LA: Negative regulation of TLR4 via targeting of the
proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat
Immunol. 11:141–147. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng P, Li Z and Liu X: Reduced expression
of miR-23a suppresses A20 in TLR-stimulated macrophages.
Inflammation. 38:1787–1793. 2015. View Article : Google Scholar : PubMed/NCBI
|