Introduction
Experimental autoimmune encephalomyelitis (EAE) is a
kind of autoimmune disease mediated by cluster of differentiation
4+T cell (1), whose characteristic
is that mononuclear cell infiltration occurs in the central nervous
system (CNS) and around the small vessels, while inflammation is
the vital cause for the occurrence and development of EAE (2,3). The
pathogenesis of the disease is very complicated and is associated
with the activation of immune cells, destruction of blood-brain
barrier and activation of glial cells (4).
Astrocytes are the satellite cells of neurons and a
category of glial cells with the largest volume (5). Many long and branched processes
generated from the body of astrocyte extend and fill up the space
between the bodies of nerve cells and their processes, playing a
part in supporting and separating the nerve cells (6). In addition, astrocytes can exert
neuroprotective effects through the mechanisms of downregulating
the inflammatory responses of the CNS and alleviating the oxidative
stress (7). Neuroglia cells
participate in the pathophysiological processes of inflammations;
for instance, the activation of microglia is an important
participant of removing necrotic debris and other foreign
substances (8). However, the
hyperactivation of astrocytes in the EAE models can accelerate the
activation of inflammatory transcription factors and multiple
inflammatory mediators and initiate inflammatory cascades, thus
promoting the progression of disease (9).
This study aimed to analyze the activation
conditions of the astrocytes in the EAE models and the association
of inflammatory factor levels expressed by them with the
disease.
Materials and methods
Laboratory animals and EAE
modeling
Wistar rats (weighing 200±18 g, male) were purchased
from and fed at the Jiangsu Laboratory Animal Center. The rats were
fed in a specific pathogen-free environment at a room temperature
of 25±2°C, with free intake of food and water. The study was
approved by the Ethics Committee of Daqing Longnan Hospital
(Daqing, China).
The EAE models were established according to foreign
literature (10): Myelin
oligodendrocyte glycoprotein 35–55 (MOG35-55) (article number:
2568, R&D Systems Europe, Ltd., Abingdon, UK, 400 µg),
mycobacterium tuberculosis H37Ra (400 µg), phosphate-buffered
saline (PBS) 200 µl; and complete Freund's adjuvant (CFA) solution
100 µl were mixed; and the mixture was made into milky-white
suspension through ultrasonic emulsification. The rats in the EAE
group were intraperitoneally injected with immunopotentiator
pertussis toxin (500 ng/rat) at 0 and 2 days, followed by 0.1 ml
MOG35-55 emulsion. Rats in the control group were injected with an
equivalent volume of normal saline. The clinical symptoms of the
rats were observed for 20 days, and scores were recorded in
accordance with the Wear neurological function scale as: 0 point,
no signs; 1, limp tail and ataxia; 3, paralysis of one hind limb;
4, paralysis of both hind limbs; and 5, dying or death.
Hematoxylin and eosin (H&E)
staining
The rats were anaesthetised with pentobarbital (2.5
mg/100 g) then sacrificed by decapitation. The brains of the two
groups of rats were fixed in an appropriate amount of formalin
overnight, which were then dehydrated in graded alcohol, cleared,
wax-impregnated and embedded in paraffin, after which the paraffin
tissues were sliced to 0.4 µm. The sections were baked in an oven
at 65°C for 3–5 h, followed by dewaxing in xylene reagent and
rehydration with graded ethanol. H&E staining, rehydration with
graded ethanol and drying of sections were conducted in sequence,
and the sections were mounted with neutral balsam. The
histomorphology of the sections was observed and photographed under
a microscope (DM-5000B, Leica Microsystems, Wetzlar, Germany).
Detection of activation conditions of
astrocytes via immunofluorescence
The brain tissue sections of the rats were
permeabilized with 0.1% Triton X-100 for 10 min after dewaxing and
rehydration with ethanol, which were then blocked in 5% standard
protein bovine serum albumin for 30 min. The rabbit anti-rat
primary antibodies to glial fibrillary acidic protein (GFAP)
polyclonal antibody were added to the sections in drops and blocked
at 4°C overnight (1:200; cat. no. ab7260; Abcam, Cambridge, UK).
The sections were washed in PBS with Tween-20 three times the next
day, followed by incubation with Alexa Fluor-labeled fluorescent
goat anti-rabbit secondary polyclonal antibody (1:1,000; cat. no.
A-11034; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at room
temperature for 1 h. The sections were mounted after 4′,
6-diamidino-2-phenylindole was added for visualization of cell
nuclei for 3 min, which were observed and photographed under an
inverted fluorescence microscope (DM-6000B, Leica
Microsystems).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was applied to extract the total RNA in the brain
tissue of the rats. The RNA concentration was detected using a
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
RNA (1 µg) was withdrawn for reverse transcription (Takara Bio,
Inc., Otsu, Japan), and the obtained complementary DNA was stored
at −20°C. The messenger RNA (mRNA) levels of various indexes were
measured in accordance with the instructions of an All-in-One™ qPCR
Mix (GeneCopoeia, Inc., Rockville, MD, USA) kit. Reaction
conditions of RT-qPCR were: Step 1, at 90°C for 10 min; 2, at 95°C
for 10 sec; 3, at 60°C for 20 sec and 4, at 70°C for 20 sec; 45
cycles in total. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
was selected as the internal reference. The formula for the mRNA
relative expression levels of the indexes was: 2−ΔΔCq
[ΔCq = Cq (target gene) - Cq (GAPDH)]. The corresponding primer
sequences are shown in Table I.
 | Table I.Primer sequences for RT-qPCR. |
Table I.
Primer sequences for RT-qPCR.
| Gene name | Primer sequence | Length (bp) |
|---|
| IL-10 |
5′-GAGATCTCCGAGATGCCTTCAG-3′ | 248 |
|
|
5′-CAAGGACTCCTTTAACAACAAGTTGT-3′ |
|
| TNF-α |
5′-CAGGGGCCACCACGCTCTTC-3′ | 189 |
|
|
5′-CTTGGGGCAGGGGCTCTTGAC-3′ |
|
| IFN-γ |
5′-GCTGTCATAATAATATTCAGAC-3′ | 273 |
|
|
5′-CGAGCTTTAAAAGATAGTTCC-3′ |
|
| GAPDH |
5′-AGGTCGGTGTGAACGGATTTG-3′ | 254 |
|
|
5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
|
Western blot analysis
Brain tissues (5 g) of the rats were cut up and
placed into a 2.5 ml Eppendorf tube, to which 150 µl mixture of
radio immtmoprecipitation assay lysis buffer and protease inhibitor
Cocktail (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added,
followed by homogenizing in a tissue homogenizer for 10 min. After
centrifugation at 12,000 × g for 15 min (at 4°C), the upper liquid,
namely, tissue protein, was absorbed. The concentration of the
protein was measured using a bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology, Haimen, China). After being
denatured, the total protein was separated by 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, which was transferred
to the nitrocellulose membrane (Merck KGaA). The bands were blocked
in 5% skim milk for 1 h and then incubated in monoclonal antibodies
overnight. Interleukin-10 (IL-10; Abcam, 1:1,000), tumor necrosis
factor-α (TNF-α; Abcam, 1:2,000) and interferon-γ (IFN-γ; Abcam,
1:1,000) were added. The bands were then incubated in secondary
antibodies of anti-rabbit immunoglobulin G for 1 h after the
membrane was washed, and enhanced chemiluminescence system (Bio-Rad
Laboratories, Inc.) was used to reveal the bands of target
proteins.
Statistical analysis
GraphPad Prism software (Version 5.01; GraphPad
Software, Inc., La Jolla, CA, USA) was utilized for analysis.
Independent-sample t-test was applied to compare the differences in
the indexes between the two groups of samples. P<0.05 was
considered to indicate a statistically significant difference.
Results
Wear scores for neurological functions
of rats
There was no onset of disease in rats of the control
group from beginning to end. However, in the EAE group, the disease
occurred in the 10 rats at 9 days after immunization, with an
average onset time of 5.4±1.5 days. Eight rats were attacked by the
disease, 1 rat was not attacked and 1 rat was dead; the incidence
rate was 80%. During the onset of disease, the Wear score of the
rats in EAE group was markedly increased (P<0.05) (Fig. 1). In addition, the weight of the rats
in EAE group at 9 days after immunity was markedly lower than that
in the control group (P<0.05) (Fig.
2).
Detection of brain histomorphology of
rats via HE staining
As shown in Fig. 3,
the brain tissue sections of the control and EAE groups were
compared, and it was indicated that the inflammatory lesion was
mainly located in the region of brain parenchyma in rats of the EAE
group, which was presented as local infiltration of inflammatory
cells dominated by lymphocytes and monocytes. It confirmed that the
EAE model had been successfully established.
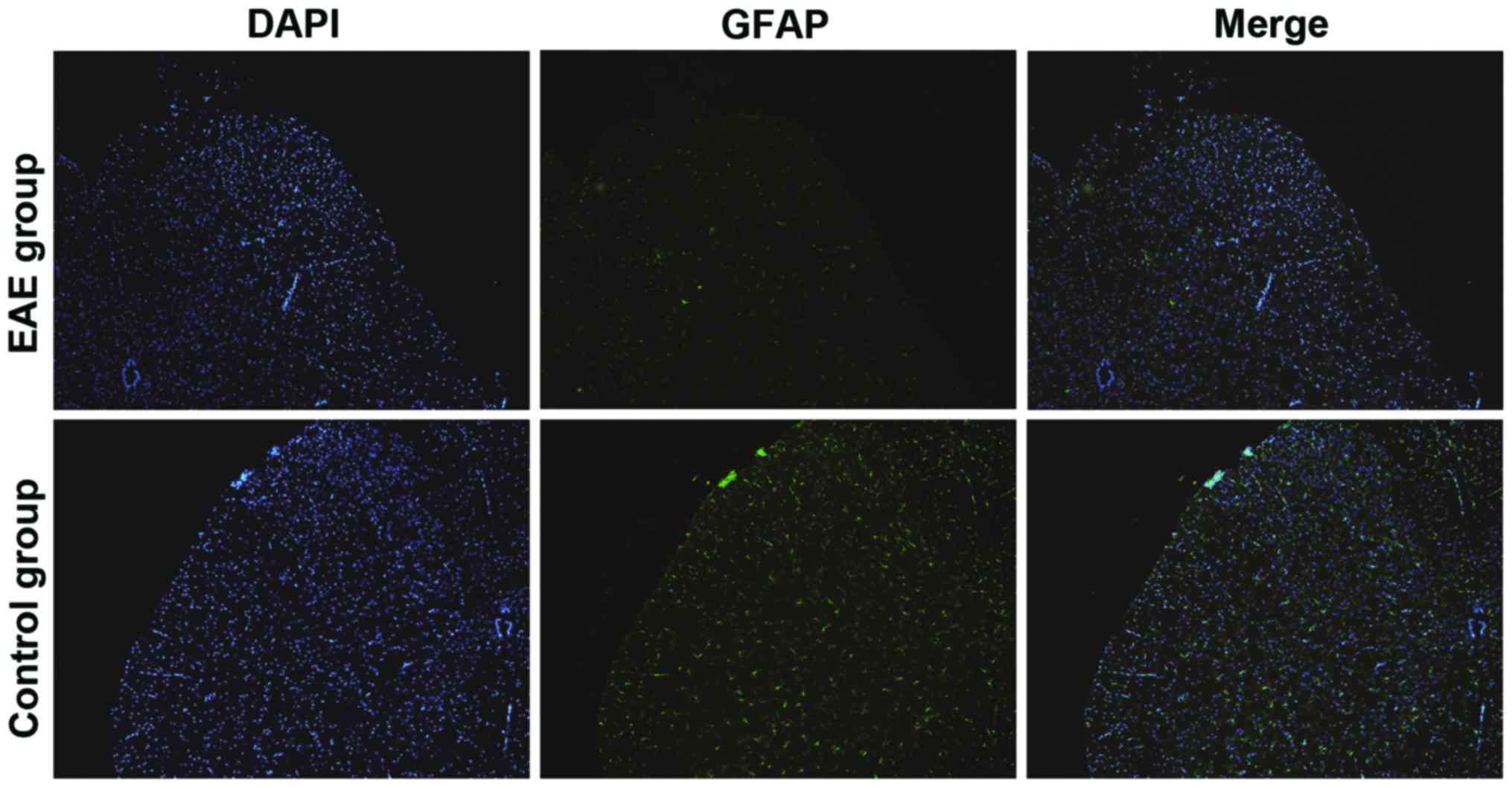
Detection of activation conditions of
astrocytes via immunofluorescence
The activation degree of the astrocytes in the rat
CNS was detected using GFAP specificity. In Fig. 4, the green fluorescence intensity
represented the expression level of GFAP. The GFAP level in the
brain sections of rats in EAE group was notably elevated compared
with that in control group, suggesting that the activation degree
of the astrocytes was significantly increased in the EAE rat
models.
Detection of inflammatory cytokine
expressions via RT-qPCR
As shown in Fig. 5,
compared with those in control group, the mRNA expression level of
IL-10 in the brains of the rats in EAE group was lowered obviously
(P<0.05), while the mRNA expression levels of IFN-γ and TNF-α
were elevated notably (P<0.05).
Detection of inflammatory cytokine
expressions via western blot analysis
In order to further investigate the expression of
inflammatory factors in the EAE models, western blot analysis was
performed to measure the levels of inflammatory factors in the
brain tissues of the two groups of rats. Fig. 6 shows that, the protein level of
IL-10 in the brains of rats in the EAE group was decreased
significantly (P<0.05), while those of IFN-γ and TNF-α were
markedly increased (P<0.05).
Discussion
The pathogenesis of EAE is very complex, and the T
helper cell type 1 (Th1)/Th2 imbalance which has been identified
currently is the main feature of the EAE pathological process
(11), in which Th1 plays a major
role. As a group of small molecular polypeptides regulating the
immune responses, cytokines play vital parts in the regulation of
the immune system (12). The
encephalitogenic cytokine IFN-γ and TNF-α which are associated with
inflammatory responses mediated by Th1 can promote the activation
and infiltration of T cells and macrophages and directly damage the
blood-cerebrospinal fluid barrier and myelin sheaths surrounding
the neurons, further expanding the inflammatory lesion (13,14).
TNF-α is mainly secreted by the monocytes in the CNS, most of which
have been identified as phenotype cells of microglia and
macrophages. Inducing the proliferation of astrocytes can increase
the severity of EAE (15).
IL-10 is a type of cytokine secreted by Th2 and has
the immunosuppressive function; it can inhibit the Th1-mediated
immune responses (16). Furthermore,
Ouyang et al (17) revealed
that IL-10 also has an anti-inflammatory effect. Specifically,
IL-10 may influence the pathogenesis of EAE at the initiating
stages of the T cell, including recruitment of inflammatory cells
for the CNS and destruction of the CNS tissues. IL-10 can
significantly strengthen these cells to suppress the inflammatory
lesion of the CNS and improve the capability of autoimmune
reaction, thus further decreasing the damage to the myelin sheaths
(17,18). Furthermore, IL-10 can enhance the
differentiation of transplanted neural stem cells into neurons and
lower the possibility of astrocyte hyperplasia at the same time. It
can also accelerate the growth of myelin sheaths and axons, which
is also an important factor for the pathogenesis of EAE (19).
Previous findings have indicated that the action of
astrocytes is involved in the EAE progression (20). Therefore, in this research, the EAE
rat models were successfully established by injecting MOG33-35 into
the spinal cord, which was consistent with the report of Wang et
al (21); EAE rats had loss of
myelin sheaths and infiltration of inflammatory cells in the brain;
the weight changes were recorded at the same time; the disease
occurred in the rats in EAE group one after another at 10 days
after immunization, of which the clinical manifestations and Wear
scores met the modeling standards. According to the report of Wang
et al (21), the brain
tissues of the rats were fixed at 21 days after immunization. Since
GFAP is the skeleton protein of the astrocytes, its expression
level can reflect the degrees of astrocyte hyperplasia and
necrosis; the activation conditions of the astrocytes in the rat
brain tissues was determined by means of immunofluorescence in this
research, and the expression levels of the inflammatory factors in
the brain tissue cells of the two groups of rats were detected
simultaneously. It was found that the number of activated
astrocytes in the brains of EAE rat models was increased obviously;
furthermore, RT-qPCR and western blot analysis experiments
suggested that the level of anti-inflammatory cytokine IL-10 which
was expressed by the astrocytes of EAE rats was markedly decreased,
while that of inflammatory factors TNF-α and IFN-γ was elevated
significantly.
In conclusion, the results of the present study are
that, the expression of inflammatory factors IFN-γ and TNF-α were
markedly increased, while the expression of anti-inflammatory
cytokine IL-10 was decreased during the development of EAE,
accompanied with the significant activation of astrocytes at the
same time. It is suggested that astrocytes and inflammatory factors
(IL-10, TNF-α and IFN-γ) play crucial roles in the occurrence and
development of EAE.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and HX were responsible for immunofluorescence.
LZ and LC helped with PCR and western blot analysis. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Daqing Longnan Hospital (Daqing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumar V, Stellrecht K and Sercarz E:
Inactivation of T cell receptor peptide-specific CD4 regulatory T
cells induces chronic experimental autoimmune encephalomyelitis
(EAE). J Exp Med. 184:1609–1617. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reboldi A, Coisne C, Baumjohann D,
Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A,
Engelhardt B and Sallusto F: C-C chemokine receptor 6-regulated
entry of TH−17 cells into the CNS through the choroid
plexus is required for the initiation of EAE. Nat Immunol.
10:514–523. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kroenke MA, Carlson TJ, Andjelkovic AV and
Segal BM: IL-12-and IL-23-modulated T cells induce distinct types
of EAE based on histology, CNS chemokine profile, and response to
cytokine inhibition. J Exp Med. 205:1535–1541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lees JR, Golumbek PT, Sim J, Dorsey D and
Russell JH: Regional CNS responses to IFN-γ determine lesion
localization patterns during EAE pathogenesis. J Exp Med.
205:2633–2642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian X, Shen Q, Goderie SK, He W, Capela
A, Davis AA and Temple S: Timing of CNS cell generation: A
programmed sequence of neuron and glial cell production from
isolated murine cortical stem cells. Neuron. 28:69–80. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakakibara S and Okano H: Expression of
neural RNA-binding proteins in the postnatal CNS: Implications of
their roles in neuronal and glial cell development. J Neurosci.
17:8300–8312. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raff M, Hart I and Lillien L: Glial cell
deversification in the rat optic nerve. Cell Differ Dev.
27:1721989. View Article : Google Scholar
|
|
8
|
Doucette R: Olfactory ensheathing cells:
Potential for glial cell transplantation into areas of CNS injury.
Histol Histopathol. 10:503–507. 1995.PubMed/NCBI
|
|
9
|
Nishiyama A, Chang A and Trapp BD:
NG2+ glial cells: A novel glial cell population in the
adult brain. J Neuropathol Exp Neurol. 58:1113–1124. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furlan R, Bergami A, Cantarella D,
Brambilla E, Taniguchi M, Dellabona P, Casorati G and Martino G:
Activation of invariant NKT cells by alphaGalCer administration
protects mice from MOG35-55-induced EAE: Critical roles for
administration route and IFN-γ. Eur J Immunol. 33:1830–1838. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steinman L: A brief history of
TH17, the first major revision in the
TH1/TH2 hypothesis of T cell-mediated tissue
damage. Nat Med. 13:139–145. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Charlton B and Lafferty KJ: The Th1/Th2
balance in autoimmunity. Curr Opin Immunol. 7:793–798. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arican O, Aral M, Sasmaz S and Ciragil P:
Serum levels of TNF-α, IFN-γ, IL-6, IL-8, IL-12, IL-17, and IL-18
in patients with active psoriasis and correlation with disease
severity. Mediators Inflamm. 2005:273–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hernandez-Pando R and Rook GA: The role of
TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2
cytokine balance. Immunology. 82:591–595. 1994.PubMed/NCBI
|
|
15
|
Saha RN, Liu X and Pahan K: Up-regulation
of BDNF in astrocytes by TNF-α: A case for the neuroprotective role
of cytokine. J Neuroimmune Pharmacol. 1:212–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fiorentino DF, Zlotnik A, Vieira P,
Mosmann TR, Howard M, Moore KW and O'Garra A: IL-10 acts on the
antigen-presenting cell to inhibit cytokine production by Th1
cells. J Immunol. 146:3444–3451. 1991.PubMed/NCBI
|
|
17
|
Ouyang W, Rutz S, Crellin NK, Valdez PA
and Hymowitz SG: Regulation and functions of the IL-10 family of
cytokines in inflammation and disease. Annu Rev Immunol. 29:71–109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tagore A, Gonsalkorale WM, Pravica V,
Hajeer AH, McMahon R, Whorwell PJ, Sinnott PJ and Hutchinson IV:
Interleukin-10 (IL-10) genotypes in inflammatory bowel disease.
Tissue Antigens. 54:386–390. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benveniste EN, Tang LP and Law RM:
Differential regulation of astrocyte TNF-α expression by the
cytokines TGF-β, IL-6 and IL-10. Int J Dev Neurosci. 13:341–349.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun D and Wekerle H: Ia-restricted
encephalitogenic T lymphocytes mediating EAE lyse
autoantigen-presenting astrocytes. Nature. 320:70–72. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang ZW, Yang J, Kong Q, Zhan XX, Lailong
M, Li LL, Sun B, Zhang Y, Wang GY, Li LH, et al: Curative effect of
calcitriol on experimental autoimmune encephalomyelitis and
relevant mechanism. J Chin Bio. 11:242011.(In Chinese).
|